Abstract
Objective
To assess the magnitude of loss to follow-up in smear- or culture-positive tuberculosis patients before treatment initiation and outcomes among patients who were traced.
Methods
Ovid Medline and Global Health databases were searched for studies published between 1994 and January 2013 that described pre-treatment loss to follow-up in patients with smear- or culture-positive tuberculosis in routine national tuberculosis programmes (NTPs) in low- and lower-middle-income countries and in countries with a high burden of tuberculosis. Data on the proportion of patients who did not initiate treatment after their tuberculosis diagnosis were extracted from studies meeting inclusion criteria. Where available, data on causes and outcomes, including initiation of tuberculosis treatment at another facility, were investigated. Heterogeneity and publication bias were assessed and random-effects meta-analyses by subgroup (region) were performed.
Findings
Twenty-three eligible studies were identified, with a total of 34 706 smear- or culture-positive tuberculosis patients from 14 countries (8 in Africa, 5 in Asia and 1 in the western Pacific). Most studies were retrospective and linked laboratory and treatment registers to identify pre-treatment loss to follow-up. Pre-treatment loss to follow-up varied from 4 to 38% and was common in studies from Africa (random-effects weighted proportion, WP: 18%; 95% confidence interval, CI: 13–22) and Asia (WP: 13%; 95% CI: 10–15).
Conclusion
Pre-treatment loss to follow-up, common in most settings, can hinder tuberculosis control efforts. By not counting individuals who are lost to follow-up before treatment when reporting standard programme indicators, NTPs underestimate case detection rates and mortality and overestimate cure rates.
Résumé
Objectif
Évaluer l’ampleur du manque de suivi chez les patients atteints d’une tuberculose à frottis positif ou culture positive avant le début du traitement, ainsi que les résultats chez les patients qui ont été suivis.
Méthodes
Des études publiées entre 1994 et janvier 2013, qui décrivaient le manque de suivi des patients atteints de tuberculose à frottis positif ou à culture positive dans des programmes nationaux courants de lutte contre la tuberculose (NTP) dans les pays à revenu faible et à revenu moyen inférieur fortement touchés par la tuberculose ont été recherchées dans les bases de données Ovid, Medline et Global Health. Des données concernant le taux de patients qui n’ont pas débuté le traitement après leur diagnostic de tuberculose ont été extraites des études satisfaisant les critères d’inclusion. Le cas échéant, les données portant sur les causes et les résultats, y compris le début d’un traitement contre la tuberculose dans un autre établissement, ont été examinées. L’hétérogénéité et le parti pris dans les publications ont été évalués, et des méta-analyses à effets aléatoires par sous-groupe (région) ont été réalisées.
Résultats
Vingt-trois études éligibles ont été identifiées pour un total de 34 706 patients atteints de tuberculose à frottis positif ou à culture positive dans 14 pays (8 en Afrique, 5 en Asie et 1 dans le Pacifique occidental). La plupart des études étaient rétrospectives et associaient des registres de laboratoire et de traitement pour identifier le manque de suivi avant le traitement. Celui-ci variait de 4% à 38% et s’est révélé commun dans les études provenant d’Afrique (proportion pondérée à effets aléatoires, PP: 18%; intervalle de confiance à 95%, IC: 13–22) et d’Asie (PP: 13%; IC à 95%: 10–15).
Conclusion
Le manque de suivi avant le traitement, commun dans la plupart des contextes, peut entraver les efforts fournis en matière de lutte contre la tuberculose. Comme les individus qui ne sont pas suivis avant le traitement ne sont pas pris en compte lors de l’élaboration des rapports sur les indicateurs de programme standard, les programmes nationaux de lutte contre la tuberculose sous-estiment les taux de dépistage des cas et la mortalité, mais surestiment les taux de guérison.
Resumen
Objetivo
Evaluar la magnitud de la pérdida de seguimiento de los pacientes con tuberculosis que dieron positivo en el análisis o el cultivo antes del inicio del tratamiento y los resultados entre los pacientes que se sometieron a un seguimiento.
Métodos
Se realizó una búsqueda en las bases de datos Ovid, Medline y Global Health de estudios publicados entre 1994 y enero de 2013 que describían pérdidas de seguimiento antes del tratamiento en pacientes que dieron positivo en el análisis o el cultivo de tuberculosis en los programas nacionales contra la tuberculosis (PNT) ordinarios en países de ingresos medios y bajos y en países con carga alta de tuberculosis. Se extrajeron datos sobre la proporción de pacientes que no inició un tratamiento después del diagnóstico de la tuberculosis de estudios que cumplían los criterios de inclusión. Siempre que fue posible, se investigaron los datos sobre las causas y resultados, incluida la iniciación del tratamiento de la tuberculosis en otro centro. Se evaluó el sesgo de las publicaciones y la heterogeneidad, y se realizaron metanálisis de efectos aleatorios por subgrupos (región).
Resultados
Se identificaron veintitrés estudios que cumplían los criterios, con un total de 34 706 pacientes que dieron positivo en el análisis o cultivo de tuberculosis de 14 países (8 de África, 5 de Asia y 1 del Pacífico occidental). La mayoría de los estudios eran retrospectivos y relacionaban los registros de laboratorio con los registros de tratamiento para identificar la pérdida de seguimiento antes del tratamiento, que osciló entre el 4 y 38 % y fue frecuente en los estudios de África (proporción ponderada de efectos aleatorios, WP: 18 %; intervalo de confianza del 95 %, IC: 13–22) y Asia (WP: 13 %; IC del 95 %: 10–15).
Conclusión
La pérdida de seguimiento antes del tratamiento, común en la mayoría de los entornos, puede obstaculizar los esfuerzos de control de la tuberculosis. Sin contar los pacientes cuyo seguimiento se pierde antes del tratamiento, los programas nacionales contra la tuberculosis subestiman la mortalidad y las tasas de detección de casos, a la vez que sobrestiman las tasas de curación al informar sobre los indicadores del programa estándar.
ملخص
الغرض
تقييم حجم الفقدان المقرر متابعته في مرضى السل الذين ثبتت إيجابية نتائج اختبار اللطاخة أو المزرعة الخاص بهم قبل بدء العلاج والحصائل بين المرضى الذين تم تتبعهم.
الطريقة
تم البحث في قواعد بيانات Ovid و Medlineو Global Healthعن الدراسات التي تم نشرها في الفترة من 1994 إلى يناير 2013 التي وصفت الفقدان في مرحلة ما قبل العلاج المقرر متابعته في مرضى السل الذين ثبتت إيجابية نتائج اختبار اللطاخة أو المزرعة الخاص بهم في البرامج الوطنية الروتينية لمكافحة السل في البلدان المنخفضة الدخل وبلدان الشريحة الدنيا من الدخل المتوسط والبلدان التي ترزح تحت العبء الثقيل للسل. وتم استخلاص البيانات بشأن نسبة المرضى الذين لم يبدؤا العلاج بعد تشخيصهم بالسل من الدراسات التي لبت معايير الإدراج. وتم تحري البيانات المعنية بالأسباب والحصائل، بما في ذلك بدء علاج السل في مرفق آخر، حيثما كان ذلك متاحاً. وتم تقييم التغايرية والتحيز في النشر وإجراء التحليلات الوصفية للتأثيرات العشوائية حسب الفئة الفرعية (الإقليم).
النتائج
تم تحديد ثلاث وعشرين دراسة مؤهلة اشتملت على إجمالي 34706 مريضاً من مرضى السل الذين ثبتت إيجابية نتائج اختبار اللطاخة أو المزرعة الخاصة بهم من 14 بلداً (8 في أفريقيا و5 في آسيا وواحدة في غرب المحيط الهادئ). وكانت معظم الدراسات استرجاعية وربطت بين المختبر وسجلات العلاج لتحديد الفقدان في مرحلة ما قبل العلاج المقرر متابعته. وتراوح الفقدان في مرحلة ما قبل العلاج المقرر متابعته من 4 % إلى 38 % وكان شائعاً في دراسات أفريقيا (النسبة المرجحة للتأثيرات العشوائية: 18 %؛ فاصل الثقة 95 %، من 13 إلى 22) وآسيا (النسبة المرجحة: 13 %؛ فاصل الثقة 95 %، من 10 إلى 15).
