Abstract
Objective
To assess, by socioeconomic setting, the effect of nationwide vaccination against species A rotavirus (RVA) on childhood diarrhoea-related hospitalizations in Mexico.
Methods
Data on children younger than 5 years who were hospitalized for diarrhoea in health ministry hospitals between 1 January 2003 and 31 December 2011 were collected from monthly discharge reports. Human development indexes were used to categorize the states where hospitals were located as having generally high, intermediate or low socioeconomic status. Annual rates of hospitalization for diarrhoea – per 10 000 hospitalizations for any cause – were calculated. Administrative data were used to estimate vaccine coverage.
Findings
In the states with high, intermediate and low socioeconomic status, coverage with a two-dose monovalent RVA vaccine – among children younger than 5 years – had reached 93%, 86% and 71%, respectively, by 2010. The corresponding median annual rates of hospitalization for diarrhoea – per 10 000 admissions – fell from 1001, 834 and 1033 in the “prevaccine” period of 2003–2006, to 597, 497 and 705 in the “postvaccine” period from 2008 to 2011, respectively. These decreases correspond to rate reductions of 40% (95% confidence interval, CI: 38–43), 41% (95% CI: 38–43) and 32% (95% CI: 29–34), respectively. Nationwide, RVA vaccination appeared to have averted approximately 16 500 hospitalizations for childhood diarrhoea in each year of the postvaccine period.
Conclusion
Monovalent RVA vaccination has substantially reduced childhood diarrhoea-related hospitalizations for four continuous years in discretely different socioeconomic populations across Mexico.
Résumé
Objectif
Évaluer, par contexte socio-économique, les effets de la vaccination nationale contre le rotavirus A (RVA) sur les hospitalisations liées à la diarrhée infantile au Mexique.
Méthodes
Les données chez les enfants de moins de 5 ans qui ont été hospitalisés pour cause de diarrhée dans les hôpitaux du ministère de la santé entre le 1er janvier 2003 et le 31 décembre 2011 ont été recueillies à partir des rapports mensuels de sortie des hôpitaux. Des indices de développement humain ont été utilisés pour classer les états dans lesquels les hôpitaux sont situés comme des états ayant un statut socio-économique généralement élevé, intermédiaire ou bas. Les taux annuels d'hospitalisation pour cause de diarrhée – par 10 000 hospitalisations, quelle qu'en soit la cause – ont été calculés. Les données administratives ont été utilisées pour estimer la couverture vaccinale.
Résultats
Dans les états présentant des statuts socio-économiques élevés, intermédiaires et bas, la couverture avec un vaccin monovalent contre le RVA administré en 2 doses – parmi les enfants de moins de 5 ans – a atteint 93%, 86% et 71%, respectivement, en 2010. Les taux annuels médians d'hospitalisation correspondants pour cause de diarrhée – par 10 000 admissions – ont chuté de 1001, 834 et 1033 dans la période «pré-vaccinale» de 2003 à 2006, à 597, 497 et 705 dans la période «post-vaccinale» de 2008 à 2011, respectivement. Ces diminutions correspondent à des réductions de taux de 40% (Intervalle de Confiance (IC) à 95%: 38–43), 41% (IC à 95%: 38–43) et 32% (IC à 95%: 29–34), respectivement. Dans tout le pays, la vaccination contre le RVA semble avoir évité environ 16 500 hospitalisations pour cause de diarrhée infantile chaque année au cours de la période post-vaccinale.
Conclusion
La vaccination monovalente contre le RVA a substantiellement réduit les hospitalisations liées à la diarrhée infantile pendant quatre ans de suite dans des populations socio-économiques différentes et distinctes dans tout le Mexique.
Resumen
Objetivo
Evaluar, por el contexto socioeconómico, el efecto de la vacunación a escala nacional contra el rotavirus de tipo A (RVA) en las hospitalizaciones vinculadas a la diarrea infantil en México.
Métodos
Se recopilaron datos sobre niños menores de 5 años hospitalizados por diarrea en hospitales del Ministerio de Salud entre el 1 de enero de 2003 y el 31 de diciembre de 2011 a partir de los informes de altas hospitalarias mensuales. Se usaron índices de desarrollo humano para clasificar los estados donde los hospitales estaban situados según un nivel socioeconómico generalmente alto, intermedio o bajo. Se calcularon las tasas anuales de hospitalización por diarrea, por 10 000 hospitalizaciones por cualquier causa. Se emplearon datos administrativos para estimar la cobertura de la vacuna.
Resultados
En los estados con nivel socioeconómico alto, intermedio y bajo, la cobertura de la vacuna monovalente contra el rotavirus de tipo A de dos dosis había alcanzado entre los niños menores de 5 años el 93 %, 86 % y 71 %, respectivamente, en el año 2010. Las tasas anuales medias correspondientes de hospitalización por diarrea (por 10 000 ingresos) cayeron, respectivamente, de 1001, 834 y 1033 en el periodo «prevacuna» entre 2003 y 2006, a 597, 497 y 705 en el periodo «posvacuna» entre 2008 y 2011. Estas disminuciones se corresponden con reducciones de las tasas del 40 % (intervalo de confianza del 95 %, IC: 38–43), 41 % (IC del 95 %: 38–43) y el 32 % (IC del 95 %: 29–34), respectivamente. A nivel nacional, la vacunación contra el rotavirus de tipo A parece haber evitado unas 16 500 hospitalizaciones por diarrea infantil cada año del periodo «posvacuna».
