Abstract
Primary salivary type lung cancer are extremely rare intrathoracic malignancies. Mucoepidermoid tumor is one of the salivary gland tumor which originates from submucosal glands of tracheobronchial tree. These are very slow growing low grade malignant tumors. Surgery is the mainstay of treatment and rarely requires adjuvant therapy. In this case report, we describe a case of a young male who presented with cough and hemoptysis. On further investigation he was found to have mucoepidermoid tumor originating from the left bronchus.
Keywords: Mucoepidermoid carcinoma, Luftsichel sign, Pneumonectomy
1. Introduction
Primary salivary gland type lung cancers are slow growing, low grade malignant neoplasms which are derived from the submucosal glands of the tracheobronchial tree and bear structural homology with exocrine salivary glands. These tumors commonly involve major and minor salivary glands, but lung involvement is quite uncommon. Primary salivary gland type lung cancers are extremely rare intrathoracic malignancies and account for approximately 0.1–0.2% of thoracic malignancies. Surgical resection is the treatment of choice. Complete surgical resection is associated with excellent prognosis. In this report, we describe the case of a 26 year old young male who presented with chronic cough and an endobronchial lesion in the left upper lobe bronchus which was diagnosed as mucoepidermoid carcinoma of the lung.
2. Case presentation
A 26 year old male was admitted with fever, chills and worsening cough for 2 weeks. He had a chronic cough which started 2 years prior to presentation and became progressively worse 2 weeks prior to presentation. He reported purulent sputum production with occasional streaks of blood in the sputum. There was no history of tuberculosis or tuberculosis exposure. He was tested tuberculin negative. His past medical history was not significant and his family history was noncontributory. His physical exam was remarkable for reduced air entry in the left upper lung field. Laboratory studies showed leukocytosis of 18,300 cells/ul. Sputum and blood cultures were negative. Sputum smear and culture for acid fast bacilli (AFB) were negative.
Chest radiograph (CXR) (Fig. 1A) demonstrates luftsichel sign, which signifies left upper lobe collapse with an area of lucency around the aortic arch created by the hyperinflated left lower lobe, a portion of which wraps around the medial side of the collapsed left upper lobe. However, overall there is no significant volume loss of left upper lobe due to the presence of an expansive underlying mass. Lateral Chest radiograph (Fig. 1B) showed major fissure pulled anteriorly with hyper-inflated left lower lobe. Computed Tomography (CT) (Fig. 2A and B) of chest showed a large heterogenous mass with an endobronchial component and dilated cystic spaces. These cystic spaces demonstrate a branching pattern and appear to be the dilated bronchus filled with mucous secretions. There are signs on CT chest which helped to distinguish it as a lung mass
-
A.
The mass is separated medially from the vessels by the mediastinal fat plane and is posteriorly outlined by the major fissure
-
B.
Presence of endobronchial lesion and dilated cystic spaces suggest a bronchus filled with mucous.
Fig. 1.
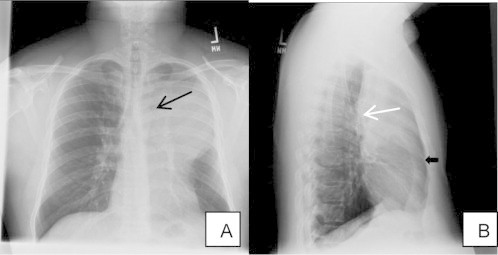
A – Chest radiograph frontal view: A large left upper lobe opacity obliterating the left heart border with luftsichel sign (black arrow). B – Chest radiograph lateral view: Major fissure is pulled anteriorly (white arrow) with hyperinflated left lower lobe (black arrow).
Fig. 2.

A – CT chest cross sectional view: Large heterogenous left upper lobe mass (white arrow) with endobronchial lesion (black arrow). B – CT chest cross sectional view: The mass is separated medially from the vessels by the mediastinal fat plane (arrow head), posteriorly outlined by the major fissure (white arrow) and contains dilated cystic spaces with branching pattern suggestive of dilated bronchus filled with mucus (black arrow).