الاستنتاج
من الممكن أن يعرقل الفقدان في مرحلة ما قبل العلاج المقرر متابعته، والذي يشيع في معظم البيئات، جهود مكافحة السل. وتقلل البرامج الوطنية لمكافحة السل من تقدير معدلات الكشف عن الحالات والوفيات وتزيد من تقدير معدلات الشفاء عند عدم عد الأشخاص الذين فقدت متابعتهم قبل العلاج عند الإبلاغ عن مؤشرات البرامج الموحدة.
摘要
目的
评估在治疗开始之前痰涂片或菌培阳性肺结核病人失访的量级和被追踪病人的结果。
方法
搜索奥维德(Ovid)、联机医学文献和检索系统(Medline)以及全球卫生(Global Health)数据库,寻找在1994 年和2013 年1 月之间发表的描述中低收入国家和肺结核病高负担国家在常规国家结核病规划(NTP)中对痰涂片阳性或者菌培阳性肺结核病人治疗前失访情况的研究。从满足入选标准的研究中,提取肺结核诊断之后没有开始治疗的患者比例方面的数据。如果有的话,还调查原因和结果数据(包括在另一个医疗设施中开始肺结核治疗的情况)。评估异质性和发表偏倚,执行子群(地区)随机荟萃分析。
结果
确认了23 个符合要求的研究,其中包括14 个国家(非洲8 个,亚洲5 个,西太平洋1 个)的总计34706 名痰涂片阳性或者菌培阳性肺结核病人。大多数研究是回顾性研究,与实验室和治疗登记相关,用以识别治疗前失访情况。治疗前失访率为4%到38%不等,常见于非洲(随机加权比例WP:18%;95%置信区间,CI:13-22)和亚洲(WP:13%;95% CI:10-15)的研究中。
结论
常见于大多数环境中的治疗前失访会妨碍肺结核控制工作。在报告标准项目指标时,由于没有计算治疗之前失访的个人数量,NTP低估了病例发现率和死亡率,高估了治愈率。
Резюме
Цель
Произвести количественную оценку числа случаев отсутствия наблюдения за мокрото- или культуропозитивными пациентами, страдающими туберкулезом легких, с момента диагностирования заболевания до начала лечения, а также обработать результаты по пациентам, которых удалось отследить.
Методы
В базах данных Ovid, Medline и Global Health был произведен поиск исследований, проведенных в рамках стандартных национальных программ по борьбе с туберкулезом в странах с низкими доходами и доходами ниже среднего уровня, а также в странах с высоким бременем туберкулеза, опубликованных в период с 1994 года по январь 2013 года, и содержащих описания случаев отсутствия наблюдения до начала лечения за мокротопозитивными или культуропозитивными пациентами, страдающими туберкулезом легких. Данные по пропорциональному количеству пациентов, которые не начали лечение после диагностирования туберкулеза, были извлечены из исследований, соответствующих критериям включения в данный анализ. По мере возможности, также были исследованы данные по причинам и последствиям, включая прохождение лечения в другом медицинском учреждении. Также была проведена оценка разнородности и систематических ошибок, связанных с предпочтительной публикацией положительных результатов исследований, а также мета-анализ случайных эффектов по подгруппам (регионам).
Результаты
Было выявлено 23 исследования, соответствующие критериям включения в данный анализ, которые охватывали в целом 34 706 мокротопозитивных или культуропозитивных пациентов, страдающих туберкулезом легких из 14 стран (8 в Африке, 5 в Азии и 1 в регионе Западной части Тихого океана). Большинство исследований были ретроспективными и включали анализ журналов регистраций результатов лабораторных анализов и прохождения лечения, что позволило выявить случаи непрохождения последующего наблюдения до начала лечения. Показатель непрохождения последующего наблюдения до начала лечения колебался от 4 до 38%, где наиболее высокие значения были отмечены в исследованиях, проводимых в Африке (взвешенная пропорция случайных эффектов, ВП: 18%; 95% доверительный интервал, ДИ: 13–22) и Азии (ВП: 13%; 95% ДИ: 10–15).
Вывод
Непрохождение пациентами наблюдения от момента обнаружения заболевания до начала лечения, при схожих значениях остальных параметров, может снижать эффект от принимаемых мер по борьбе с туберкулезом. Неучет лиц, за которыми не велось наблюдение до начала лечения, и их невключение в отчеты со стандартными показателями программы приводит к тому, что национальные программы по борьбе с туберкулезом занижают показатели выявления случаев заболевания и смертности, одновременно завышая оценки показателей эффективности лечения.
Introduction
Since tuberculosis was declared a global emergency in 1993 by the World Health Organization (WHO), new cases of tuberculosis and deaths from the disease have dropped dramatically in several countries with a high burden of the disease.1 All six WHO regions are on track to meet the Millennium Development Goal target of reducing tuberculosis incidence and deaths from tuberculosis by half between 1990 and 20151,2 and, with the sole exception of the African Region, all are on track to halve tuberculosis mortality rates.2 Nevertheless, the situation remains precarious.3 Twenty-two predominantly low- and middle-income countries were estimated to account for 82% of the 5.7 million tuberculosis cases notified in 20101 and high rates of death from tuberculosis among people living with human immunodeficiency virus (HIV) infection prevail in much of sub-Saharan Africa.4,5
Rapid case identification of individuals with sputum smear-positive tuberculosis and rapid initiation of anti-tuberculosis chemotherapy are key to controlling tuberculosis6 and are promoted as part of the DOTS strategy model of passive case-finding that has been adopted by most national tuberculosis programmes (NTPs).7 From the patient’s perspective, the tuberculosis diagnostic and care pathway (Fig. 1) begins with a recognition of symptoms that prompt care seeking. Individuals may drop out of care during the diagnostic process (“loss to follow-up during diagnostic period”), before initiating treatment (“pre-treatment loss to follow-up”, formerly known as “initial default”) or after treatment has begun. Patients diagnosed with smear-positive tuberculosis who do not initiate treatment represent an important failing in the provision of care.8,9 High rates of mortality are reported in this group.10 Moreover, bringing these patients into care could reduce tuberculosis transmission to others.11 Patients with a diagnosis of tuberculosis who are lost to follow-up before they receive treatment are not included in routine reporting by NTPs. Thus, programme effectiveness may be overestimated.8
Fig. 1.
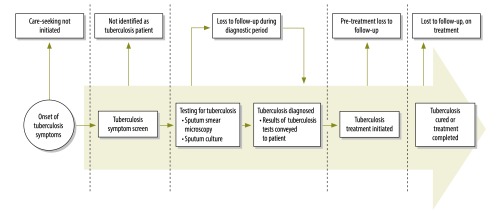
The diagnostic and care pathway for tuberculosis
Efforts to improve tuberculosis case detection rates have centred on ensuring rapid treatment for all individuals diagnosed with smear-positive tuberculosis.12,13 With this goal in mind, WHO has recently changed its policy, which now calls for two sputum specimens instead of three and same-day collection.13,14 However, assessing the impact of these changes on linkage to treatment has been hampered by a lack of understanding of the extent of pre-treatment loss to follow-up8 and of the patient, provider and health system factors that contribute to it.15
Although nearly 50 years have passed since high rates of pre-treatment loss to follow-up were first identified as a potential major contributor to the failure of tuberculosis control programmes, researchers and policy-makers have paid little attention to the fate of patients who do not access treatment after receiving a diagnosis of tuberculosis.16,17,18 Indeed, the “Piot model” used to describe loss to care at different stages for any disease was first developed for tuberculosis.18
This study had two main objectives: (i) to systematically quantify pre-treatment loss to follow-up in low- and lower-middle income countries and in countries with a high burden of tuberculosis; and (ii) to describe the reasons for drop-out and the outcomes seen in individuals with a tuberculosis diagnosis who do not initiate treatment. A secondary objective was to assess the quality of the studies reporting on pre-treatment loss to follow-up.
Methods
Definitions
We followed PRISMA reporting guidelines for systematic reviews.19 To define the points at which tuberculosis patients drop out of care, we developed the tuberculosis diagnostic and care pathway described in Fig. 1 using terms recommended recently that replace previously used terms such as “initial default”.9
For this study, patients in a national tuberculosis care programme who received a diagnosis of tuberculosis on the basis of at least one positive sputum smear or culture but did not start tuberculosis treatment were defined as having pre-treatment loss to follow-up. This included individuals who died before initiating treatment.