Conclusión
La vacunación monovalente contra el RVA ha reducido sustancialmente las hospitalizaciones relacionadas con la diarrea infantil durante cuatro años seguidos en poblaciones socioeconómicas ligeramente distintas de todo México.
مخلص
الغرض
تقييم تأثير التطعيم على الصعيد الوطني ضد الفيروس العجلي من النوع أ (RVA)، حسب البيئة الاقتصادية الاجتماعية، على حالات الإدخال إلى المستشفيات ذات الصلة بالإسهال لدى الأطفال في المكسيك.
الطريقة
تم جمع البيانات بشأن الأطفال الأقل من 5 سنوات الذين تم إدخالهم إلى المستشفيات إثر إصابتهم بالإسهال في مستشفيات وزارة الصحة في الفترة من 1 كانون الثاني/ يناير 2003 إلى 31 كانون الأول/ ديسمبر 2011 من تقارير الخروج الشهرية. وتم استخدام أدلة التنمية البشرية لتصنيف الولايات التي تقع بها المستشفيات من حيث الوضع الاقتصادي والاجتماعي المرتفع أو المتوسط أو المنخفض بشكل عام. وتم حساب معدلات الإدخال السنوية إلى المستشفيات إثر الإصابة بالإسهال لكل 10 آلاف حالة إدخال لأي سبب. وتم استخدام البيانات الإدارية لتقدير التغطية باللقاح.
النتائج
في الولايات ذات الوضع الاقتصادي والاجتماعي المرتفع والمتوسط والمنخفض، وصلت التغطية بلقاح الفيروس العجلي من النوع أ أحادي التكافؤ ثنائي الجرعة – بين الأطفال الأقل من 5 سنوات – 93 % و86 % و71 %، على التوالي بحلول 2010. وانخفض متوسط المعدلات السنوية المقابلة لحالات الإدخال إلى المستشفى إثر الإصابة بالإسهال – لكل 10 آلاف حالة إدخال – من 1001 و834 و1033 حالة في فترة "ما قبل التطعيم" من 2003 إلى 2006، إلى 597 و497 و705 في فترة "ما بعد التطعيم" من 2008 إلى 2011، على التوالي. وتتوافق هذه الانخفاضات مع الانخفاضات في المعدل التي تبلغ 40 % (فاصل الثقة 95 %، فاصل الثقة: من 38 إلى 43) و41 % (فاصل الثقة 95 %، فاصل الثقة: من 38 إلى 43) و32 % (فاصل الثقة 95 %، فاصل الثقة: من 29 إلى 34)، على التوالي. وبدا أن التطعيم بلقاح الفيروس العجلي من النوع أ قد حال دون إدخال 16500 حالة تقريباً إلى المستشفيات إثر الإصابة بالإسهال لدى الأطفال في كل سنة من سنوات فترة "ما بعد التطعيم".
الاستنتاج
أدى التطعيم بلقاح الفيروس العجلي من النوع أ أحادي التكافؤ إلى تقليل حالات الإدخال إلى المستشفيات ذات الصلة بالإسهال لدى الأطفال بشكل كبير لأربع سنوات متواصلة في فئات سكانية ذات وضع اقتصادي واجتماعي مختلف على نحو متمايز في أنحاء المكسيك.
摘要
目的
按照社会经济背景,评估墨西哥全国A群轮状病毒疫苗接种(RVA)对儿童腹泻相关住院治疗的影响。
方法
从每月出院报告中收集2003 年1 月1 日至2011 年12 月31 日因腹泻在卫生部医院接受住院治疗的五岁以下儿童的数据。使用人类发展指数将医院所在的各个州分为一般性的高、中或低级三类社会经济地位。计算年腹泻住院率(占万例所有原因住院病例比例)。使用行政数据来估计疫苗覆盖率。
结果
在具有高、中和低级社会经济地位的州中,到2010 年五岁以下两剂量单价RVA疫苗覆盖率分别达到93%、86%和71%。相应的平均每年腹泻住院率(每万例住院病例数)从2003-2006 年接种前期间的1001、834 和1033 分别降低为2008 至 2011 年接种后期间的597、497 和705 例。这些降低分别对应40%(95%置信区间,CI:38-43)、41%(95% CI:38-43)和32%(95% CI:29-34)的住院率降低。RVA疫苗接种看来在后期间避免了全国范围内每年大约16500 例儿童腹泻住院。
结论
单价RVA疫苗接种连续四年显著减少了墨西哥不同社会经济群体儿童腹泻相关住院。
Резюме
Цель
Оценить с социально-экономической точки зрения воздействие общенациональной кампании по вакцинации от ротавируса типа A на госпитализацию детей в связи с диареей в Мексике.
Методы
Были собраны данные ежемесячных отчетов о выписках детей в возрасте до 5 лет, которые находились на стационарном лечении в больницах министерства здравоохранения в связи с диареей, за период с 1 января 2003 г. по 31 декабря 2011 г. Для определения категорий штатов, в которых были расположены больницы, использовались индексы человеческого развития; штаты были распределены по категориям с высоким, средним и низким общим социально-экономическим статусом. Были рассчитаны годовые уровни госпитализации в связи с диареей на 10 000 госпитализаций по любым причинам. Для оценки покрытия вакцинации использовались административные данные.
