Abstract
We report here a 66-year-old woman diagnosed with bronchioloalveolar carcinoma of the right lung cT4N2M0. The patient was from the Philippines, had never smoked, and tested positive for an EGFR mutation. She received gefitinib as neoadjuvant therapy for two months and displayed a partial response. The tumour was resected by performing a right pneumonectomy. The residual viable tumour accounted for less than 10%. Adjuvant chemotherapy with carboplatin-taxol was administered for four cycles. Fifteen months post-surgery, two brain metastases were found. Gefitinib was prescribed, and one month later complete radiological response was assessed. The patient remains asymptomatic and without visible disease four months later. Controlled randomised trials are needed to clarify the role of these target therapies in the neoadjuvant setting.
Keywords: Lung cancer, Gefitinib, Neadjuvant treatment
1. Case report
A 66-year-old woman from the Philippines, a never-smoker consulted for chest pain, dyspnea and weight loss. A CT scan revealed a condensation that almost entirely occupied the right lung (Fig. 1). A PET scan revealed uptake throughout the right lung, with an SUV of 10 and mediastinal lymphadenopathy. Bronchoscopy revealed partial obstruction of the LM and LIF of the bronchus intermedius. A transbronchial biopsy indicated that the tumour was bronchioloalveolar carcinoma. An EGFR mutation test was positive (exon 21). The patient was diagnosed with bronchioloalveolar carcinoma stage IIIB, and gefitinib treatment with 250 mg/once per day was prescribed. One month later, the patient was asymptomatic, and a CT scan revealed a response greater than 50% (Fig. 2). PET/TAC analysis indicated a response of >80%, with an SUV of 3 and no mediastinal adenopathy. When a right pneumonectomy was performed post-treatment, the amount of residual viable tumour found was less than 10%, and reparative fibrous lesions were present (Fig. 3). Adjuvant chemotherapy with carboplatin-taxol was then administered for four cycles. The patient came to our clinic regularly and did not display disease relapse until 15 months after diagnosis, when she experienced blurred vision and phosphenes in the inferior inner quadrant of the right eye. A brain MRI scan revealed two lesions that were consistent with metastases in the left occipital and frontal subcortical regions. Gefitinib was prescribed, and one month later complete radiological response was observed. The patient remains asymptomatic and without visible disease four months later.
Fig. 1.
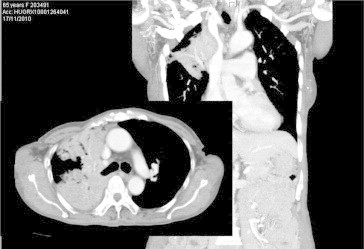
A condensation that occupied nearly the entire right lung was observed at diagnosis.
Fig. 2.
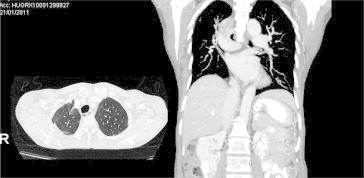
A response of greater than 50% was observed one month after starting Gefitinib treatment.
Fig. 3.
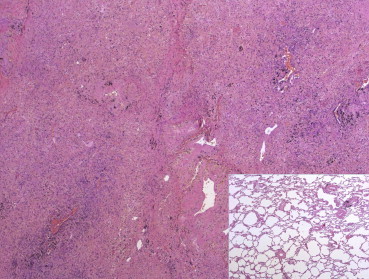
Panoramic image of the lung parenchyma. After treatment, most lung tissue was replaced by fibrous cellular sparingly, histiocytes with pigment and some inflammatory cells. At the bottom right side, normal parenchyma preserved to compare similar microscope magnifications.