The recruitment period was defined as the time during which patients with a diagnosis of tuberculosis were recruited to studies or during which data from such patients were extracted from national programme registers. For studies with individual follow-up, the follow-up period was defined as the time between diagnosis and the most recent date of active follow-up. For studies in which tuberculosis treatment registers were checked retrospectively, we present the minimum and maximum follow-up periods available. Because studies had different follow-up periods and varying temporal definitions for pre-treatment loss to follow-up, we used the definition given in each study rather than a time-delineated definition. However, we did require a follow-up period of at least 4 weeks to allow enough time for patients to link to care and treatment.
Inclusion and exclusion criteria
Studies were included in the review if they reported on the proportion of patients having smear- or culture-positive tuberculosis who experienced pre-treatment loss to follow-up in NTPs in low- or lower-middle-income countries as defined by the World Bank on 1 July 2011,20 or in any of the 22 countries with a high burden of tuberculosis as defined by the Stop TB department of WHO.1 Studies that reported on clinical trials, including randomized and non-randomized active case-finding studies, were excluded because participants in these studies would be more likely to receive intensive follow-up and tracing and would not be representative of patients with tuberculosis diagnosed routinely. Studies that reported only on paediatric patients – i.e. children 15 years of age or younger – were excluded. Studies that recruited both adults and children were included even if the data were not disaggregated by age group.
Search strategy
We systematically searched the Ovid, Medline and Global Health databases for studies published between 1 January 1994 and 31 January 2013. Our search strategy is outlined in Table 1. We also hand searched the abstracts of the Union World Conference on Lung Health from 2009 to 2012. We identified additional studies through reference lists and annotated bibliographies and by corresponding with researchers in the field. If the manuscript did not give the absolute number of individuals with pre-treatment loss to follow-up, we contacted the authors to obtain the data.
Table 1. Systematic strategy used to search for studies on pre-treatment loss to follow-up in tuberculosis patients.
| Set | MEDLINE | Global Health |
|---|---|---|
| 1 | tuberculosis | tuberculosis |
| 2 | TB | patient refusal of treatment |
| 3 | TUBERCULOSIS | dropout |
| 4 | Sets 1–3 were combined with “OR” | dropouts |
| 5 | PATIENT DROPOUT | referral |
| 6 | DELAYED DIAGNOSIS | delayed |
| 7 | REFERRAL AND CONSULTATION | attrition |
| 8 | DIAGNOSTIC SERVICES | retain |
| 9 | TUBERCULOSIS, PULMONARY DIAGNOSIS | treatment programme |
| 10 | initial delay* | initial delay |
| 11 | initial default* | initial default |
| 12 | drop out | retention |
| 13 | attrition | diagnostic delay |
| 14 | retention | treatment delay |
| 15 | retain* | care seeking |
| 16 | diagnostic delay* | loss to follow-up |
| 17 | treatment delay* | lost to follow-up |
| 18 | treatment seek* | Sets 2–17 were combined with “OR” |
| 19 | care seek* | Sets 1 and 18 were combined with “AND” |
| 20 | loss to follow-up | Set 19 was limited to 1994–2013 |
| 21 | lost to follow-up | |
| 22 | loss to follow-up | |
| 23 | lost to follow-up | |
| 24 | Sets 1–23 were combined with “OR” | |
| 25 | Sets 4 and 24 were combined with “AND” | |
| 26 | Set 25 was limited to 1994–2013 |
Note: Words written in capital letters were used as MeSH headings; the others were used as free text. The asterisks are the truncation symbol in Medline (i.e. any possible ending after the preceding text).
Three authors (KK, PM, RH) reviewed titles and abstracts to obtain the full texts of relevant articles. All three assessed the full texts to determine their suitability and based their final inclusion in the review on consensus as a team. PM and KK extracted data from included studies using a pre-designed table.
Quality of selected studies
One researcher (PM) used a modified version of the Newcastle-Ottawa scale to assess studies in terms of quality and of the risk of bias in the selection of participants and in the ascertainment of outcomes. Each study could score up to six points in each of these two categories, each having six items. The section for the selection of comparison groups was removed from the Newcastle-Ottawa scale because no study had a comparison group. The factors considered included: the representativeness of the patients recruited with respect to the underlying population of tuberculosis patients diagnosed in the routine health-care system; the test used to ascertain the diagnosis of tuberculosis; the method of identification of pre-treatment loss to follow-up; and the adequacy of follow-up (judged in terms of the proportion of participants whose outcomes were ascertained, with > 85% being adequate). In studies in which laboratory and treatment registers were linked, we evaluated the process and variables used for linkage (including personal identifiers and dates).
Data analysis and statistical methods
For each included study, we report on the number of patients who received a diagnosis of smear- or culture-positive tuberculosis and the proportion who initiated antituberculosis treatment. For patients identified as having experienced pre-treatment loss to follow-up, we report the duration of follow-up and, if available, the proportion who were successfully traced and their outcomes (alive but not on treatment; alive after starting treatment; deceased; or transferred to another facility but treatment and vital status unknown). To calculate summary estimates of pre-treatment loss to follow-up, we classified as treatment initiators those tuberculosis patients who were classified as having experienced pre-treatment loss to follow-up but who, on tracing, were found to have initiated treatment at an alternative site. We assessed heterogeneity using the I2 statistic. On initial analysis, we found substantial heterogeneity between studies. Therefore, we estimated the pooled proportion of patients with a diagnosis of tuberculosis and pre-treatment loss to follow-up (and the corresponding 95% confidence intervals) using a random-effects model, weighting for the inverse of the variance and stratification by study region. Stata 12.1 (Statacorp, College Station, Texas, USA) was used to analyse the data.
Ethics statement
Ethical approval was not required for this study.
Results
Study characteristics
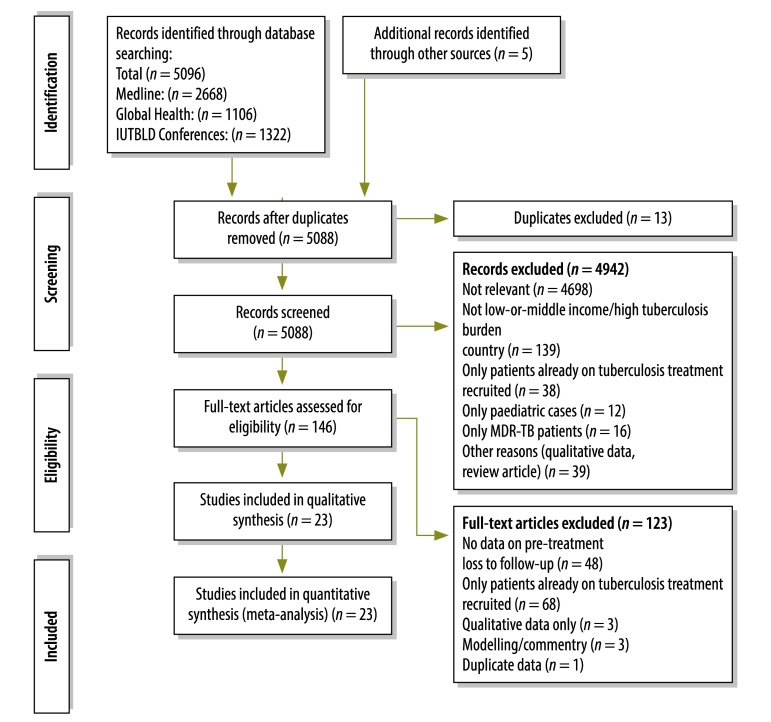
We identified 5096 potentially relevant studies, of which 23 were eligible for inclusion in the analysis (Fig. 2).10,21–42 These reported on a total of 34 706 patients with smear- or culture-positive tuberculosis, 3474 of whom had experienced pre-treatment loss to follow-up. The characteristics of the included studies are summarized in Table 2. There were 13 studies from sub-Saharan Africa (8 countries), 9 from Asia (5 countries) and 1 from the western Pacific (1 country).
Fig. 2.
Flowchart for the selection of studies on pre-treatment loss to follow-up in patients with a diagnosis of tuberculosis
IUTBLD, International Union Against Tuberculosis and Lung Disease; MDR-TB, multidrug resistant-tuberculosis.
