Abstract
Hybrid sterility hinders the transfer of useful traits between Oryza sativa and O. glaberrima. In order to further understand the nature of interspecific hybrid sterility between these two species, a strategy of multi-donors was used to elucidate the range of interspecific hybrid sterility in this study. Fifty-nine accessions of O. glaberrima were used as female parents for hybridization with japonica cultivar Dianjingyou 1, after several backcrossings using Dianjingyou 1 as the recurrent parent and 135 BC6F1 sterile plants were selected for genotyping and deducing hybrid sterility QTLs. BC6F1 plants containing heterozygous target markers were selected and used to raise BC7F1 mapping populations for QTL confirmation and as a result, one locus for gamete elimination on chromosome 1 and two loci for pollen sterility on chromosome 4 and 12, which were distinguished from previous reports, were confirmed and designated as S37(t), S38(t) and S39(t), respectively. These results will be valuable for understanding the range of interspecific hybrid sterility, cloning these genes and improving rice breeding through gene introgression.
Keywords: Oryza sativa, Oryza glaberrima, interspecific hybrid, sterility
Introduction
Rice (Oryza sativa L.) is a staple food crop for more than half of the world’s population. Narrow genetic diversity has been considered as a bottleneck for further yield increase (Tanksley and McCouch 1997); thus, mining and introgression of favorable alleles from relatives of O. sativa to enhance its genetic diversities have been attracting increasing attention.
O. glaberrima, an African cultivated rice species with the same AA genome, is closely related to O. sativa. It contains various valuable traits for resistance to biotic stress, such as rice yellow mottle virus (Attere et al. 1983), blast (Silue et al. 1991), bacterial leaf blight (Khush 1989), African rice gall midge (Olga 2002), Heterodera sacchari (Reversat et al. 1995) and leafhopper (Khush 1989), and it is also tolerant for abiotic factors, such as drought, salinity and acidity (Ghesquiere et al. 1997, Lorieux et al. 2000, Sano et al. 1984). Meanwhile, high-yield genes or alleles of O. glaberrima could be used to break the yield ceiling of O. sativa cultivars. Thus, it was considered as an excellent gene pool to improve Asian cultivated rice (Jones et al. 1997, Xu et al. 2005).
However, hybrid sterility between O. sativa and O. glaberrima hinders the transfer of useful genes between the two species. To overcome hybrid sterility, genetic factors affecting sterility must be identified and characterized to better understand the nature of the sterility barrier.
So far, several genetic models have been proposed for hybrid sterility in plants, including the one-locus allelic interaction model (Ikehashi et al. 1986, Oka 1974, Sano 1990, Sano et al. 1979), duplicate gametophytic lethal model (Oka 1974) and Bateson-Dobzhansky-Muller (BDM) model (Bateson 1909, Dobzhansky 1936, Muller 1942). The one-locus allelic interaction model can explain the genetic behavior of most hybrid sterile loci in indica-japonica or O. glaberrima-japonica hybrids. Recently, with the development of rice genomics and molecular markers, a large number of loci affecting hybrid sterility between O. sativa and O. glaberrima were identified, such as gamete eliminator S1 and pollen killer S3, S18, S19, S20, S21, S29(t) (Doi et al. 1998, 1999, Hu et al. 2006, Sano 1983, 1986, Taguchi et al. 1999); however, only a few genes have been cloned and characterized in rice. S5 is a major gene controlling female fertility with wide compatibility in O. sativa L. ssp. indica-japonica hybrid. Chen et al. (2008) proposed a triallelic system to explain the molecular interactions among indica (S5-i), japonica (S5-j) and wide compatibility (S5-n) alleles, but Yang et al. (2012) found a killer-protector system at the S5 locus encoded by three tightly linked genes regulating fertility in indica-japonica hybrids. Sa, a locus for indica-japonica hybrid male sterility, comprises two adjacent genes, SaM and SaF, a two-gene/three-component interaction model was proposed for Sa (Long et al. 2008). Both S27 and S28, the reciprocal loss of duplicated genes, encode a mitochondrial ribosomal protein L27 (mtRPL27), which controls hybrid pollen sterility in F1 hybrids between O. sativa and its wild relative O. glumaepatula (Yamagata et al. 2010). Additionally, a model in which incompatibilities in epistatic interactions between S1 and additional factors are the cause of the female sterility barrier between O. sativa and O. glaberrima was developed to explain female sterility and the transmission ratio distortion mediated by S1 (Garavito et al. 2010).