2. Discussion
In 2004, three research groups identified somatic mutations in the tyrosine-kinase domain of EGFR that were associated with high TKI-response rates.1–3 Eighty-five percent of all EGFR mutations in non-small cell lung cancer (NSCLC) include exon 19 deletions and replacement of leucine 858 by arginine (L858R) in exon 21. These mutations were found more often in groups of patients who displayed specific clinical characteristics (female, Asian, adenocarcinomas, non-smokers).4
Published data from 1179 patients showed that more than 70% of patients with EGFR mutations responded to treatment with TKIs, whereas only 10% of patients without mutations responded to this type of treatment.5 An initial assessment conducted by the Spanish Lung Cancer Group (SLCG) indicated that patients with EGFR mutations who received second-line treatment with gefitinib had a 60–90% response, with survival approaching 13 months, whereas patients without EGFR mutations had a response rate below 10% as well as statistically lower survival.6,7
EGFR mutations may be associated with distinct sensitivity to TKIs. Various studies have demonstrated that response and survival after erlotinib and gefitinib treatment are significantly different in patients with exon 19 deletions than in those with exon 21 mutations.8–10
The SLCG evaluated the feasibility of large-scale EGFR mutation screening in NSCLC patients and analysed the association between EGFR mutations and clinical outcomes following treatment with erlotinib.11 From April 2005 through November 2008, a total of 2105 patients with NSCLC from 129 institutions were prospectively screened for EGFR mutations. EGFR mutation assessment was performed centrally at the Catalan Institute of Oncology. Mutations in the EGFR gene were detected in 350 of the 2105 patients screened (16.6%). Mutations were detected more frequently in women (30%), never-smokers (37.7%) and patients with adenocarcinomas (17.3%). However, mutations were also observed in men (8.2%), former smokers (9.5%) current smokers (5.8%), and patients with large-cell carcinomas (11.5%).
Erlotinib was administered to 217 patients, 113 of whom received erlotinib as first-line therapy and 104 received erlotinib as second- or third-line therapy. EGFR exon 19 deletion mutations were detected in 135 tumours, and the L858R mutation was detected in 82 tumours. Of the 164 patients in whom EGFR mutations were also assessed in serum, 97 carried mutations: exon 19 deletions were present in 64 patients and L858R mutations were present in 33 patients. The overall response rate was 70.6%, of which 12.2% were complete responses. The responses to erlotinib treatment in patients with exon 19 deletion mutations were better than those in patients with the L858R mutation (odds ratio, 3.08; 95% CI, 1.63 to 5.81; P = 0.001) and in patients 61–70 years of age (odds ratio, 2.55; 95% CI, 1.32 to 4.96; p = 0.006). The median PFS was 14 months. The duration of response was similar for patients receiving first- or second-line therapy. The median overall survival was 28 months for patients receiving first-line therapy and 27 months for those receiving second-line therapy. The median PFS was 16 months for women and 9 months for men (P = 0.003). The median overall survival was 29 months for women and 18 months for men. There were no significant differences in PFS on the basis of performance status (PS), age, first-line versus second- or third-line therapy, or smoking history.
Multivariate analysis revealed associations between poor PFS and male sex and the presence of the L858R mutation. In the multivariate analysis of overall survival, PS 1, male sex, the presence of the L858R mutation, brain metastases, and the presence of bronchioloalveolar adenocarcinoma were associated with poor prognosis.
Large-scale screening of patients for EGFR mutations, with subsequent customisation of erlotinib treatment, was demonstrated to be feasible and to improve outcomes. Subsequently, two phase III trials of Asian patients with EGFR mutations demonstrated that progression-free survival was longer for patients receiving gefitinib treatment than for those receiving chemotherapy.12,13
The EURTAC trial—the most recently published randomised phase III trial—was conducted in patients with metastatic lesions. Overall, 174 patients with EGFR mutations were enrolled from 42 hospitals in France, Italy, and Spain. They were randomised to receive either erlotinib- or standard-platinum based chemotherapy as first-line therapy for metastases. Median PFS in the erlotinib treated group was significantly higher than that in the group receiving standard chemotherapy (9.7 months versus 5.2 months, HR 0.37; p < 0.0001). Median survival was 18.8 months in the chemotherapy arm and 22.9 months in the erlotinib arm (HR, 0.80; P = 0.42). This study was the first phase III trial conducted in Western populations to demonstrate the superiority of oral target therapy to platinum-based chemotherapy as a first-line treatment for metastases.14
No randomised phase III trials have been conducted in the neoadjuvant setting with gefitinib or erlotinib, but some favourable data already exist, most of which are in the form of case reports.15–19
In 2009, a phase II study of preoperative gefitinib administration during clinical stage I NSCLC was published.20 Thirty-six patients received 250 mg/d gefitinib treatment for one month prior to surgery. A partial response was observed in four patients (11%), and disease progression was observed in three patients (9%). The strongest predictor of response was the presence of an EGFR mutation, which was found in 17% of the patients studied.
