Abstract
We describe the case of a 47-year-old Caucasian male patient who developed sarcoidosis 18 months after he was diagnosed with pulmonary tuberculosis for which he was treated according to guidelines. The presentation of sarcoidosis was very similar to his first presentation when he was diagnosed with tuberculosis.
Mycobacterium tuberculosis as a possible aetiological agent in sarcoidosis has been point of debate since many years and has been studied thoroughly. Recent advances in immunologic and molecular techniques have strengthened the association between mycobacteria and sarcoidosis.1
Sarcoidosis is a systemic inflammatory disorder of unknown aetiology, characterised by the presence of non-caseating epitheloid cell granulomas. It is generally agreed that this is a tissue reaction to environmental agents in a genetically susceptible individual.2 Tuberculosis is an infectious disease caused by M. tuberculosis and characterised by caseating granulomas. In both clinical and histopathological features sarcoidosis is remarkably similar to tuberculosis and therefore can be difficult to distinguish.
First, this case report demonstrates the need of diagnostic testing when reactivation of tuberculosis is suspected. And second the role of M. tuberculosis in the aetiology of sarcoidosis will be discussed.
Keywords: Tuberculosis, Sarcoidosis, Aetiology
1. Introduction
Sarcoidosis is a systemic inflammatory disorder of unknown aetiology, characterised by the presence of non-caseating epitheloid cell granulomas. It is generally agreed that this is a tissue reaction to environmental agents in a genetically susceptible individual.2 Tuberculosis is an infectious disease caused by Mycobacterium tuberculosis and characterised by caseating granulomas. In both clinical and histopathological features sarcoidosis is remarkably similar to tuberculosis and therefore can be difficult to distinguish.
M. tuberculosis as a possible aetiological agent in sarcoidosis has been point of debate since many years and has been studied thoroughly. Recent advances in immunologic and molecular techniques have strengthened the association between mycobacteria and sarcoidosis.1
Herein we demonstrate the need of diagnostic testing when reactivation of tuberculosis is suspected. Furthermore the role of M. tuberculosis in the aetiology of sarcoidosis will be discussed.
2. Case presentation
A 47-year-old male with no medical history was referred to the St. Catharins Hospital (Eindhoven, the Netherlands) in May 2010 following a two-week history of fever and dyspnoea. There were no complaints of cough and no history of smoking. His mother died in an other hospital three months before this episode due to an unknown pulmonary infection.
On physical examination, the patient was dyspnoeic with 24 breaths per minute and an oxygen saturation of 88% on ambient air. His blood pressure was 115/70 mmHg with a heart rate of 105 beats/min. Body temperature was 38.8 degrees Celsius. Apart from bilateral inspiratory fine crackles no other physical abnormalities were observed.
Laboratory investigations showed a mild normocytic anaemia (haemoglobin 7.9 mmol/L), white blood count 3.7/nL and C-reactive protein 200 mg/L. Alanine-aminotransferase, aspartate-aminotransferase, alkalic phosphatase and gamma-glutamyl-transferase were all raised tenfold the upper limit. Serum electrolyte levels and renal function indices were normal.
Arterial gas analysis indicated pH 7.42, pO2 32 mmHg, pCO2 74 mmHg and bicarbonate 20.4 mmol/L.
Chest X-ray on admission disclosed bilateral consolidations (Fig. 1). Under suspicion of an atypical pulmonary infection broncho-alveolar lavage (BAL) was performed in the middle lobe. Direct smear microscopy examination of the sample and sputum for the presence of acid-resistant bacilli by using Ziehl-Neelsen method was negative. However the Polymerase Chain Reaction (PCR) for M. tuberculosis complex (MTBC) DNA was positive. Patient was tested HIV negative and no signs of immunosuppressive disorders were found.
Fig. 1.