Результаты
В штатах с высоким, средним и высоким социально-экономическим статусом покрытие двухфазной вакцинацией с применением моновалентной ротавирусной вакцины против вируса типа A среди детей младше 5 лет к 2010 г. составило 93%, 86% и 71% соответственно. Соответствующие средние годовые уровни госпитализации в связи с диареей на 10 000 случаев госпитализации сократились от 1001, 834 и 1033 в период до вакцинации (с 2003 по 2006 г.) до 597, 497 и 705 в период после вакцинации (с 2008 по 2011 г.) соответственно. Такое сокращение соотносится с сокращением уровней в 40% (доверительный интервал 95%, CI: 38–43), 41% (95 %, CI: 38–43) и 32% (95 %, CI: 29–34) соответственно. На общенациональном уровне ротавирусная вакцинация против вируса типа A позволила предотвратить приблизительно 16 500 госпитализаций детей в связи с диареей ежегодно за период после вакцинации.
Вывод
Проведение вакцинации с применением моновалентной ротавирусной вакцины против вируса типа A существенно сократило число госпитализаций детей в связи с диареей за четыре последовательных года в разграниченных по социально-экономическому статусу популяциях по всей Мексике.
Introduction
Species A rotavirus (RVA) is the leading cause of severe childhood gastroenteritis worldwide and accounts for about 2 million hospitalizations and 453 000 deaths among young children annually.1,2 In 2006, the World Health Organization (WHO) recommended including two RVA vaccines in national immunization programmes in the Americas and Europe – after both vaccines had been found to show high efficacy and safety in clinical trials in these regions.2 By 2009, the same vaccines had been found to be efficacious in Africa and Asia and WHO therefore expanded its recommendation to include all children worldwide.2 Nearly 40 countries – most of them high- or middle-income – have introduced an RVA vaccine into their national programmes. Early evaluations after the introduction of these vaccines in high- or middle-income countries have shown a drop in hospitalizations for diarrhoea ranging from 33 to 50%.3–9 RVA vaccines have appeared to perform less well in low-income countries.10–12 The impact of RVA vaccination in different socioeconomic groups within a single country has not yet been investigated.
The evaluation of the impact of RVA vaccination in “real-world” settings is a public health priority. Although the direct protection conferred by RVA vaccines against RVA infection needs to be assessed, the broader public health benefits of such vaccines may be better appreciated by evaluating the impact of RVA vaccination on hospitalizations for diarrhoea.
The states of Mexico vary in the level of development that they have achieved and this heterogeneity provides a useful opportunity to examine possible differences in the impact of an RVA vaccine across several socioeconomic strata.13 After the national introduction of a monovalent RVA vaccine in 2007, substantial declines in diarrhoea-related mortality and morbidity among Mexican children were observed.14,15 In one study in Mexico, the incidence of hospitalization for diarrhoea was found to have declined since 2007.16 However, this decline could not be attributed to RVA vaccination unequivocally because at the time “postvaccine” data had only been collected for two years and the incidence of infection with RVA shows considerable inter-annual variation in the absence of any interventions.16
In this study we describe trends in hospitalization for diarrhoea among young children in Mexico before and after the introduction of RVA vaccination. The primary aims of this analysis were to document the long-term effect of RVA vaccination on diarrhoea-related hospitalizations and to compare the impact of such vaccination in the poorly developed states of Mexico with that seen in the country’s more developed states.
Methods
Data sources
All-cause and diarrhoea-related hospitalizations
Monthly data on all-cause and diarrhoea-related hospitalizations among children younger than 5 years were collected – from an electronic database of Mexico’s National System for Health Informatics17 – for the period from 1 January 2003 to 31 December 2011 and for all of Mexico’s 591 health ministry hospitals.14,16 No attempt was made to evaluate hospitalizations specifically for RVA infection because testing for RVA is rare in Mexico. However, studies in Mexico and other Latin American countries have indicated that 62 to 68% of hospitalizations for diarrhoea occur during the winter months and that about 40% of such hospitalizations throughout the year are due to rotavirus infection.18,19
To allow for a thorough examination of the temporal trends in hospitalization for diarrhoea, we confined our analysis to data from the 346 health ministry hospitals that had no lapses in reporting over our study period of 2003–2011. These 346 hospitals are located in 31 (97%) of the 32 Mexican states. Together they represent all levels of socioeconomic development in the country and cover about 38% of the hospitalizations nationwide. Included (n = 346) and excluded hospitals (n = 245) did not differ in terms of the proportion of their patient populations lacking social security (25.1% versus 25.4%; P = 0.7).
For the period of 2004–2010, the National System for Health Informatics expanded to include records of health care encounters at all hospitals throughout Mexico. This expansion allowed us to estimate the national reductions in diarrhoea-related hospitalizations among children younger than 5 years that could be reasonably attributed to RVA vaccination.20
Vaccine coverage and socioeconomic categories
Administrative data kept by Mexico’s National Centre for Child and Adolescent Health [Centro Nacional para la Salud de la Infancia y la Adolescencia] were used to estimate RVA vaccine coverage. Through government health facilities, the National Centre supplies RVA vaccine to about 50 to 61% of infants in Mexico; the rest is provided by the Mexican Social Security Institute [Instituto Mexicano de Seguro Social] and the Institute for Social Security and Services for State Workers [Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado]. Two doses of the monovalent RVA vaccine – given at the ages of 2 and 4 months – are recommended. Every month each government health facility sends to the National Centre’s vaccine registration system the numbers of first and second doses of the RVA vaccine that the facility has administered. Coverage with two doses of the RVA vaccine in 2010 was estimated by dividing the number of second doses given by the population of infants served by the National Centre in the same year.21
To investigate possible differences in vaccine impact by level of development, the Mexican states represented by the study hospitals were grouped into low (n = 10), intermediate (n = 11) and high socioeconomic status (n = 10) using the human development indexes reported for these states in 2007.22,23 The corresponding population-weighted mean human development indexes24 were 0.77 (range: 0.73–0.79), 0.81 (range: 0.80–0.83) and 0.87 (range: 0.84–0.91), respectively. In general, the level of socioeconomic development of the southern states of Mexico was lower than that of the central and northern states.