Table 2. Characteristics of studies included in the review and proportion of smear-positive tuberculosis patients who initiated treatment.
| Study | Year(s) study conducted | Country | Setting | Diagnostic criterion | Recruitment period | No. with diagnosis of tuberculosis | Follow-up period | Temporal definition of pre-treatment loss to follow-up | Method used to confirm start of treatment | No. (%) of patients initiating treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Creek, 200027 | 1997 | Botswana | Gaborone, outpatient department of public hospital and 13 PHCs | ≥ 1 positive smear | 5 months | 184 | 5–22 months | 2 weeks | Retrospective linking of laboratory register and national electronic tuberculosis register | 165 (90) |
| Dembele, 200628 | 2001 | Burkina Faso | 6 districts (including the capital) | Any smear positive | 1 year | 31 | NR | ND | Retrospective linking of laboratory and treatment registers | 27 (87) |
| Afutu, 201221 | 2009 | Ghana | Regional hospital | Smear positive not further specified | 1 year | 84 | 9–27 months | ND | Retrospective linking of laboratory and treatment registers | 52 (62) |
| Glynn, 199830 | 1986–1994 | Malawi | Rural PHCs, one district hospital | ≥ 1 positive smear | 90 months | 682 | Up to 110 months | ND | Prospective monthly follow-up as part of Demographic and Health Survey | 642 (94) |
| Nyirenda, 199832 | 1997 | Malawi | National | Smear positive, not further specified | 6 months | 3482 | 2–8 monthsa | ND | Retrospective linking of laboratory and treatment registers | 2980 (86) |
| Squire, 200510 | 2000 | Malawi | Rural, 31 PHCs, one district hospital | Smear positive, not further specified | 6 months | 157 | 0–6 months | ND | Retrospective linking of laboratory and treatment registers; home tracing of patients with missing treatment information | 134 (85) |
| Uchenna, 201236 | 2009 | Nigeria | 5 states in southern Nigeria | Smear positive, not further specified | 3 months | 323 | Up to 3 months | 2 days | Retrospective collation of total number of tuberculosis patients diagnosed in laboratory registers and treated in treatment registers (individual records not linked) | 268 (83) |
| Botha, 200823 | 2004–2005 | South Africa | 13 PHCs | ≥ 2 positive smears | 1 year | 367 | 3–15 months | 3 months | Retrospective linking of sputum collection register and treatment register | 303 (83) |
| Botha, 200824 | 2005 | South Africa | 11 PHCs in the Western Cape province (8 in Cape Town metropolitan area) | ≥ 2 positive smears | 3 months | 227 | 4–16 months | 2 months | Retrospective linking of laboratory and treatment registers | 203 (89) |
| Claassens, 201037 | 2009 | South Africa | 133 PHCs in 5 provinces | Smear positive, not further specified | 5 months | 3020 | NR | 1 month | Retrospective linking of laboratory and treatment registers | 2268 (75) |
| Dunbar, 201129 | 2007 | South Africa | Two community clinics | Bacteriologically confirmed | 1 year | 306 | Up to 24 monthsb | 2 months | Retrospective linking of laboratory and treatment registers | 243 (79) |
| Davis, 201141 | 2009 | Uganda | Five PHCs | ≥ 1 positive smear | 1 year | 81 | NR | ND | Prospective cohort follow-up, with additional retrospective linkage of laboratory and treatment registers for confirmation | 62 (77) |
| Chadambuka, 201126 | 2006 | Zimbabwe | Gokwe district | Smear positive, not further specified | 1 year | 112 | 1 month | ND | Retrospective linking of laboratory and treatment registers/”stock cards” | 82 (73) |
| Balasubramanian, 200422 | 1998–2001 | India | PHCs | ≥ 2 positive smears | 2 years | 833 | 3 months | 3 months | Retrospective linking of laboratory register and patient records | 713 (86) |
| Gopi, 200531 | 2001–2003 | India | One PHC | Smear positive, not further specified | 31 months | 1049 | 2 months | 2 months | Prospective reconciliation of laboratory and treatment registers | 893 (85) |
| Sai Babu, 200834 | 2006 | India | 20 districts in Andhra Pradesh state | ≥ 2 positive smears | 3 months | 15 361 | Cross-sectional: identification of all initial defaulters in one quarter of 2006 | ND | Extraction of data from laboratory register; home tracing of patients with missing treatment information | 14 676 (96) |
| Razia, 201139 | 2009 | Pakistan | One district, including 16 peripheral centres and five tertiary centres | Smear positive, not further specified | 1 year | 1698 | Up to one year | ND | Retrospective linking of laboratory and treatment registers | 1597 (94) |
| Rao, 200933 | 2007–2008 | Pakistan | Chest clinic, Karachi | Smear positive, not further specified | 5 months | 224 | Patients prospectively recruited during a 5-month period; time before tracing undertaken not defined | ND | Telephone tracing of patients who did not return for treatment | 162 (72) |
| Rao, 201140 | 2010 | Pakistan | Chest clinic, Karachi | Smear positive, not further specified | 6-months | 1121 | Up to 6 months | ND | Telephone tracing of patients who did not return for treatment | 947 (84) |
| Korobitsyn, 201038 | 2008–2009 | Tajikistan | Four districts | Smear positive, not further specified | 1 year | 254 | Up to one year | ND | Retrospective linking of laboratory and treatment registers | 209 (82) |
| Uthaivoravit, 200335 | 1995 | Thailand | Provincial referral hospital | ≥ 1 positive smear | 60 months | 212 | “Mid-1996” to “the end of 2000”. Reported in yearly cohorts | ND | Prospective linking of laboratory, treatment register and medical records | 168 (79) |
| Buu, 200325 | 2000 | Viet Nam | District tuberculosis units | ≥ 1 positive smear | 1 year | 4208 | 1 month | 1 month | Retrospective linking of laboratory and treatment registers | 3859 (92) |
| Ram, 201242 | 2001–2010 | Fiji | 4 laboratories and 2 DOTS sites | ≥ 1 positive smear | 9 years | 690 | NR | ND | Retrospective linking of laboratory and treatment registers | 579 (84) |
ND, not defined; NR, not reported; PHC, primary-health-care centre.
a The period during which tuberculosis laboratory registers were reconciled with tuberculosis treatment registers is not reported. The “data collection period” is given as ranging between 2 and 8 months.
b The treatment records of all individuals with bacteriologically confirmed tuberculosis during 2007 were identified by searching electronic treatment registers for 2007 and 2008.
Most studies reported on pre-treatment loss to follow-up among smear-positive patients only. Two studies included patients who were either smear- or culture-positive.29,30 In some studies smear positivity was defined as at least 125,27,28,30,35,41,42 or at least 222–24,34 positive smears, whereas others did not provide any definition.10,21,26,31–33,36–40 A study from South Africa stratified rates of reported pre-treatment loss to follow-up by smear status (smear-positive or smear-negative but culture-positive),23 whereas another study, also from South Africa, reported on pre-treatment loss to follow-up in tuberculosis patients whose diagnosis was established clinically and/or bacteriologically.29
Quality of included studies
The quality of the included studies varied (Table 3). Only a few studies (n = 4) showed a low risk of bias or scored full marks across all items assessing patient selection and ascertainment of outcomes. The methods for ascertaining pre-treatment loss to follow-up were suboptimal or poorly described in most studies; only seven studies adequately described the follow-up period allotted to each participant. The majority of studies (n = 19) identified patients diagnosed with tuberculosis by extracting data from laboratory or sputum collection registers (Table 2). Such extraction was performed retrospectively in 17 studies and prospectively in two. In the remaining 4 studies, patients with a diagnosis of tuberculosis were identified as part of ongoing epidemiological surveillance30 or were prospectively recruited for follow-up from a chest clinic33,40 or from primary-health-care centres.41 The recruitment periods ranged from 3 months24,34,36 to 90 months.30 Only 9 studies22–25,27,29,31,36,37 applied a cut-off for time since diagnosis – ranging from 1 month to 3 months – to define pre-treatment loss to follow-up.