These efforts allow further understanding of the nature of hybrid sterility. Meanwhile, it would be interesting and useful to find an interspecific neutral allele or wide compatibility allele, such as S5-n, which does not cause gamete abortion in hybrids (Ikehashi et al. 1986, Tao et al. 2003). Unfortunately, there is still no publication report regarding this issue (Deng et al. 2010). An alternative method of solving this problem is to use a bridge parent with a sterile gene allele (Deng et al. 2010); however, when a single sterile gene or a pyramid of several sterile genes was transferred to Asian cultivar species as a bridge parent to cross with African species, F1 was still highly sterile (Heuer et al. 2003, Sigrid et al. 2003). These results suggested that our understanding of the nature of interspecific hybrid sterility is far from complete. Currently, most hybrid sterility studies using one O. glaberrima experimental population can only obtain a few individuals due to high sterility in F1 and BC1F1 of interspecific hybrids; thus, it is likely that the current diverse studies are not sufficient to cover the range of interspecific hybrid sterile loci. Therefore, it is necessary to adopt a multiple donor approach to determine the number of loci and allelic variations responsible for hybrid fertility (Zhou et al. 2010) within accessions of O. glaberrima (Semon et al. 2005).
In this study, 59 accessions of O. glaberrima were used as donors to detect interspecific hybrid sterile loci. The results partially confirm previously described sterility loci and allow the identification of some new loci. This research will be benefit for further understanding the range of interspecific hybrid sterility, overcoming the sterility barrier between O. sativa and O. glaberrima and transferring genes controlling desirable traits such as high yield and drought tolerance from O. glaberrima to Asian cultivated rice varieties.
Materials and Methods
Materials
Fifty-nine accessions of O. glaberrima from the International Rice Research Institute (IRRI) as the maternal and donor parents, one O. sativa ssp. japonica variety, Dianjingyou 1 (DJY 1), from Yunnan province, P. R. China, as the paternal and recurrent parent, were used to obtain F1 (Late Crop Season in 2001, July to November), BC1F1 (Late Crop Season, 2002), BC2F1 (Winter Crop Season, November–March 2002) and BC3F1 (Early Crop Season, March–June 2003) progenies in Sanya, Hainan province, P. R. China. At BC1F1, about 20 plants of each cross were grown and used for backcrossing. Between BC2F1 and BC3F1, 10 plants of each family were grown, and about 3 plants of each family were randomly selected for backcrossing without selection. From BC4F1 (2003 Late Crop Season) after investigation of pollen grain fertility, sterile individuals (pollen grain fertility below 90%) were selected to backcross with the recurrent parent DJY 1 to obtain BC5F1 and BC6F1 (2003 Winter Crop Season, 2004 Late Crop Season). In BC6F1, 135 semi-sterile plants selected from 142 families of 59 cross combinations of O. glaberrima/Dianingyou 1/7/Dianjingyou 1 were used for genotyping (7 families without any sterile individual, sterile individuals from each donor ranged from 1 to 9), and raising BC7F1 mapping populations. BC7F1 populations were planted in Sanya in the 2004 Winter Crop Season.
Based on marker-assisted selection, NILs for the homozygous sterile gene were obtained from self-fertilized progenies of sterile plants from corresponding BC7F1 mapping populations. NILs were backcrossed with the recurrent parent DJY 1 to obtain BC8F1 for phenotype confirmation. Data for NILs and BC8F1 were collected in Late Crop Season, 2009.
Pollen grain and spikelet fertility check
Pollen grain fertility was measured using anthers collected from spikelets 1 to 2 days before anthesis and stored in 70% ethanol (Doi et al. 1998). Pollen grain fertility was estimated as the percentage of pollen grains that could be stained with 1% I-KI solution. Sterile types were further classified as typical, spherical and stained abortion types (Li 1980). Five independent microscopic fields were scored for counting the percentage of fertile pollen grains in each plant. Spikelet fertility was only investigated for parents and mapping populations.
Genotype and segmental linkage group construction
Leaves from each plant were sampled to extract DNA. The 135 BC6F1 semi-sterile plants were genotyped with 225 SSR markers based on the results of polymorphism detection for 344 SSR primer pairs tested between two parents, O. sativa DJY 1 and O. glaberrima IRGC102203. The selected markers were evenly distributed on 12 chromosomes. Since interspecific sterility was heterozygous sterility and selection was according to sterility, if the heterozygous rate of a marker in advanced backcross progeny is significantly higher than that of the theoretical predication from binomial distribution, this marker is likely to be linked to a QTL for sterility (Bernardo 2004, Li et al. 2008, 2011), provided there is no differentiation among these 59 cultivars in regards to hybrid sterility, and sterility is controlled by a dominant gene(s). In order to avoid the high probability of false QTL from multiple tests, a high significance level of 0.0005 was used.