Data analysis
Overall numbers of diarrhoea-related hospitalizations from 1 January 2003 to 31 December 2011 were examined. Because the catchment populations of the study hospitals were not known, rates of hospitalization for diarrhoea per 10 000 hospitalizations from all causes were calculated. The median annual rate of diarrhoea-related hospitalizations during the baseline, “prevaccine” period of 2003 to 2006 was compared with the corresponding value for the “postvaccine” period of 2008 to 2011. Since RVA vaccination was still being rolled out in Mexico during the year, 2007 was considered a transitional year and excluded from this part of the analysis.
Rate ratios (RR) were calculated so that monthly and annual diarrhoea-related hospitalization rates in each of the postvaccine years could be compared with the corresponding prevaccine rates. The RRs were stratified by age group – 0–11, 12–23 and 24–59 months – and socioeconomic category. Although RVA testing or coding is not routinely conducted in the study hospitals, in Mexico approximately 60 to 70% of the hospitalizations for laboratory-confirmed RVA infection occur during the months of December to May.19 Seasonal changes in diarrhoea-related hospitalizations were therefore specifically assessed before and after the introduction of the RVA vaccine.
Ninety-five per cent confidence intervals (95% CI) were calculated around the relative reductions in the rates of diarrhoea-related hospitalization. Statistical significance in χ2 tests was assessed. A P-value of less than 0.05 was considered indicative of a statistically significant difference.
By extrapolating the rates of diarrhoea-related hospitalization per 10 000 all-cause admissions – observed in the 346 study hospitals – to the total number of hospital admissions for all causes observed countrywide, the national reductions in diarrhoea-related hospitalizations in Mexico that could reasonably be attributed to the RVA vaccinations were estimated. The numbers of reported hospital admissions for the entire country were available for the years 2004 to 2006 (prevaccine) and 2008 to 2010 (postvaccine) and for two age groups of young children: those younger than 12 months and those aged from 12 to 59 months.
All analyses were performed using SAS version 9.2 (SAS Institute, Chicago, United Sates of America) and Excel (Microsoft, Redmond, USA).
Results
Coverage
By 2010, a total of 3 830 932 second doses of monovalent RVA vaccine had been administered to the National Centre for Child and Adolescent Health’s target population of 4 677 341 children younger than 5 years. This was equal to a national two-dose coverage of 82% (Table 1). Two-dose coverage was at least 69% across all the age groups and socioeconomic categories that we considered (Table 1). In general, coverage was relatively higher among children younger than 24 months and in the states with high or intermediate socioeconomic status.
Table 1. Annual rates of diarrhoea-related hospitalizations in children younger than 5 years, Mexico, 2003–2011.
| Socioeconomic categorya | Age (months) | Vaccine coverageb (%) | No. of DR hospitalizationsc |
Rate reductiond % (95%CI) | |
|---|---|---|---|---|---|
| 2003–2006 | 2008–2011 | ||||
| High | ≤ 11 | 100 | 823 | 400 | 51 (48–54) |
| 12–23 | 100 | 2050 | 1049 | 49 (45–53) | |
| 24–59 | 88 | 792 | 698 | 12 (4–19) | |
| 0–59 | 93 | 1001 | 597 | 40 (38–43) | |
| Intermediate | ≤ 11 | 84 | 580 | 302 | 48 (44–51) |
| 12–23 | 100 | 2200 | 1113 | 49 (45–53) | |
| 24–59 | 79 | 850 | 638 | 25 (18–31) | |
| 0–59 | 86 | 834 | 497 | 41 (38–43) | |
| Low | ≤ 11 | 85 | 668 | 401 | 40 (36–44) |
| 12–23 | 100 | 2703 | 1531 | 43 (39–47) | |
| 24–59 | 51 | 1077 | 893 | 17 (10–23) | |
| 0–59 | 71 | 1033 | 705 | 32 (29–34) | |
| All | ≤ 11 | 89 | 684 | 358 | 48 (46–50) |
| 12–23 | 100 | 2301 | 1195 | 48 (46–50) | |
| 24–59 | 69 | 888 | 733 | 18 (13–21) | |
| 0–59 | 82 | 945 | 590 | 38 (36–39) | |
CI, confidence interval; DR, diarrhoea-related; RVA, species A rotavirus.
a The socioeconomic status of the state in which the reporting hospital was located, based on the human development index of the state for the year 2007.
b RVA vaccine coverage with two doses, as recorded in 2010. As the Ministry of Health may deliver vaccine to a region larger than that planned, the recorded coverage, which is based on the size of the planned target population, can exceed 100%.
c Per 10 000 all-cause admissions. Prevaccine and postvaccine rates – shown for 2003–2006 and 2008–2011, respectively – are based on the sums of the monthly median numbers of diarrhoea-related hospitalizations and all-cause admissions at 346 health ministry hospitals. To account for differences in vaccine coverage by age group and postvaccine year, the postvaccine values for children aged ≤ 11, 12–23 and 24–59 months were based on the number of diarrhoea-related hospitalizations over 2008–2011, 2009–2011 and 2010–2011, respectively.
d Rate reduction in the postvaccine period with respect to the prevaccine period.