Table 3. Modified Newcastle-Ottawa Scale for assessment of the quality of the studies included in the review of pre-treatment loss to follow-up in tuberculosis patientsa.
| Author | Country | Selectionb | Outcomec |
|---|---|---|---|
| Creek27 | Botswana | *** | ** |
| Dembele28 | Burkina Faso | *** | * |
| Afutu21 | Ghana | *** | ** |
| Glynn30 | Malawi | *** | *** |
| Nyirenda32 | Malawi | *** | * |
| Squire10 | Malawi | *** | ** |
| Uchenna36 | Nigeria | *** | * |
| Botha23 | South Africa | *** | *** |
| Botha24 | South Africa | *** | *** |
| Claassens37 | South Africa | *** | * |
| Dunbar29 | South Africa | *** | *** |
| Davis41 | Uganda | *** | * |
| Chadambuka26 | Zimbabwe | ** | |
| Balasubramanian22 | India | ** | |
| Gopi31 | India | ** | * |
| Sai Babu34 | India | *** | * |
| Razia39 | Pakistan | *** | * |
| Rao33 | Pakistan | ** | |
| Rao40 | Pakistan | ||
| Korobitsyn38 | Tajikistan | *** | * |
| Uthaivoravit35 | Thailand | *** | * |
| Buu25 | Viet Nam | *** | ** |
| Ram42 | Fiji | *** | * |
a A study can be awarded a maximum of one star for each of three items within the “selection” and “outcome” categories.
b Assessment of patient selection comprised three items (those that score stars are shown): (i) representativeness of the cohort (truly representative,* somewhat representative,* selected group of users, no description of derivation); (ii) ascertainment of tuberculosis diagnosis (secure records/registers,* structured interviews,* written self-report, no description); (iii) demonstration that treatment for tuberculosis was not being taken at recruitment (secure records/registers,* structured interviews,* written self-report, no description).
c Assessment of outcome comprised three items (those that score stars are shown): (i) ascertainment of pre-treatment loss to follow-up (secure records/registers,* structured interviews,* written self-report, no description); (ii) sufficient follow-up time to allow outcome to occur (4 weeks) (yes,* no); (iii) adequacy of follow-up (complete*, follow-up > 80%*, follow-up < 80%, no description).
Although most studies (n = 16) used retrospective linkage of laboratory and treatment registers to identify patients who initiated treatment for tuberculosis10,21–29,32,37–39,41,42 – and so scored full marks for this item – the quality of the procedures used to ensure accurate linkage varied considerably. Only one study27 described the variables used to link records and gave the proportion of records that were reliably matched.
Pre-treatment loss to follow-up
The proportion of patients with a diagnosis of tuberculosis who experienced pre-treatment loss to follow-up ranged from 4 to 38%.21,34 In studies from Africa pre-treatment loss to follow-up ranged from 6 to 38%, whereas in studies from Asia it ranged from 4 to 28%. Studies that reported on data from a single clinical site21,22,26,29,31,33,35,40 had higher rates of pre-treatment loss to follow-up (range: 14–38%) than studies reporting on national or regional data (range: 4–25%).10,23–25,27,28,30,32,34,36–39,41,42
In total, 10 studies10,24,25,30–34,38,40 attempted to trace tuberculosis patients with pre-treatment loss to follow-up (Table 4). One of them did not detail the tracing method used.38 Tracing rates were rather poor on average. The proportion of patients who could not be traced ranged from 0%30 to 77%.32 This limited our ability to draw inferences about the fate of tuberculosis patients with pre-treatment loss to follow-up.
Table 4. Outcomes observed in studies of pre-treatment loss to follow-up in tuberculosis patients.
| Author | Country | No. lost to follow-up before treatment | Patients not traced |
Patients traced |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % (95% CI) | No. | Deceased |
No. treated elsewhere (private sector) | No. transferred, treatment status unknown | Alive, not on treatment |
Losta
|
||||||||
| No. | % (95% CI) | No. | % (95% CI) | No. | % 95% CI) | ||||||||||
| Glynn30 | Malawi | 40 | 0 | 0 (0.00–0.09) | 40 | 25 | 0.63 (0.47–0.76) | 0 | 0 | 0 | 0 (0.00–0.09) | 15 | 0.38 (0.24–0.53) | ||
| Nyirenda32 | Malawi | 502 | 386 | 0.77 (0.73–0.80) | 116b | 40 | 0.34 (0.26–0.44) | 27 (outside district) | 23 | 2 | 0.02 (0.00–0.06) | 20 | 0.17 (0.11–0.25) | ||
| Squire10 | Malawi | 20 | 3 | 0.15 (0.05–0.36) | 17 | 14 | 0.82 (0.59–0.94) | 0 | 1 | 2 | 0.12 (0.04–0.40) | 0 | 0 (0.00–0.18) | ||
| Botha24 | South Africa | 58 | 26 | 0.45 (0.33–0.58) | 32 | 14 | 0.44 (0.28–0.61) | 0 | 0 | 18 | 0.56 (0.39–0.72) | 0 | 0 (0.00–0.11) | ||
| Gopi31 | India | 156 | 79 | 0.51 (0.43–0.58) | 77 | 23 | 0.30 (0.21–0.41) | 13 (outside area) | 0 | 33 | 0.39 (0.29–0.45) | 8 | 0.09 (0.05–0.17) | ||
| Sai Babu34 | India | 685 | 402 | 0.59 (0.55–0.62) | 278c | 152 | 0.55 (0.49–0.60) | 38 (private) | 22 | 28 | 0.10 (0.07–0.14) | 38 | 0.13 (0.10–0.18) | ||
| Rao33 | Pakistan | 62 | 7 | 0.11 (0.06–0.22) | 55 | 0 | 0 (0.00–0.06) | 6 (private) | 0 | 25d | 0.45 (0.33–0.58) | 0 | 0 (0.00–0.06) | ||
| Rao40 | Pakistan | 173 | 82 | 0.47 (0.40–0.55) | 91 | 1 | 0.01 (0.00–0.06) | 15 (private) | 0 | 0e | 0 (0.00–0.04) | NS | NS | ||
| Korobitsyn38 | Tajikistan | 45 | 27 | 0.60 (0.45–0.73) | 18 | 2 | 0.11 (0.03–0.33) | 0 | 0 | 3 | 0.17 (0.06–0.39) | 3 | 0.72 (0.49–0.88) | ||
| Buu25 | Viet Nam | 349 | 174 | 0.50 (0.45–0.55) | 175f | NS | NS | 108 (private) | NS | 67 | 0.38 (0.31–0.46) | NS | NS | ||
CI, confidence interval; NS, not stated.
α Patients with known addresses, but when the home visit was conducted they had moved away but were known to be alive.
b Four patients were discharged from hospital before smear result was reported.
c Nineteen patients were follow-up cases, not new diagnoses; 24 were chronic cases, not new diagnoses.
d Twenty-four patients were traced and put on tuberculosis treatment.
e Ninety patients were traced and put on tuberculosis treatment.
f Three patients did not want to participate in the study and five patients were judged not to have tuberculosis.
Note: The 95% CIs were calculated by authors using data in selected studies.
Six studies – five of them from Asia – reported that patients who had initially been classified as being lost to follow-up before being treated had in fact initiated treatment for tuberculosis at another clinical facility.25,31,32,33,34,40 In the Asian studies, transfer to a private clinic for tuberculosis treatment was the commonest reason for pre-treatment loss to follow-up; from 0 to 62% of patients were found to have been treated at private clinics, although only one such study successfully traced more than 80% of the patients.33 In the only study from Africa that traced individuals and recorded if they initiated treatment elsewhere, 23% of tuberculosis patients who were initially classified as lost to follow-up before treatment in Malawi had started treatment for tuberculosis in another district.32
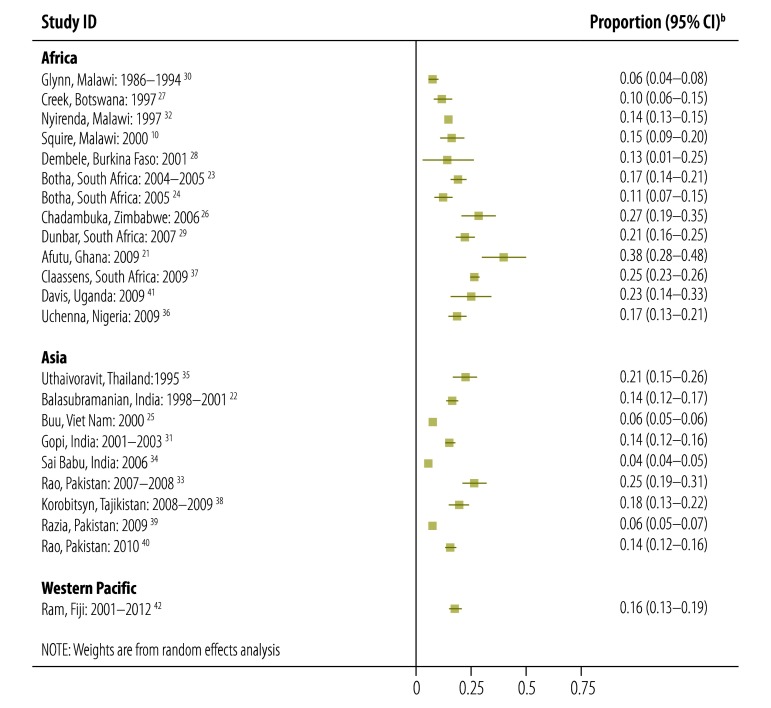
When we counted traced individuals who had initiated treatment at an alternative site as tuberculosis treatment initiators, we noted substantial heterogeneity between studies in rates of pre-treatment loss to follow-up (I2 = 98.4%; P < 0.001). This remained after stratifying by study region (Africa: I2 = 96.1%; P < 0.001; Asia: I2 = 98.0%; P < 0.001; western Pacific: one study only). The funnel plot showed asymmetry, suggestive of publication bias (Egger’s statistic: P < 0.001). Fig. 3 shows a forest plot for the included studies, stratified by region. In random-effects meta-analysis, the overall inverse-weighted proportion of patients with a diagnosis of tuberculosis who experienced pre-treatment loss to follow-up was 16% (95% confidence interval, CI: 13–18). Although this proportion was 18% in studies from Africa (95% CI: 13–22) and hence higher than in Asian studies, where it was 13% (95% CI: 10–15%), the CIs overlapped.