The same pathological samples were studied in order to define the pathological features associated with response to TKIs in NSCLC. The tumours studied included 7 squamous cell carcinomas, 27 adenocarcinomas, one adenosquamous carcinoma and one large-cell carcinoma. Six of the adenocarcinomas harboured EGFR mutations. After gefitinib treatment, the tumours possessing EGFR mutations demonstrated lower tumour cellularity and a lower proliferative index, as well as large areas of fibrosis, compared with adenocarcinomas and non-adenocarcinomas in individuals who did not possess mutations. However, there were no significant correlations between the degree of fibrosis or whether the tumours had undergone the epithelial to mesenchymal transition and radiological changes in tumour size.21
A phase II study of preoperative erlotinib treatment in patients from four hospitals in the Netherlands who had early stage NSCLC was recently reported.22 Sixty patients received 150 mg of erlotinib once daily for three weeks before surgery. PET scans revealed that 27% of the patients displayed metabolic responses, and CT scans indicated that 5% responded to treatment when RECIST criteria were used. There was a group within the patient population (female never-smokers with nonsquamous carcinomas) in whom the response rate was 34%. No unexpected complications occurred in surgery.
An important issue is the gold standard test to evaluate the response to TKIs in the neoadjuvant setting. As we have described previously, the radiological response observed in CT scans with respect to RECIST criteria does not correlate adequately with pathological response. In an effort to address this discrepancy, Aukema et al. developed a study to prospectively evaluate the use of 18F-FDG PET/CT in the early identification of response to neoadjuvant erlotinib treatment.23 In the study, 23 patients diagnosed with NSCLC received erlotinib treatment once daily for three weeks. A PET/CT scan was performed before and at one week after erlotinib administration. The metabolic responses were compared with the pathologic responses. Twenty-six percent of patients had a partial metabolic response, 70% of patients had stable disease and 1 patient had progressive disease. The median percentage of necrosis in the metabolic responder group was 70%, and the median percentage of necrosis was 40% in the non-responder group. The results of this study suggest that PET/CT scans can predict response to erlotinib treatment in patients with NSCLC.
The patient in our report met all the clinical criteria that most common in patients with EGFR mutations: female sex, Asian ethnicity, never-smoker status and presence of adenocarcinoma. Because of the large size of the tumour, neither pulmonary nor mediastinal radical surgery was possible. We opted to use TKI therapy because of the presence of an EGFR mutation and the excellent results TKIs have shown in the treatment of metastases. We performed an early reevaluation of the patient using both CT and PET scans. We observed a partial response, both radiologic and metabolic, that had a good correlation with the pathological findings. The PFS was 17 months, with brain symptomatic relapse. The patient achieved complete radiologic response when gefitinib was introduced again.
Given the important results in the metastatic setting and the favourable data obtained in some neoadjuvant cases, randomised phase III trials are needed to clarify the role of EGFR TKIs as neoadjuvant therapy for patients with activated EGFR mutations. Other aspects that remain to be clarified are the role of certain mutations that are insensitive to TKIs, the resistance factors to TKIs and a gold standard test that can be used to evaluate patient response to TKIs.
Conflicts of interest
None.
References
- 1.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W. Activating mutations in the epidermal growth factor receptor underlying response of non-small-celllung cancer to Gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez J.G., Jänne P.A., Lee J.C., Tracy S., Greulich H., Gabriel S. EGFR mutations in lung cancer: correlation with clinical response to Gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W., Miller V., Zakowski M., Doherty J., Politi K., Sarkaria I. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to Gefitinib and Erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitsudomi T., Kosaka T., Yatabe Y. Biological and clinical implications of EGFR mutations in lung cancer. Int J Clin Oncol. 2006;11:190–198. doi: 10.1007/s10147-006-0583-4. [DOI] [PubMed] [Google Scholar]