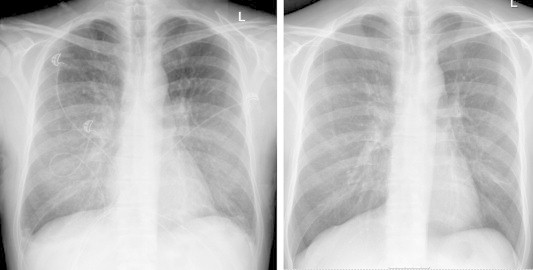
Chest radiogram with diffuse bilateral reticular interstitial densities in the middle and lower lung lobes (left) and a normal chest radiogram after treatment (right).
During the first day of hospitalisation the patient became respiratory insufficient and tracheal intubation was necessary for five days. Treatment was started with broad spectrum antibiotics and on suspicion of a human tuberculosis infection standard anti-tuberculosis drug regimen was started. (isoniazid (H) 300 mg daily, rifampin (R) 600 mg daily, pyrazinamide (Z) 2000 mg daily, ethambutol (E) 1600 mg daily and pyridoxin 600 mg daily). Three weeks after admission the culture of the BAL fluid was positive for M. tuberculosis. Antibiogram showed a normal sensitivity pattern so the ethambutol was discontinued. In total the patient was treated with HRZ for two months followed by four months of HR.
14 days after initiating therapy sputum conversion was reached as presence of acid-resistant bacilli by using Ziehl-Neelsen method was negative in sputum sample, during 3 consecutive days. 10 Days after discharge from the intensive care unit patient was discharged from the ward. Clinical parameters all improved rapidly during his treatment. Furthermore the liver enzymes normalised during his hospital stay and remained normal during the total treatment period.
One month after initiating treatment for tuberculosis patient complained of lumbar back pain. Further radiologic evaluation using MRI showed abnormal signal intensity of L4, which was interpreted as spondylitis for which patient was referred to the orthopaedic surgeon. No further actions were taken and complaints resolved. At radiologic follow-up 3 months after MRI sclerosis was seen, but no signs of spondylitis anymore.
After treatment of the M. tuberculosis infection no abnormalities were seen on radiologic evaluation using X-ray of the chest, especially no parenchymal abnormalities or lymphadenopathy.
In November 2011 the patient was referred to the ophthalmologist by the general practitioner with complaints of red eye which did not resolve with chloramphenicol. The ophthalmologist diagnosed uveitis and initiated treatment with ocular prednisolon and atropine. Under this treatment uveitis resolved completely except for remaining synechia. Under suspicion of reactivation of tuberculosis the patient was referred to our outpatient clinic. At that time he had no pulmonary complaints, in particular no cough, weight loss or dyspnoea. He did not appear to be ill with a blood pressure of 115/70 mmHg, heart rate 105 beats/min and oxygen saturation 98% on ambient air. No physical abnormalities were observed. His body temperature was 37.1 degrees Celsius.
Laboratory investigations showed normal electrolytes including calcium and renal function. Erythrocyte sedimentation rate was 5 mm/h and C-reactive protein was <6.0 mmol/L. Angiotensin converting enzyme was 86 U/L (normal range 12–82 U/L).
Chest radiogram disclosed suspicion of mediastinal and hilar lymphadenopathy and interstitial densities in the right lobe which was confirmed on computed tomography of the thorax (Fig. 2).
Fig. 2.
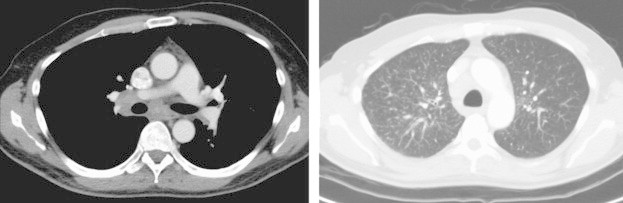
Computed tomogram of the thorax showing mediastinal lymphadenopathy (left) and multiple nodules in the apical segment of the right upper lobe.