Fig. 3.
Proportion of patients with a diagnosis of tuberculosis who were lost to follow-up before treatmenta in 23 studies from Africa, Asia and the western Pacific
CI, confidence interval.
a All studies combined represent 34 706 patients with a diagnosis of tuberculosis; 3267 were lost to follow-up before treatment.
b The 95% CIs were calculated by authors from data provided in selected studies.
Note: In studies in which patients were traced, individuals with a diagnosis of tuberculosis who were lost to follow-up before treatment but who had started treatment for tuberculosis elsewhere are included as having initiated treatment.
Case fatality
Among traced tuberculosis patients with pre-treatment loss to follow-up, the case fatality rate ranged from 0% (95% CI: 0–6)40 to 82% (95% CI: 59–94).10 The risk of death was highest in studies from Africa but varied widely and low rates of tracing rendered it unreliable. Only the study from Malawi described the time from diagnosis to death among 19 patients who were traced:10 a median of 3.5 weeks (range: 2–12) in 14 deceased patients.
Reasons for loss to follow-up
Factors associated with an increased risk of pre-treatment loss to follow-up were male sex, older age,31 living in an urban area,25 diagnosis in a hospital or stationary clinic (rather than a mobile clinic),23 geographical location of the tuberculosis laboratory (regional versus local),42 and being diagnosed with smear-negative but culture-positive tuberculosis.24 However, distance to treatment site was not associated with the risk of pre-treatment loss to follow-up in Ghana.21
Of the nine studies that traced patients with pre-treatment loss to follow-up, one undertook in-depth qualitative interviews to determine the reasons for drop-out,10 and the other eight were based on structured patient interviews, either in person24,25,30–32,34,40 or by telephone.33,40 Health-system-related obstacles for not starting treatment for tuberculosis included dissatisfaction with long waiting times in health services, the need for repeated visits, and delays in receiving the results of sputum smears.10,25,29,31 Some reasons for not starting treatment for tuberculosis were patient-related (e.g. difficulty getting time off from work or a lack of understanding of tuberculosis, its severity or the potential benefits of treatment).10,24,31,33,40 Other reasons were disease-related (e.g. weakness and fatigue).31,32
Discussion
This review highlights the paucity of data on pre-treatment loss to follow-up among patients with a diagnosis of tuberculosis, despite high prevalence and mortality rates. Only 23 studies from 14 countries were identified over a period of 17 years, in sharp contrast with the 37 studies on HIV care programmes in low-resource settings that were published in a period of 5 years.43 Yet pre-treatment loss to follow-up in patients with smear-positive tuberculosis is an important problem for tuberculosis programmes because these patients are highly infectious44 and experience high morbidity and mortality.45,46
In the studies identified in this review, pre-treatment loss to follow-up was high – from 4 to 38% – and was higher in sub-Saharan Africa (18%) than in Asia (13%). Given the very high risk of death among tuberculosis patients who are not promptly treated, minimizing treatment delay and losses at all stages in the diagnostic and care pathway is critically important.12 Although tracing was suboptimal in most studies, the main reason for pre-treatment loss to follow-up was death, especially in countries in sub-Saharan Africa with generalized epidemics of HIV infection, perhaps because of the high mortality among patients having both tuberculosis and HIV infection.47,48 It is difficult to ascertain whether these deaths are caused by or result from lack of treatment. Only one study reported the time between diagnosis and death in patients who did not start tuberculosis treatment; the median of 3.5 weeks found in the study suggests that patients were severely ill at the time of diagnosis.10
The diagnostic and care pathway is often costly and long, even in settings where health care and diagnostic tests are free at the point of delivery. Reducing costs and time for the patient might improve linkage to treatment. Thus, NTPs should consider the following measures: (i) reducing the number of sputum samples for initial diagnosis from three to two;49 (ii) replacing “spot-morning-spot” sputum collection (requiring visits to the facility on two separate days) with collection of two spot sputum samples one hour apart;14 (iii) preparing two smears from the same sputum specimen;50 and (iv) introducing same-day light-emitting diode (LED) microscopy51 or automated nucleic acid molecular diagnostics,52 shown to be more sensitive and associated with reduced time to diagnosis and lower pre-treatment loss to follow-up. Further evaluation of the impact of these interventions on reducing pre-treatment loss to follow-up is required.
Health system factors, particularly relating to the recording and registration of suspected and confirmed tuberculosis cases, were found to be important contributors to pre-treatment loss to follow-up in several studies. Moreover, in many studies researchers were required to reconcile laboratory registers with treatment registers to determine the pre-treatment loss to follow-up rate, a task not easy to perform regularly under routine programmatic conditions. These issues could be addressed by using a single patient identifier for the entire diagnostic and care pathway for tuberculosis. Patients attending a facility with a positive screening for symptoms of tuberculosis would be recorded in a “cough register”53 for subsequent monthly tracing of those whose smear results had not been received and of smear-positive patients who had not returned for treatment.
By not including individuals lost to follow-up before treatment when reporting standard programme indicators, NTPs incorrectly report case detection, cure and case fatality rates. For example, with DOTS strategy targets of 70% case detection and 85% cure rate, including individuals who experience pre-treatment loss to follow-up (using 18% in Africa and 13% in Asia, for illustration), would result in the true case detection rate rising from 70% to 85% in African countries and 70% to 80% in Asian countries, as those diagnosed but not started on treatment are included (Table 5). The cure rate however would drop from 85% to 70% in Africa and from 85% to 74% in Asia, as those detected but not started on treatment are counted as “not cured”. Moreover, in African countries, where the higher death rate in individuals with pre-treatment loss to follow-up could be attributable to HIV infection, the reported death rate would increase from 6 to 12%. In NTPs from Asia, the death rate would be unchanged at 3%. These numbers better reflect NTP’s actual performance – they are finding more cases than thought, but are not performing so well at providing treatment.
Table 5. Impact of including rates of pre-treatment loss to follow-up on national tuberculosis programme indicators in hypothetical programmes in Africa and Asia with 100 000 individuals and DOTS strategy targets (70% case detection, 85% cure) theoretically achieved, 2011.
| Indicator | Africa |
Asia |
|||
|---|---|---|---|---|---|
| Outcomes reported under current WHO targets | After including tuberculosis patients lost to follow-up before treatment | Outcomes reported under current WHO targets | After including tuberculosis patients lost to follow-up before treatment | ||
| No. of cases | 100 000 | 100 000 | 100 000 | 100 000 | |
| Cases detected, no. (%) | 70 000 (70) | 85 366a (85) | 70 000 (70) | 80 460a (80) | |
| Tuberculosis patients lost to follow-up before treatment, no. (%) | Unknown | 15 366 (18) | Unknown | 10 460 (13) | |
| Cases started on treatment, no. (%) | Unknown | 70 000 (82)b | Unknown | 70 000 (87)b | |
| Patients cured, no. (%) | 59 500 (85) | 59 500 (70)c | 59 500 (85) | 59 500 (74)c | |
| Deceased tuberculosis patients, no. (%) | 4200d (6) | 12 498e (12) | 2100d (3) | 3251e (3) | |
WHO, World Health Organization.
a Calculated as [1 ÷ (1 − fraction lost to follow-up before treatment)] × number of cases detected. For Africa: [1 ÷ 0.82] × 70 000; for Asia: [1 ÷ 0.87] × 70 000.
b Percentage calculated as number of cases initiating tuberculosis treatment divided by the number of cases detected. For Africa: 70 000 ÷ 85 366; for Asia: 70 000 ÷ 80 460.
c Percentage calculated as the number of patients who successfully completed treatment divided by the number of cases detected. For Africa: 59 500 ÷ 85 366; for Asia: 59 500 ÷ 80 460.
d Number obtained from WHO country database.
e Calculated as the number of deceased tuberculosis patients plus the product of the number of cases lost to follow-up before treatment and the median case fatality rate found in this review. For Africa: 4200 + (15 366 × 0.54); for Asia: 2100 + (10 460 × 0.11).