Pulmonary function test was consistent with mild obstructive airway disease with FEV1 of 2.87 L (76% predicted). Lung volumes were normal. Positron emission tomography (PET) scan with 18F–flurodeoxyglucose showed increased uptake only over left hemi-thorax with no other areas of uptake indicating metastasis. Flexible bronchoscopy was performed for further evaluation of this mass. There was a large pinkish polypoidal mass obstructing the left upper lobe bronchus with thick purulent secretions (Fig. 3). Endobronchial biopsy showed (Fig. 4A and B) malignant epithelial neoplasm infiltrating the fibrous hyaline stroma. The neoplasm is composed of multiple glandular structures lined by mucous secreting cells with interspersed stroma which has squamoid and clear cells with minimal mitosis which is consistent with the diagnosis of low grade primary salivary type lung cancer: mucoepidermoid carcinoma. Thoracic surgery was consulted for left upper sleeve lobectomy. Unfortunately there were extensive adhesions which limited the separation of vascular planes between the left upper and lower lobe and thus complete pneumonectomy was performed. Extensive lymph node sampling did not reveal any regional spread. His postoperative course was uneventful and did not require any adjuvant therapy.
Fig. 3.
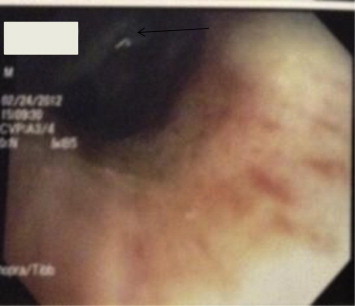
A 2 cm large exophytic, sessile luminal mass with intact mucosa obstructing the left upper lobe bronchus and covered with thick purulent secretions.
Fig. 4.
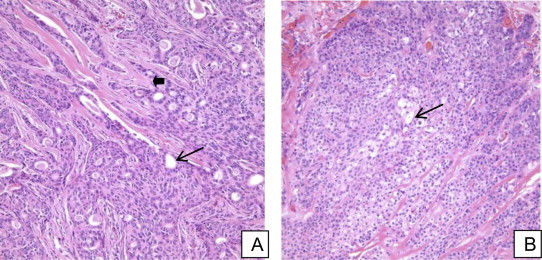
A – High power view: Multiple glandular structures lined by mucin producing goblet cells (black arrow) in the background of stroma (arrow head). B – High power view: Stroma mixed with intermediate and clear cells (black arrow).
3. Discussion
Mucoepidermoid tumor affects males and females equally and the median age of presentation is 40 years. They commonly present with cough, dyspnea, hemoptysis, wheezing and pneumonia. Most salivary-type lung cancers present as a mass in the trachea, carina or in a main stem bronchi and occasionally as a peripheral nodule. In contrast to adenoid cystic lesions, mucoepidermoid tumors involve lobar and main stem bronchi more commonly than trachea and cause post-obstructive pneumonia and atelectasis.
The diagnosis is often delayed for more than a year due to slow growth, non-specific signs and symptoms and subtle findings on thoracic imaging, unless hemoptysis due to tumor growth or mucosal erosion prompts bronchoscopic evaluation. Chest radiographs are rarely helpful, may show distal atelectasis or pneumonia. Axial CT typically shows non-spherical, smooth lobulated polypoidal mass associated with dilated distal bronchi, mucoid impaction and distal atelectasis. At bronchoscopy, mucoepidermoid carcinomas of the trachea appear as pink, polypoid masses that can be confused with a carcinoid tumor. The diagnosis is made by histo-pathological analysis of the biopsy specimen which typically shows variable proportions of mucus-secreting cells, squamous cells, intermediate cells and intercellular bridges. On the basis of pathological findings mucoepidemoid tumors can be categorized into low grade and high grade tumors. Mitoses, nuclear pleomorphism, and necrosis are usually absent or minimal in low-grade mucoepidermoid carcinomas and it rarely metastasizes to regional lymph nodes or distant organs.