Bronchoscopy was performed with lavage in the right upper lobe in combination with trans-bronchial needle aspiration (TBNA) of a pretracheal lymph node. No acid-resistant bacilli were found in the fluid lavage or in the cytologic material of TBNA. PCR for M. tuberculosis DNA was negative. Nevertheless since there was high suspicion on reactivation of pulmonary tuberculosis anti-tuberculosis drug therapy was initiated.
Two months following initiating treatment for M. tuberculosis another computed tomogram of the thorax was performed which was completely unchanged in comparison to the earlier scan, except now there were multiple nodules located at the right major fissure.
An endoscopic ultrasound with fine needle aspiration of a subcarinal lymph node was performed. Microscopic analysis revealed a population of lymphoid cells with spread granulomas. Necrosis was not observed. PCR for MTBC DNA was negative and acid-resistant bacilli were not present. Apart from the fine needle aspiration, a biopsy of an erythematosquamous lesion on the left upper arm was taken which revealed granulomatous structures, consisting of epitheloid histiocytes and few polynucleid giant cells, surrounded by small atypic lymphocytes. No central necrosis or caseiting was seen.
The patient was diagnosed with sarcoidosis and anti-tuberculosis therapy was discontinued.
Cardiac magnetic resonance imaging revealed no cardiac granulomas.
Diffusion capacity for carbon monoxide was normal and during exercise testing no oxygen uptake problem could be observed.
As there was no indication for initiating immunosuppressive therapy patient was seen at our outpatient clinic every three months for follow-up.
3. Discussion
At first presentation the patient was diagnosed with pulmonary miliary tuberculosis. Because of the rapid deterioration, it was very well possible that during admission at the hospital a bacterial superinfection was present, although no other infectious agent was determined. Treatment of the tuberculosis infection was performed according to the standard anti-TB drug regimen. Directly observed therapy (DOT) was performed during the six months therapy, so we can assume adequate therapy was given, although randomized controlled trials provide no assurance that the routine use of DOT improves cure or treatment completion.3
18 months after his initial presentation the clinical features and diagnostic imaging which was performed could very well be matched with a reactivation of tuberculosis as uveitis and mediastinal lymphadenopathy in combination with nodules located in the right upper lobe were present. The normal erythrocyte sedimentation rate and no physical or respiratory complaints argue with this statement. The first TBNA that was performed during the hospitalisation 12 months after discontinuation of the anti-tuberculous therapy did not show lymphoid material meaning the results of negative cultures and the absence of acid-resistant bacilli as well as the negative PCR for MTB DNA should be taken with caution.
Guided by the above-discussed results diagnosis of pulmonary sarcoidosis and uveitis was the correct diagnosis.
The percentage of sarcoidosis patients with eye involvement varies widely with approximately 30% in the United States of America and England to approximately 70% in Japan.4 Uveitis is also frequently seen in M. tuberculosis infection, so this does not differentiate between sarcoidosis and tuberculosis.
The use of endobronchial ultrasound could have been considered in an earlier phase, as the TBNA was not representative. If the PCR for MTBC DNA and Ziehl-Neelsen were also negative discontinuation of anti-TB drug therapy was justified earlier.
As stated in the introduction the role of mycobacteria in the aetiology of sarcoidosis has been extensively studied. Initial studies had focused on an epidemiological link and tried to demonstrate presence of mycobacteria using cultures or histological evaluation. More recent studies demonstrate that molecular assays can detect genetic material from mycobacteria in sarcoidosis samples with high sensitivity. Gazouli et al. reported that in a Greek sarcoidosis population 72% of tissues contained amplifiable MTB DNA and Saboor and colleagues found MTB DNA in half of sarcoidosis lung samples using PCR.5,6
Furthermore, several studies using immune assays and the isolation of antigens (e.g. Mycobacterial catalase-peroxidase antigen, mKatG) have lead to a robust step in associating sarcoidosis and mycobacteria. mKatG may lead to a T-cell response which leads to formation of granulomas and might therefore be a pathogenic antigen.7 These studies may indicate that the presence of mycobacterial antigens is enough to elicit immune reaction in a susceptible host.