Note: Estimates for the western Pacific region not included, as only one study was identified.
In studies from Asia, where more private practitioners offer tuberculosis treatment services alongside NTPs,54,55 a small number of patients lost to follow-up initiated treatment with private providers. Since they would not be included in NTP reports of outcomes, the success of the national programme would be underestimated. Interventions to improve links and data sharing between NTPs and private providers have proved effective in increasing case detection rates in studies from Asia56,57 and are promoted by WHO.58 Further expansion of such interventions will help to ensure that programme outcomes are accurately reported at the national level.
A limitation of this analysis is the poor quality of outcome ascertainment in several studies. The small number of traced individuals who had initiated treatment under a different provider underscores the need to tailor tuberculosis services to the individual patient and the difficulty of accurately estimating outcomes at the programme level. The varying length of follow-up of tuberculosis patients in cohort studies and the absence of time-delineated definitions for pre-treatment loss to follow-up make it difficult to draw firm conclusions. Following the framework set out in Fig. 1, NTPs should strive to adopt and routinely report retention in care throughout the diagnostic and care pathway.16,59 A focus on retention could enhance the reporting of the pre-treatment loss to follow-up rate (e.g. the proportion of smear-positive patients not initiating treatment for tuberculosis within 3 months) as part of the regular quarterly reporting system, in addition to allowing comparison within and between NTPs.
A second limitation is that negative publication bias may have resulted in an under- or overestimation of pre-treatment loss to follow-up in this review. Although we undertook a systematic literature search, we may have missed some studies reporting on pre-treatment loss to follow-up if this was not the main focus of the study.
Because the studies identified were so heterogeneous, the summary estimates should be interpreted cautiously. Our ability to draw conclusions on the risk factors or reasons for pre-treatment loss to follow-up among people with tuberculosis is limited by the poor reporting of the baseline characteristics of study participants and low numbers of traced patients in several studies. We identified studies from 8 of the world’s 22 countries with a high burden of tuberculosis. Although the data from these countries are helpful in showing the important contribution of pre-treatment loss to follow-up to suboptimal NTP performance, data from a broader range of countries and regions are urgently needed. In particular, no studies from Latin American countries or the Russian Federation were identified, perhaps because these countries have produced no studies or because we limited our search to English-language sources. To facilitate comparisons between studies and regions, all studies reporting outcomes in patients with a diagnosis of tuberculosis should specify the proportion that is lost to follow-up before getting treated.
In conclusion, there is a paucity of evidence on the magnitude and clinical consequences of pre-treatment loss to follow-up in tuberculosis patients. The limited data available suggest that pre-treatment loss to follow-up is common and that it entails a high risk of death. There is an urgent need to improve the recording and reporting of pre-treatment loss to follow-up and to evaluate and scale up interventions to reduce this problem.
Acknowledgements
Peter MacPherson and Elizabeth L Corbett are also affiliated with TB/HIV Group, Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre, Malawi.
Funding:
PM (the corresponding author) was funded by the Wellcome Trust (grant number: WT089673). RMGJH was funded by the Bill & Melinda Gates Foundation. ELC was funded by a Wellcome Trust Senior Research Fellowship in Clinical Science (grant number: WT091769). The funders had no role in the design or analysis of the study, or in the writing or decision to submit for publication. PM confirms that he had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests:
None declared.
References
- 1.Global tuberculosis control Geneva: World Health Organization; 2011. [Google Scholar]
- 2.The Millennium Development Goals report New York: United Nations; 2011. [Google Scholar]
- 3.Lönnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, Glaziou P, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–29. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 4.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–7. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 5.Harries AD, Lawn SD, Getahun H, Zachariah R, Havlir DV. HIV and tuberculosis–science and implementation to turn the tide and reduce deaths. J Int AIDS Soc. 2012;15:17396. doi: 10.7448/IAS.15.2.17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dye C, Bassili A, Bierrenbach AL, Broekmans JF, Chadha VK, Glaziou P, et al. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis. 2008;8:233–43. doi: 10.1016/S1473-3099(07)70291-8. [DOI] [PubMed] [Google Scholar]
- 7.The Stop TB Strategy Geneva: World Health Organization. Stop TB Partnership; 2006. [Google Scholar]
- 8.Harries AD, Rusen ID, Chiang CY, Hinderaker SG, Enarson DA. Registering initial defaulters and reporting on their treatment outcomes. Int J Tuberc Lung Dis. 2009;13:801–3. [PubMed] [Google Scholar]
- 9.Zachariah R, Harries AD, Srinath S, Ram S, Viney K, Singogo E, et al. Language in tuberculosis services: can we change to patient-centred terminology and stop the paradigm of blaming the patients? Int J Tuberc Lung Dis. 2012;16:714–7. doi: 10.5588/ijtld.11.0635. [DOI] [PubMed] [Google Scholar]
- 10.Squire SB, Belaye AK, Kashoti A, Salaniponi FM, Mundy CJ, Theobald S, et al. ‘Lost’ smear-positive pulmonary tuberculosis cases: where are they and why did we lose them? Int J Tuberc Lung Dis. 2005;9:25–31. [PubMed] [Google Scholar]
- 11.Dowdy DW, Chaisson RE. The persistence of tuberculosis in the age of DOTS: reassessing the effect of case detection. Bull World Health Organ. 2009;87:296–304. doi: 10.2471/BLT.08.054510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis JL, Dowdy DW, den Boon S, Walter ND, Katamba A, Cattamanchi A. Test and treat: a new standard for smear-positive tuberculosis. J Acquir Immune Defic Syndr. 2012;61:e6–8. doi: 10.1097/QAI.0b013e3182614bc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Same-day diagnosis of tuberculosis: policy statement Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 14.Cuevas LE, Yassin MA, Al-Sonboli N, Lawson L, Arbide I, Al-Aghbari N, et al. A multi-country non-inferiority cluster randomized trial of frontloaded smear microscopy for the diagnosis of pulmonary tuberculosis. PLoS Med. 2011;8:e1000443. doi: 10.1371/journal.pmed.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26:2059–67. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 18.Piot MA. A simulation model of case finding and treatment in tuberculosis control programmes Geneva: World Health Organization; 1967. (WHO/TB/Technical Information/67.53). [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The World Bank [Internet]. How we classify countries. Washington: WB; 2011. Available from: http://data.worldbank.org/about/country-classifications [accessed 9 December 2013].