Diarrhoea-related hospitalizations
Overall trends
Before the introduction of RVA vaccination nationwide, diarrhoea-related hospitalizations were distinctly seasonal in Mexico (Fig. 1). About 67% of such hospitalizations occurred between the months of December and May – i.e. during the autumn–winter peak of RVA activity in Mexico.19 In 2008, a moderate decrease in the number of diarrhoea-related hospitalizations was noted among children younger than 12 months – that is, among the first birth cohort that received the RVA vaccine. After 2009, clear blunting of the seasonal peaks extended to include all children aged less than 24 months. By 2011, declines were also noted among children between the ages of 24 and 59 months. The number of diarrhoea-related hospitalizations observed among children younger than 5 years in each of the four years following vaccine introduction was substantially less than that observed in any study year before the vaccine was introduced.
Fig. 1.
Monthly numbers of diarrhoea-related hospitalizations among young children, Mexico, 2003–2011
Note: the numbers are the totals reported by 346 health ministry hospitals.
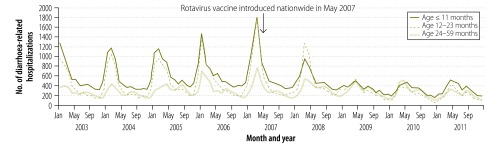
Changes in the seasonality of diarrhoea-related hospitalizations were also observed following the introduction of the RVA vaccine. In the prevaccine period, monthly rates of such hospitalizations peaked in January and February (Fig. 1). However, the monthly rates did not peak until March in 2007 and 2008 and not until April and May in 2009 to 2011.
Prevaccine versus postvaccine years
During the prevaccine period, a median of 15 398 diarrhoea-related hospitalizations were reported annually among children younger than 5 years at the 346 study hospitals. This corresponds to an annual rate of hospitalization for diarrhoea of 945 cases per 10 000 hospital admissions (Table 1). During the postvaccine period, the corresponding median annual number of diarrhoea-related hospitalizations was 10 577 – corresponding to an annual rate of 590 cases per 10 000 admissions; by comparison with the prevaccine values, this represented a rate reduction of 38% (P < 0.001). Overall, an absolute reduction of 4821 childhood hospitalizations was observed each year across the 346 study hospitals following vaccine introduction. About 93% of this reduction – 4463 of the 4821 hospitalizations – was observed in the months of December to May. Although significant postvaccine reductions in the rates of diarrhoea-related hospitalization were seen in all the age groups that we investigated, the greatest declines were observed among children younger than 24 months (Table 1).
Among children younger than 5 years, the rates of diarrhoea-related admission to the study hospitals in the states with high, intermediate and low socioeconomic status fell from 1001, 834 and 1033 per 10 000 admissions in 2003 to 2006 to 597, 497 and 705 per 10 000 admissions in 2008 to 2011, respectively. These changes correspond to rate reductions of 40% (P < 0.001), 41% (P < 0.001) and 32% (P < 0.001), respectively (Table 1). Substantial and significant reductions – ranging from 40 to 51% – were seen in all regions among children younger than 24 months. Although significant reductions were also noted among children between the ages of 24 and 59 months, the corresponding CIs were wider and these reductions represented only about 5% of the overall fall that was observed.
Changes in diarrhoea-related hospitalizations with respect to the prevaccine period were evident in each postvaccine year (Appendix A, available at: https://dl.dropboxusercontent.com/u/97573266/Appendix%20A.pdf). In 2008, statistically significant reductions were only observed among children younger than 12 months. Starting in 2009, significant reductions were also seen among children between 12 and 23 months of age. By 2010, the reductions seen among children 24 months of age or older had also reached statistical significance. The magnitude of the reductions increased with each postvaccine year. The RRs for the years 2008, 2009, 2010 and 2011 – 0.95, 0.66, 0.57 and 0.55, respectively – were all statistically significant (P < 0.001).
In all of the socioeconomic categories and age groups considered, the postvaccine reductions in diarrhoea-related hospitalizations largely occurred during the autumn and winter seasons, which is when hospitalizations peaked in the prevaccine period (Fig. 2, Fig. 3 and Appendix A). The greatest reductions were seen in January in states with high or intermediate socioeconomic status and in February in states with low socioeconomic status (Fig. 3). Nationwide, the greatest reductions were seen in January across all age groups (Appendix A). During 2008, statistically significant RRs that exceeded 1 were noted in all regions, especially in March and April (Fig. 3). However, the results of age-stratified analysis indicated that the increases seen in diarrhoea-related hospitalizations in March–April 2008 were the result of hospitalizations of children who were then more than 12 months of age (Appendix A). Few such children had received the RVA vaccine by April 2008. After 2009, significant reductions in diarrhoea-related hospitalizations of children aged less than 5 years were evident in all months of each year (Fig. 3). For children aged 24–59 months, statistically significant RRs that exceeded 1 were noted during April and May in 2008, 2009 and 2010 (Appendix A) – when vaccine uptake was still increasing in this age cohort. In 2011, no increase was apparent during April and May. In this year, the reductions observed from December to March continued, leading to an annual RR of 0.76 (Appendix A).