In BC7F1 mapping populations, targeted markers and their linked markers for corresponding BC6F1 plants were employed to construct segmental linkage groups. Linkage analysis was performed using MAPMAKER version 3.0 (Lander et al. 1987), with a logarithm of odds (LOD) score of >3.0 for the segregating markers. The recombination frequency was converted to cM using the Kosambi function. Segmental linkage maps of target genes were drawn by Map Chart 2.2 (Voorrips 2002).
Results
Distribution of pollen grain fertility from BC4F1 to BC6F1 in the progenies of O. glaberrima/Dianjingyou 1
Even though several plants from each cross combination were randomly selected for backcrossing from BC1F1 to BC3F1 without selection, the results showed enough sterile plants for selection in BC4F1. The distribution was continuous in BC4F1 and BC5F1 and fertile, semi-sterile and sterile plants could still be found, but only fertile and semi-sterile plants could be found in BC6F1 (Table 1). These results indicate that interspecific hybrid sterility controlled by polygenes in the preliminary populations was gradually dissected as a simple inheritance mode after three times phenotypic selections and continuous backcrossing; thus, it was easy to detect and confirm QTLs in advanced backcross populations.
Table 1.
Distribution of pollen grain fertility from BC4F1 to BC6F1 in O. glaberrima/DJY 1 in 2003 Late Crop Season, 2003 Winter Crop Season and 2004 Late Crop Season, respectively, in Sanya, Hainan, P. R. China
| Generation | Population | Pollen grain fertility | Population size | Means of pollen grain fertility (%) | STDEV of pollen grain fertility | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2.5 | 7.5 | 12.5 | 17.5 | 22.5 | 27.5 | 32.5 | 37.5 | 42.5 | 47.5 | 52.5 | 57.5 | 62.5 | 67.5 | 72.5 | 77.5 | 82.5 | 87.5 | 92.5 | 97.5 | |||||
| BC4F1 | Preliminary population | 47 | 4 | 19 | 14 | 17 | 20 | 18 | 14 | 42 | 34 | 30 | 32 | 59 | 41 | 33 | 15 | 6 | 6 | 2 | 1 | 222 | 676 | 60.33 | 33.39 |
| Selected population | 35 | 1 | 7 | 5 | 4 | 8 | 6 | 4 | 15 | 8 | 10 | 9 | 24 | 12 | 15 | 1 | 1 | 0 | 0 | 0 | 3 | 168 | 36.99 | 25.67 | |
| BC5F1 | Preliminary population | 5 | 2 | 6 | 10 | 13 | 14 | 24 | 61 | 51 | 70 | 123 | 117 | 91 | 79 | 44 | 23 | 16 | 13 | 14 | 12 | 431 | 1219 | 67.78 | 27.06 |
| Selected population | 1 | 1 | 1 | 1 | 2 | 3 | 3 | 9 | 5 | 12 | 20 | 25 | 25 | 17 | 10 | 3 | 2 | 1 | 1 | 0 | 0 | 142 | 50.79 | 14.79 | |
| BC6F1 | Preliminary population | 0 | 0 | 1 | 1 | 5 | 8 | 6 | 10 | 40 | 58 | 87 | 150 | 73 | 38 | 10 | 6 | 1 | 1 | 3 | 11 | 285 | 794 | 67.74 | 34.43 |
| Selected population | 0 | 0 | 1 | 1 | 3 | 2 | 1 | 3 | 14 | 12 | 25 | 37 | 23 | 8 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 135 | 48.78 | 11.43 | |
Detection of QTLs for hybrid sterility in BC6F1
According to binomial distribution, significant probability was observed in six regions on chromosome 1, 2, 3, 4, 6, 12 and two regions on chromosome 7. We deduced that there were eight hybrid sterile QTLs corresponding to these regions and denoted them tentatively as qSS1, qSS2, qSS3, qSS4, qSS6, qSS12 and qSS7a, qSS7b (Table 2). Among these, qSS1, qSS4 and qSS12 were not reported between O. sativa and O. glaberrima in previous publications and these three new QTLs were detected from 3, 5, 5 different donors, respectively. Thus, three BC6F1 sterile plants 2004H2E137-2 (donor, IRGC101854), 2004H2E245-1 (donor, Acc102528), 2004H2E185-2 (donor, IRGC103466), harboring qSS1, qSS4 and qSS12, respectively and fewer heterozygous markers were used to raise the corresponding BC7F1 populations 2004H3E142 (qSS1), 2004H3E244 (qSS4) and 2004H3E188 (qSS12) for the confirmation and mapping of QTLs.