- 21.Afutu FK, Zachariah R, Hinderaker SG, Ntoah-Boadi H, Obeng EA, Bonsu FA, et al. High initial default in patients with smear-positive pulmonary tuberculosis at a regional hospital in Accra, Ghana. Trans R Soc Trop Med Hyg. 2012;106:511–3. doi: 10.1016/j.trstmh.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Balasubramanian R, Garg R, Santha T, Gopi PG, Subramani R, Chandrasekaran V, et al. Gender disparities in tuberculosis: report from a rural DOTS programme in south India. Int J Tuberc Lung Dis. 2004;8:323–32. [PubMed] [Google Scholar]
- 23.Botha E, den Boon S, Lawrence KA, Reuter H, Verver S, Lombard CJ, et al. From suspect to patient: tuberculosis diagnosis and treatment initiation in health facilities in South Africa. Int J Tuberc Lung Dis. 2008;12:936–41. [PubMed] [Google Scholar]
- 24.Botha E, Den Boon S, Verver S, Dunbar R, Lawrence KA, Bosman M, et al. Initial default from tuberculosis treatment: how often does it happen and what are the reasons? Int J Tuberc Lung Dis. 2008;12:820–3. [PubMed] [Google Scholar]
- 25.Buu TN, Lönnroth K, Quy HT. Initial defaulting in the National Tuberculosis Programme in Ho Chi Minh City, Vietnam: a survey of extent, reasons and alternative actions taken following default. Int J Tuberc Lung Dis. 2003;7:735–41. [PubMed] [Google Scholar]
- 26.Chadambuka A, Mabaera B, Tshimanga M, Shambira G, Gombe NT, Chimusoro A. Low tuberculosis case detection in Gokwe North and South, Zimbabwe in 2006. Afr Health Sci. 2011;11:190–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Creek TL, Lockman S, Kenyon TA, Makhoa M, Chimidza N, Moeti T, et al. Completeness and timeliness of treatment initiation after laboratory diagnosis of tuberculosis in Gaborone, Botswana. Int J Tuberc Lung Dis. 2000;4:956–61. [PubMed] [Google Scholar]
- 28.Dembele SM, Ouédraogo HZ, Combary AI, Sondo B, Macq J, Dujardin B. Are patients who present spontaneously with PTB symptoms to the health services in Burkina Faso well managed? Int J Tuberc Lung Dis. 2006;10:436–40. [PubMed] [Google Scholar]
- 29.Dunbar R, Lawrence K, Verver S, Enarson DA, Lombard C, Hargrove J, et al. Accuracy and completeness of recording of confirmed tuberculosis in two South African communities. Int J Tuberc Lung Dis. 2011;15:337–43. doi: 10.5588/ijtld.10.0695. [DOI] [PubMed] [Google Scholar]
- 30.Glynn JR, Warndorff DK, Fine PE, Munthali MM, Sichone W, Pönnighaus JM. Measurement and determinants of tuberculosis outcome in Karonga District, Malawi. Bull World Health Organ. 1998;76:295–305. [PMC free article] [PubMed] [Google Scholar]
- 31.Gopi PG, Chandrasekaran V, Subramani R, Narayanan PR. Failure to initiate treatment for tuberculosis patients diagnosed in a community survey and at health facilities under a DOTS programme in a district of South India. Indian J Tuberc. 2005;52:153–6. [Google Scholar]
- 32.Nyirenda T, Harries AD, Banerjee A, Salaniponi FM. Registration and treatment of patients with smear-positive pulmonary tuberculosis. Int J Tuberc Lung Dis. 1998;2:944–5. [PubMed] [Google Scholar]
- 33.Rao NA, Anwer T, Saleem M. Magnitude of initial default in pulmonary tuberculosis. J Pak Med Assoc. 2009;59:223–5. [PubMed] [Google Scholar]
- 34.Sai Babu B, Satyanarayana AV, Venkateshwaralu G, Ramakrishna U, Vikram P, Sahu S, et al. Initial default among diagnosed sputum smear-positive pulmonary tuberculosis patients in Andhra Pradesh, India. Int J Tuberc Lung Dis. 2008;12:1055–8. [PubMed] [Google Scholar]
- 35.Uthaivoravit W, Yanai H, Tappero JW, Limpakarnjanarat K, Srismith R, Mastro TD, et al. Impact of enhanced notification of tuberculosis laboratory results to minimise treatment delay, Chiang Rai Hospital, Northern Thailand. Int J Tuberc Lung Dis. 2003;7:46–51. [PubMed] [Google Scholar]
- 36.Uchenna OU, Chukwu JN, Onyeonoro U, Oshi DC, Nwafor CC, Meka AO, et al. Pattern and magnitude of treatment delay among TB patients in five states in southern Nigeria. Ann Trop Med Pub Health. 2012;5:173. doi: 10.4103/1755-6783.98608. [DOI] [Google Scholar]
- 37.Claassens M, Lawrence K, Beyers N. Tuberculosis initial treatment default in primary healthcare facilities in South Africa. In: 41st Union World Conference on Lung Health, 11–15 November 2010, Berlin, Germany [Internet]. Paris: International Union Against Tuberculosis and Lung Disease; 2010. [Google Scholar]
- 38.Korobitsyn A, Rajabov J, Norov O, Shekhov A. Analysis of initial defaulters in selected districts in Tajikistan. In: 41st Union World Conference on Lung Health, 11–15 November 2010, Berlin, Germany [Internet]. Paris: International Union Against Tuberculosis and Lung Disease; 2010. [Google Scholar]
- 39.Razia F, Ejaz Q. Initial default common in tertiary care hospitals: descriptive study, Rawalpindi district, Pakistan. In: 41st Union World Conference on Lung Health, 11–15 November 2010, Berlin, Germany [Internet]. Paris: International Union Against Tuberculosis and Lung Disease; 2011. [Google Scholar]
- 40.Rao N, Arain A, Ara I, Anwer T. Primary default tracing at chest clinics of Ojha Institute of Chest Diseases, Karachi, Pakistan. In: 41st Union World Conference on Lung Health, 11–15 November 2010, Berlin, Germany [Internet]. Paris: International Union Against Tuberculosis and Lung Disease; 2011. [Google Scholar]
- 41.Davis J, Katamba A, Vasquez J, Crawford E, Sserwanga A, Kakeeto S, et al. Evaluating tuberculosis case detection via real-time monitoring of tuberculosis diagnostic services. Am J Respir Crit Care Med. 2011;184:362–7. doi: 10.1164/rccm.201012-1984OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ram S, Kishore K, Batio I, Bissell K, Zachariah R, Satyanarayana S, et al. Pre-treatment loss to follow-up among smear-positive pulmonary tuberculosis cases: a 10- year audit of national data from Fiji. Public Health Action. 2012;2:138–41. doi: 10.5588/pha.12.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15:17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Styblo K. The descriptive epidemiology of tuberculosis. In: Selected Papers Epidemiology of Tuberculosis The Hague: Royal Netherlands Tuberculosis Association; 1991. pp. 17–39.
- 45.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One. 2011;6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korenromp EL, Bierrenbach AL, Williams BG, Dye C. The measurement and estimation of tuberculosis mortality. Int J Tuberc Lung Dis. 2009;13:283–303. [PubMed] [Google Scholar]
- 47.Joint United Nations Programme on HIV/AIDS. Global report on the epidemic Geneva: UNAIDS; 2010. [Google Scholar]
- 48.MacPherson P, Dimairo M, Bandason T, Zezai A, Munyati SS, Butterworth AE, et al. Risk factors for mortality in smear-negative tuberculosis suspects: a cohort study in Harare, Zimbabwe. Int J Tuberc Lung Dis. 2011;15:1390–6. doi: 10.5588/ijtld.11.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crampin AC, Floyd S, Mwaungulu F, Black G, Ndhlovu R, Mwaiyeghele E, et al. Comparison of two versus three smears in identifying culture-positive tuberculosis patients in a rural African setting with high HIV prevalence. Int J Tuberc Lung Dis. 2001;5:994–9. [PubMed] [Google Scholar]
- 50.Cattamanchi A, Huang L, Worodria W, den Boon S, Kalema N, Katagira W, et al. Integrated strategies to optimize sputum smear microscopy: a prospective observational study. Am J Respir Crit Care Med. 2011;183:547–51. doi: 10.1164/rccm.201008-1207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fluorescent light-emitting diode (LED) microscopy for diagnosis of tuberculosis policy Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 52.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harries AD, Kamenya A, Schoevers MA, Boeree MJ, Nunn P, Salaniponi FM, et al. Case finding for pulmonary tuberculosis, Queen Elizabeth Central Hospital, Blantyre, Malawi. Int J Tuberc Lung Dis. 1997;1:523–7. [PubMed] [Google Scholar]
- 54.Lönnroth K, Thuong LM, Linh PD, Diwan VK. Utilization of private and public health-care providers for tuberculosis symptoms in Ho Chi Minh City, Vietnam. Health Policy Plan. 2001;16:47–54. doi: 10.1093/heapol/16.1.47. [DOI] [PubMed] [Google Scholar]
- 55.Lönnroth K, Uplekar M, Blanc L. Hard gains through soft contracts: productive engagement of private providers in tuberculosis control. Bull World Health Organ. 2006;84:876–83. doi: 10.2471/blt.06.029983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lal SS, Sahu S, Wares F, Lönnroth K, Chauhan LS, Uplekar M. Intensified scale-up of public-private mix: a systems approach to tuberculosis care and control in India. Int J Tuberc Lung Dis. 2011;15:97–104. [PubMed] [Google Scholar]
- 57.Khan AJ, Khowaja S, Khan FS, Qazi F, Lotia I, Habib A, et al. Engaging the private sector to increase tuberculosis case detection: an impact evaluation study. Lancet Infect Dis. 2012;12:608–16. doi: 10.1016/S1473-3099(12)70116-0. [DOI] [PubMed] [Google Scholar]
- 58.Public-private mix for TB care and control: a toolkit Geneva: World Health Organization, Stop TB Partnership; 2010. [Google Scholar]
- 59.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]