Fig. 2.
Seasonality in diarrhoea-related hospitalizations among young children before the introduction of a rotavirus vaccine, Mexico, 2003–2006
Note: In each plot, the median numbers of diarrhoea-related admissions in children younger than 60 months in each month of the year in 2003 to 2006 are shown as a black line. The range of values is indicated by the green shading. The figure shows the values recorded in all 346 health ministry study hospitals and – in separate plots – the values recorded in the study hospitals located in states with generally high, intermediate or low socioeconomic status.
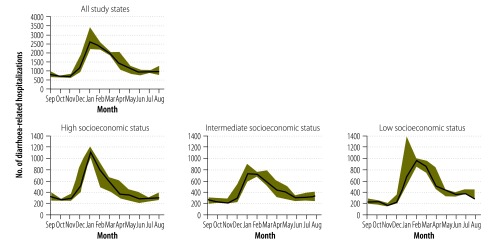
Fig. 3.
Comparison of the prevaccine and postvaccine monthly rates of diarrhoea-related hospitalization among young children, Mexico, 2007–2011
Note: Postvaccine rates were compared with the prevaccine values by calculating rate ratios. A rate ratio was calculated for each postvaccine month by dividing the rate of diarrhoea-related hospitalizations among children younger than 60 months – recorded in that month, per 10 000 admissions, in the 346 health ministry study hospitals – by the corresponding median rate recorded in 2003 to 2006. The vaccine was introduced in May 2007. Error bars indicate 95% confidence intervals. Before the vaccine was introduced, rates of diarrhoea-related hospitalization in young children peaked between December and March. These “peak” months are indicated by the green shading.
Estimated national reduction
It was estimated that 16 537 hospitalizations for diarrhoea among children younger than 5 years were averted annually in Mexico between 2008 and 2011 as the result of RVA vaccination (Table 2).
Table 2. Estimated numbers of diarrhoea-related admissions averted nationwide among children younger than 5 years after introduction of rotavirus vaccine in 2007, Mexico, 2003–2011.
| Age (months) | DR hospitalizationsa |
Annual no. of admissions for any causeb |
Estimated annual no. of DR admissionsc |
Average no. of DR admissions averted annuallyd | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2003–2006 | 2008–2011 | 2004–2006 | 2008–2010 | 2003–2006 | 2008–2011 | ||||
| ≤ 11 | 684 | 358 | 301 625 | 316 941 | 20 631 | 11 346 | 9285 | ||
| 12–59 | 1431 | 1020 | 197 853 | 196 489 | 28 313 | 20 042 | 8271 | ||
| 0–59 | 945 | 590 | 499 478 | 519 731 | 47 201 | 30 664 | 16 537 | ||
CI, confidence interval; DR, diarrhoea-related.
a Per 10 000 all-cause admissions, as reported by 346 health ministry hospitals.
b Number of admissions reported nationally for the prevaccine period (2004–2006) and the postvaccine period (2008–2010).
c Estimates made by assuming that the national annual rates of hospitalization for diarrhoea were the same as the rates recorded in the 346 study hospitals.
d Estimates for the postvaccine period of 2008–2011.
Discussion
After implementation of RVA vaccination in 2007, substantial declines in paediatric admissions for diarrhoea were observed throughout Mexico. Four observations strongly support a role of RVA vaccination in these declines. First, over 90% of the postvaccine reduction was observed over the 6 months of each year – December to May – when two thirds of childhood hospitalizations due to RVA infection are known to occur.19 Second, the impact was greatest among children who were younger than 24 months – the age group that is most at risk of severe RVA-related disease.25 Third, the declines appeared to affect each age group after that age group became eligible for RVA vaccination. Fourth, significant declines were recorded in each of the four years that followed the introduction of RVA vaccination.
Over 16 500 diarrhoea-related hospitalizations were estimated to have been averted nationally every year after the RVA vaccine was introduced in Mexico. If all children in Mexico received two doses of RVA vaccine by their fifth birthdays, one hospitalization could be prevented per 120 infants vaccinated. This analysis highlights the substantial economic and public health benefits that could result in countries that incorporate RVA vaccination into their national programmes.
Among Mexican children younger than 5 years we recorded a nationwide reduction in diarrhoea-related hospitalizations of about 38%. This level of reduction is in line with the results of Latin American trials in which the vaccine was found to be associated with 39 to 42% fewer hospitalizations for diarrhoea of any cause.5,26 Four Latin American countries recorded declines of 17 to 51% in gastroenteritis hospitalizations in the first two years after an RVA vaccine was introduced.27 Since about 40% of Mexican children who were hospitalized with diarrhoea in the prevaccine period were found to be infected with RVA,18 a 40% reduction in diarrhoea-related hospitalizations after an RVA vaccine is introduced is, presumably, the best result for Mexico that might be expected.
The peak in diarrhoea-related hospitalizations among Mexican children younger than 5 years was delayed by about two months with respect to the prevaccine period following the introduction of the RVA vaccine. In the United States, peak RVA activity was similarly delayed by about 8 weeks after vaccine introduction.28 These changes in seasonality probably reflect diminished transmission of RVA and the slower development of RVA disease in partially immunized populations. Interestingly, this hypothesis is supported by the increased rates of diarrhoea-related hospitalization recorded – in the present study – among mainly unimmunized children 24 months of age or older in April and May in 2008, 2009 and 2010. By 2011, most children in this age group had been immunized against RVA and the increase seen in April and May in the three previous years was no longer observed.