- 5.Uramoto H., Mitsudomi T. Which biomarker predicts benefit from EGFR-TKI treatment for patients with lung cancer? Br J Cancer. 2007;96:857–863. doi: 10.1038/sj.bjc.6603665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortes-Funes H., Gomez C., Rosell R., Valero P., Garcia-Giron C., Velasco A. Epidermal growth factor receptor activating mutations in Spanish Gefitinib-treated non-small cell lung cancer patients. Ann Oncol. 2005;16:1081–1086. doi: 10.1093/annonc/mdi221. [DOI] [PubMed] [Google Scholar]
- 7.Taron M., Ichinose Y., Rosell R., Mok T., Massuti B., Zamora L. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in Gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res. 2005;11:5878–5885. doi: 10.1158/1078-0432.CCR-04-2618. [DOI] [PubMed] [Google Scholar]
- 8.Mitsudomi T., Kosaka T., Endoh H., Horio Y., Hida T., Mori S. Mutations of the epidermal growth factor receptor gene predict prolonged survival after Gefitinib treatment in-patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–2520. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 9.Riely G.J., Pao W., Pham D., Li A.R., Rizvi N., Venkatraman E.S. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with Gefitinib or Erlotinib. Clin Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 10.Jackman D.M., Yeap B.Y., Sequist L.V., Lindeman N., Holmes A.J., Joshi V.A. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with Gefitinib or Erlotinib. Clin Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 11.Rosell R., Morán T., Queralt C., Porta R., Cardenal F., Camps C. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 12.Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of epidermal growth factor receptor (WJTOG 3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 13.Mok T.S., Wu Y.L., Thongprasert S., Yang C.H., Chu D.T., Saijo N. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 14.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012 Mar;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [Epub 2012 Jan 26] [DOI] [PubMed] [Google Scholar]
- 15.Liu M., Jiang G., He W., Zhang P., Song N. Surgical resection of locally advanced pulmonary adenocarcinoma after gefitinib therapy. Ann Thorac Surg. 2011 Jul;92(1):e11–e12. doi: 10.1016/j.athoracsur.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Shen H., Zhong X., Ge X.Q., Huang J.J., Yuan Y. Surgical resection of lung adenocarcinoma without EGFR mutation after neoadjuvant gefitinib treatment. Clin Respir J. 2010 Jul;4(3):192–193. doi: 10.1111/j.1752-699X.2009.00167.x. [DOI] [PubMed] [Google Scholar]
- 17.Takamochi K., Suzuki K., Sugimura H., Funai K., Mori H., Bashar A.H. Surgical resection after gefitinib treatment in patients with lung adenocarcinoma harboring epidermal growth factor receptor gene mutation. Lung Cancer. 2007 Oct;58(1):149–155. doi: 10.1016/j.lungcan.2007.04.016. [Epub 2007 Jun 4] [DOI] [PubMed] [Google Scholar]
- 18.Ong M., Kwan K., Kamel-Reid S., Vincent M. Neoadjuvant erlotinib and surgical resection of a stage iiia papillary adenocarcinoma of the lung with an L861Q activating EGFR mutation. Curr Oncol. 2012 Jun;19(3):e222–e226. doi: 10.3747/co.19.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kappers I., Klomp H.M., Burgers J.A., Van Zandwijk N. Neoadjuvant (induction) erlotinib response in stage IIIA non-small-cell lung cancer. J Clin Oncol. 2008 Sep 1;26(25):4205–4207. doi: 10.1200/JCO.2008.16.3709. [DOI] [PubMed] [Google Scholar]
- 20.Lara-Guerra H., Waddell T.K., Salvarrey M.A., Joshua A.M., Chung C.T., Paul N. Phase II study of preoperative gefitinib in clinical stage I non-small-cell lung cancer. J Clin Oncol. 2009 Dec 20;27(36):6229–6236. doi: 10.1200/JCO.2009.22.3370. [Epub 2009 Nov 2] [DOI] [PubMed] [Google Scholar]
- 21.Lara-Guerra H., Chung C.T., Schwock J., Pintilie M., Hwang D.M., Leighl N.B. Histopathological and immunohistochemical features associated with clinical response to neoadjuvant gefitinib therapy in early stage non-small cell lung cancer. Lung Cancer. 2012 May;76(2):235–241. doi: 10.1016/j.lungcan.2011.10.020. [Epub 2011 Nov 22] [DOI] [PubMed] [Google Scholar]
- 22.Schaake E.E., Kappers I., Codrington H.E., Valdés Olmos R.A., Teertstra H.J., van Pel R. Tumor response and toxicity of neoadjuvant erlotinib in patients with early-stage non-small-cell lung cancer. J Clin Oncol. 2012 Aug 1;30(22):2731–2738. doi: 10.1200/JCO.2011.39.4882. [Epub 2012 Jul 2] [DOI] [PubMed] [Google Scholar]
- 23.Aukema T.S., Kappers I., Olmos R.A., Codrington H.E., van Tinteren H., van Pel R., NEL Study Group Is 18F-FDG PET/CT useful for the early prediction of histopathologic response to neoadjuvant erlotinib in patients with non-small cell lung cancer? J Nucl Med. 2010 Sep;51(9):1344–1348. doi: 10.2967/jnumed.110.076224. [Epub 2010 Aug 18] [DOI] [PubMed] [Google Scholar]