This might explain why only few case studies have been reported where patients develop sarcoidosis after culture positive tuberculosis and that the incidence of sarcoidosis is higher in countries with high burden of latent tuberculosis. This fact also highlights the limitations of serological and molecular studies to discriminate between the two conditions.
4. Conclusion
M. tuberculosis and/or antigens very likely play a role in the aetiology of sarcoidosis in a proportion of patients as recent advances in immunological and molecular techniques have strengthened the association between mycobacteria and sarcoidosis.1,8 Further studies using new techniques could help in the understanding of immune responses caused by mycobacterial antigens and other possible infectious antigens.
This case report demonstrates that when recurrent disease of tuberculosis is suspected one has to keep in mind a broad differential diagnosis as other similar presenting diseases may be present which warrant a different approach for treatment. In the work-up of diagnostics one should always be sure that cytologic or histologic material is representative as this influences the interpretation of the results.
Only two case studies have been reported where a patient who was treated for tuberculosis developed sarcoidosis. One developed a cutaneous sarcoidosis with uveitis and no pulmonary involvement9 and the other one developed a miliary sarcoidosis.10
To our knowledge our patient is unique as he developed pulmonary stage two sarcoidosis in combination with uveitis and demonstrates a likely causative role of mycobacterial antigens in the development of sarcoidosis.
Conflict of interest
There exists no conflict of interest.
References
- 1.Brownell I., Ramirez-Valle F., Sanchez M., Prystowksi S. Translational review, evidence for mycobacteria in sarcoidosis. Am J Respir Cell Biol. 2011;45:899–955. doi: 10.1165/rcmb.2010-0433TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baughman R.P., Lower E.E., du Bois R.M. Sarcoidosis. Lancet. 2003;361:1111–1118. doi: 10.1016/S0140-6736(03)12888-7. [DOI] [PubMed] [Google Scholar]
- 3.Volmink J., Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2012;1 doi: 10.1002/14651858.CD003343. CD003343. [DOI] [PubMed] [Google Scholar]
- 4.Baughman R.P., Lower E.E., Kaufman A.H. Ocular sarcoidosis. Semin Respir Crit Care Med. 2010;31(4):452–462. doi: 10.1055/s-0030-1262213. [DOI] [PubMed] [Google Scholar]
- 5.Gazouli M., Ikonomopoulos J., Trigidou R., Foteinou M., Kittas C., Gorgoulos V. Assessment of mycobacterial, propionibacterial, and human herpesvirus 8 DNA in tissues of Greek patients with sarcoidosis. J Clin Microbiol. 2002;40:3060–3063. doi: 10.1128/JCM.40.8.3060-3063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saboor S.A., Johnson N.M., McFadden J. Detection of mycobacterial DNA in sarcoidosis and tuberculosis with polymerase chain reaction. Lancet. 1992;339:1015–1017. doi: 10.1016/0140-6736(92)90535-b. [DOI] [PubMed] [Google Scholar]
- 7.Chen E.S., Wahlstrom J., Song Z. T cell responses to mycobacterial catalase-peroxidase profile, a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008 Dec 15;181(12):8784–8796. doi: 10.4049/jimmunol.181.12.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta D., Agarwal R., Aggarwal A.N., Jindal S. Review, sarcoidosis and tuberculosis: the same disease with different manifestations or similar manifestations of different disorders. Curr Opin Pulm Med. 2012;18:506–516. doi: 10.1097/MCP.0b013e3283560809. [DOI] [PubMed] [Google Scholar]
- 9.Satyaki G., Dhiman G. Sarcoidosis following sputum positive pulmonary tuberculosis: a rare entity. Indian J Dermatol. 2012 Jan-Feb;57(1):76–78. doi: 10.4103/0019-5154.92691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatzakis K., Siafaka N.M., Bouros D. Miliary sarcoidosis following miliary tuberculosis. Respiration. 2000;67(2):219–222. doi: 10.1159/000029492. [DOI] [PubMed] [Google Scholar]