In clinical trials, the efficacy of RVA vaccines was found to be lower in low-income settings than in middle- or high-income settings.10–12 Subsequent studies in Latin America have shown that a licensed RVA vaccine contributed to a reduction of 46% in RVA-related hospitalizations in a low-income country as opposed to a reduction of 76 to 94% in three middle-income countries.27 This disparity is thought to be related to environmental and host-related factors that are common to resource-limited settings and either reduce the uptake of oral RVA vaccines or impede the development of a robust immune response – or both.29 Some of these factors include enteric infections, interference from oral polio vaccine, neutralizing breast milk and transplacental antibodies, undernutrition and comorbidities such as infection with human immunodeficiency virus.29 In the present study, the reduction in diarrhoea-related hospitalizations was relatively smaller in the Mexican states with lower levels of socioeconomic development, but it was still statistically significant. In addition, in a recent field assessment in Chiapas, the poorest of Mexico’s states, the effectiveness of the monovalent RVA vaccine was shown to be as high as that recorded in some middle- and high-income countries.30 Furthermore, RVA vaccines are expected to have the greatest impact in resource-limited settings because of the relatively high baseline rates of RVA gastroenteritis found in such settings.
The factors that reduce vaccine effectiveness in Africa and Asia – where RVA vaccination is still being rolled out – generally differ from those encountered in Latin America and may have a greater effect on vaccination success.29,31 Ongoing research focusing on strategies to improve vaccine performance – such as delaying breastfeeding at the time of vaccination, increasing the number of doses administered, and giving zinc or probiotic supplements – could prove crucial in maximizing the benefits of RVA vaccines in these settings.29,31
Our study has several important limitations. Because RVA testing is rarely conducted at health ministry facilities in Mexico, data on hospitalizations for RVA-specific gastroenteritis were not available. Thus, we cannot confirm that the observed “postvaccine” reductions in hospitalizations for diarrhoea were solely attributable to vaccination against RVA. Nonetheless, the declines occurred primarily during the peak months of RVA activity, correlated with vaccine coverage by age group and region, and showed gradual, stepwise increases – over several years – with increasing vaccine uptake. Changes in the catchment populations of the study hospitals and in referral and reporting practices may have affected the recorded temporal trends in diarrhoea-related hospitalization. However, our evaluation was limited to hospitals with continuous reporting during the study period – and the number of hospitalizations from other causes remained fairly stable over the same period. Although inter-annual “natural” variability in the prevalence of RVA infection could have had some impact on our findings, it is not likely to have led to a sustained reduction in hospitalizations for diarrhoea over a four-year period. The effect of other concurrent interventions – such as improvements in sanitation, hygiene and food and water quality – may also have contributed to the observed declines. However, these interventions do not explain the magnitude of the declines or the remarkable consistency in trends across Mexico’s regions. By examining trends in diarrhoea-related hospitalizations, we only investigated the impact of RVA vaccination on severe diarrhoea. The effects of such vaccination on less severe outcomes – such as those generally observed in outpatient and community settings – also need to be investigated. Differences across regions in baseline rates of diarrhoea-related hospitalization and in the proportions of such hospitalizations attributable to rotavirus may have affected the absolute reductions seen in diarrhoea-related hospitalizations. However, these differences should not affect the RRs that we have presented. Lastly, the heterogeneity in income, state of development and vaccine impact among different communities within states could not be evaluated.
In conclusion, the sizable declines seen in diarrhoea-related hospitalizations among Mexican children since 2007 are probably related to vaccination against RVA. Sustained reductions were observed over a four-year period and across distinct socioeconomic categories throughout Mexico. An estimated 66 000 hospitalizations have probably been averted since – and because of – the initiation of Mexico’s programme of vaccination against RVA. Our evaluation highlights the value of the RVA vaccine against diarrhoea-related hospitalizations in “real world” circumstances. It also supports WHO’s recommendation that RVA vaccination be introduced in Africa and Asia, where more than 85% of the world’s cases of severe RVA disease occur. As RVA vaccines are introduced in these challenging settings, studies of vaccine effectiveness and RVA-specific hospital-based monitoring could offer valuable measures of the vaccine’s impact. In countries that have adopted the vaccine, continued surveillance remains essential to elucidate the vaccine’s long-term impact on the burden of diarrhoeal disease.
Competing interests:
None declared.