Surgical resection is the mainstay of treatment. Complete tumor removal with nodal dissection, and preservation of functional parenchyma is the goal of the therapy. Sleeve lobectomy is commonly done and occasionally requires pneumonectomy in more extensive disease. Adjuvant radiotherapy is required in cases of unresectable or incompletely resectable tumors. Adjuvant chemotherapy is not necessary, however in few case reports tyrosine-kinase inhibitor Gefitinib has shown good response in patients with mucoepidermoid carcinoma having EGFR gene mutations. Overall survival for primary salivary gland type Lung cancer after surgical resection is excellent with 5 year and 10 year survival of 97.6% and 86.7% respectively. Molina et al. reported excellent survival in surgically resected mucoepidermoid tumors; 87% at both 5 and 10 years and poor survival in surgically resected adenoid cystic carcinomas with a 5 and 10 years survival of 57% and 45%.
Ethical approval
The study was performed at Jacobi Medical Center.
This manuscript is not under consideration in any other journal.
The authors declare that there was no funding for this study.
All authors have read the manuscript and agree to the content.
Financial/nonfinancial disclosures
The authors have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Conflict of interest
None.
Contributor Information
Amit Chopra, Email: dr.amitchopra@gmail.com.
Chang Shim, Email: Chang.shim@nbhn.net.
Nirmal Sharma, Email: nirmalshya@gmail.com.
David Gordon, Email: david.gordon@nbhn.net.
Amit Tibb, Email: amit.tibb@nbhn.net.
Suggested reading
- 1.Heitmiller R.F., Mathisen D.J., Ferry J.A., Mark E.J., Grillo H.C. Mucoepidermoid lung tumors. Ann Thorac Surg. 1989;47:394–399. doi: 10.1016/0003-4975(89)90380-9. [DOI] [PubMed] [Google Scholar]
- 2.Yousem S.A., Hochholzer L. Mucoepidermoid tumors of the lung. Cancer. 1987;60:1346–1352. doi: 10.1002/1097-0142(19870915)60:6<1346::aid-cncr2820600631>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Judson M.A., Sahn S.A. Endrobronchial lesion in HIV-infected individuals. Chest. 1994 May;105(5):1314–1322. doi: 10.1378/chest.105.5.1314. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy Michael J., Rosado-de-Christenson Melissa. Tumors of the trachea. J Thorac Imaging. 1995;10:180. doi: 10.1097/00005382-199522000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kim T.S., Lee K.S., Han J. Mucoepidermoid carcinoma of the tracheobronchial tree: radiographic and CT findings in 12 patients. Radiology. 1999;212:643–648. doi: 10.1148/radiology.212.3.r99se09643. [DOI] [PubMed] [Google Scholar]
- 6.Brzecka A., Werynska B., Dyla T. Malignant endobronchial lesions other than lung cancer. J Oncol. 2004;54(5):496–499. [Google Scholar]
- 7.Molina J.R., Aubry M.C., Lewis J.E., Wampfler J.A., Williams B.A., Midthun D.E., Yang P., Cassivi S.D. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110:2253–2259. doi: 10.1002/cncr.23048. [DOI] [PubMed] [Google Scholar]
- 8.Liu X., Adams A.L. Mucoepidermoid carcinoma of the bronchus; a review. Arch Pathol Lab Med. 2007;131:1400–1404. doi: 10.5858/2007-131-1400-MCOTBA. [DOI] [PubMed] [Google Scholar]
- 9.Han S.W., Kim A.P., Jeon Y.K., Oh D.Y., Lee S.H., Kim D.W. Mucoepidermoid carcinoma of lung: potential target of EGFR-directed treatment. Lung Cancer. 2008;61:30–34. doi: 10.1016/j.lungcan.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Kang D.Y., Yoon Y.S., Kim H.K., Choi Y.S., Kim K., Shim Y.M., Kim J. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer. 2011;72:250–254. doi: 10.1016/j.lungcan.2010.08.021. [DOI] [PubMed] [Google Scholar]