References
- 1.Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–6. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 3.Chang HG, Smith PF, Tserenpuntsag B, Markey K, Parashar U, Morse DL. Reduction in hospitalizations for diarrhea and rotavirus infections in New York state following introduction of rotavirus vaccine. Vaccine. 2010;28:754–8. doi: 10.1016/j.vaccine.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 4.Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis. 2010;201:1617–24. doi: 10.1086/652403. [DOI] [PubMed] [Google Scholar]
- 5.Linhares AC, Velázquez FR, Pérez-Schael I, Sáez-Llorens X, Abate H, Espinoza F, et al. Human Rotavirus Vaccine Study Group Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–9. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 6.Molto Y, Cortes JE, De Oliveira LH, Mike A, Solis I, Suman O, et al. Reduction of diarrhea-associated hospitalizations among children aged < 5 years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J. 2011;30(Suppl):S16–20. doi: 10.1097/INF.0b013e3181fefc68. [DOI] [PubMed] [Google Scholar]
- 7.Raes M, Strens D, Vergison A, Verghote M, Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J. 2011;30:e120–5. doi: 10.1097/INF.0b013e318214b811. [DOI] [PubMed] [Google Scholar]
- 8.Yen C, Armero Guardado JA, Alberto P, Rodriguez Araujo DS, Mena C, Cuellar E, et al. Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J. 2011;30(Suppl):S6–10. doi: 10.1097/INF.0b013e3181fefa05. [DOI] [PubMed] [Google Scholar]
- 9.Yen C, Tate JE, Wenk JD, Harris II JM, Parashar UD. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics. 2011;127:e9–15. doi: 10.1542/peds.2010-1393. [DOI] [PubMed] [Google Scholar]
- 10.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 11.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–98. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 12.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 13.Medina-Gomez OS, Lopez-Arellano O.Asociacion de los tipos de carencia y grado de desarrollo humano con la mortalidad infantil en Mexico, 2008. Cadernos de Saúde Pública 2011271603–10.Spanish. [DOI] [PubMed] [Google Scholar]
- 14.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362:299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 15.Richardson V, Parashar U, Patel M. Childhood diarrhea deaths after rotavirus vaccination in Mexico. N Engl J Med. 2011;365:772–3. doi: 10.1056/NEJMc1100062. [DOI] [PubMed] [Google Scholar]
- 16.Quintanar-Solares M, Yen C, Richardson V, Esparza-Aguilar M, Parashar UD, Patel MM. Impact of rotavirus vaccination on diarrhea-related hospitalizations among children < 5 years of age in Mexico. Pediatr Infect Dis J. 2011;30(Suppl):S11–5. doi: 10.1097/INF.0b013e3181fefb32. [DOI] [PubMed] [Google Scholar]
- 17.Dirección General de Información en Salud [Internet]. Sistema automatizado de egresos hospitalarios. Mexico City: DGIS; 2012. Available from: http://pda.salud.gob.mx/cubos/ [accessed 6 November 2013]. Spanish.
- 18.Rheingans RD, Constenla D, Antil L, Innis BL, Breuer T. Economic and health burden of rotavirus gastroenteritis for the 2003 birth cohort in eight Latin American and Caribbean countries. Rev Panam Salud Publica. 2007;21:192–204. doi: 10.1590/s1020-49892007000300002. [DOI] [PubMed] [Google Scholar]
- 19.Velázquez FR, Garcia-Lozano H, Rodriguez E, Cervantes Y, Gómez A, Melo M, et al. Diarrhea morbidity and mortality in Mexican children: impact of rotavirus disease. Pediatr Infect Dis J. 2004;23(Suppl):S149–55. doi: 10.1097/01.inf.0000142463.72442.91. [DOI] [PubMed] [Google Scholar]
- 20.Dirección General de Información en Salud [Internet]. Información dinámica. Mexico City: DGIS; 2012. Available from: http://pda.salud.gob.mx/cubos/ [accessed 6 November 2013]. Spanish.
- 21.Burton A, Monasch R, Lautenbach B, Gacic-Dobo M, Neill M, Karimov R, et al. WHO and UNICEF estimates of national infant immunization coverage: methods and processes. Bull World Health Organ. 2009;87:535–41. doi: 10.2471/BLT.08.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Human development report 2009 New York: United Nations Development Programme; 2009. Available from: http://hdr.undp.org/en/media/HDR_2009_EN_Summary.pdf [accessed 6 November 2013].
- 23.Instituto Nacional de Estadística y Geografía [Internet]. Aguascalientes: Instituto Nacional de Estadística y Geografía; 2013. Available from: http://www.inegi.org.mx/default.aspx [accessed 6 November 2013]. Spanish.
- 24.Projections of the population of Mexico 2000–2030 Mexico City: National Population Council; 2007. [Google Scholar]
- 25.Cortese MM, Parashar UD, Centers for Disease Control and Prevention Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58(RR-2):1–25. [PubMed] [Google Scholar]
- 26.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al. Human Rotavirus Vaccine Study Group Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 27.Desai R, Oliveira LH, Parashar UD, Lopman B, Tate JE, Patel MM. Reduction in morbidity and mortality from childhood diarrhoeal disease after species A rotavirus vaccine introduction in Latin America – a review. Mem Inst Oswaldo Cruz. 2011;106:907–11. doi: 10.1590/s0074-02762011000800002. [DOI] [PubMed] [Google Scholar]
- 28.Tate JE, Panozzo CA, Payne DC, Patel MM, Cortese MM, Fowlkes AL, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics. 2009;124:465–71. doi: 10.1542/peds.2008-3528. [DOI] [PubMed] [Google Scholar]
- 29.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis. 2009;200(Suppl 1):S39–48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yen C, Figueroa JR, Uribe ES, Carmen-Hernández LD, Tate JE, Parashar UD, et al. Monovalent rotavirus vaccine provides protection against an emerging fully heterotypic G9P[4] rotavirus strain in Mexico. J Infect Dis. 2011;204:783–6. doi: 10.1093/infdis/jir390. [DOI] [PubMed] [Google Scholar]
- 31.Jiang V, Jiang B, Tate J, Parashar UD, Patel MM. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin. 2010;6:532–42. doi: 10.4161/hv.6.7.11278. [DOI] [PMC free article] [PubMed] [Google Scholar]