Table 2.
Heterozygous markers and QTLs detected in 135 sterile individuals of BC6F1 derived from 59 interspecific hybridization cross combinations between Oryza sativa and O. glaberrima in 2004 Late Crop Season in Sanya, Hainan, P. R. China
| Marker | Chromosome | Position (Mb)a | No. of heterozygous plants | Percentage of heterozygous plants (%) | No. of donors | PHb | QTL | Deduced gene |
|---|---|---|---|---|---|---|---|---|
| RM562 | 1 | 14.62 | 9 | 6.7 | 9 | 1.26E-06 | qSS1 | New |
| RM595 | 1 | 15.11 | 9 | 6.7 | 9 | 1.26E-06 | ||
| RM24 | 1 | 18.97 | 12 | 8.9 | 11 | 9.11E-10 | ||
| RM236 | 2 | 21.06 | 7 | 5.2 | 6 | 9.01E-05 | qSS2 | S29(t) |
| RM22 | 3 | 15.00 | 13 | 9.6 | 13 | 6.79E-11 | qSS3 | S19 |
| RM518 | 4 | 20.22 | 12 | 8.9 | 9 | 9.11E-10 | qSS4 | New |
| RM586 | 6 | 14.77 | 69 | 51.1 | 42 | 6.90E-107 | qSS6 | S1 |
| RM587 | 6 | 22.92 | 68 | 50.4 | 36 | 9.00E-105 | ||
| RM295 | 7 | 0.41 | 20 | 14.8 | 8 | 1.10E-19 | qSS7-a | S20 |
| RM3589 | 7 | 25.05 | 8 | 5.9 | 7 | 1.14E-05 | qSS7-b | S21 |
| RM5568 | 12 | 0.71 | 7 | 5.2 | 5 | 9.01E-05 | qSS12 | New |
Chromosome map location of marker in Mb (Ref. International Rice Genome Sequencing Project (IRGSP)).
The probability of heterozygote genotypes scored by the binomial test.
Segregation of pollen grain and spikelet fertility in BC7F1 populations
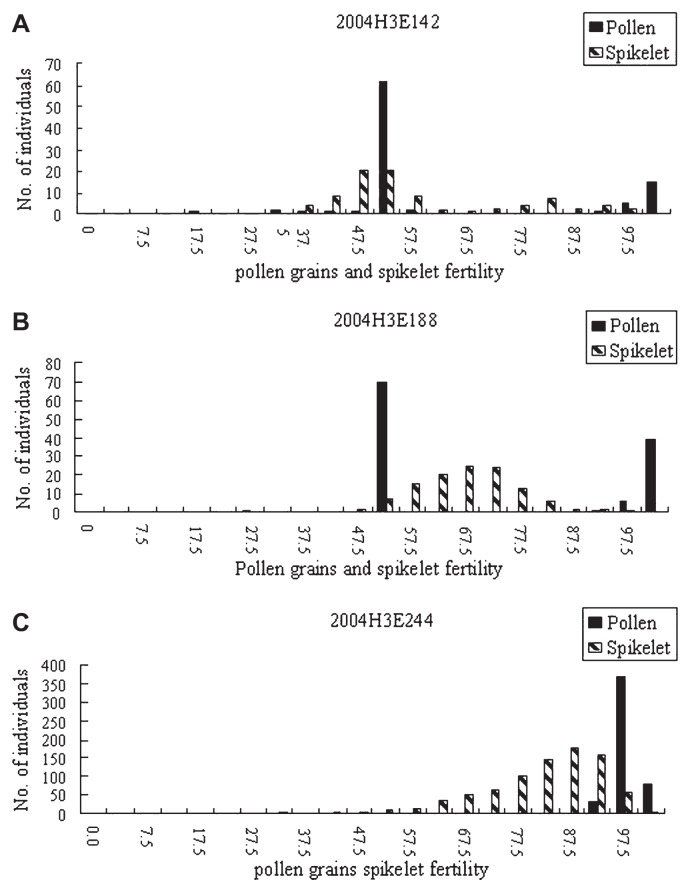
Pollen grain fertility showed similar bimodal distribution divided into semi-sterile (about 50% fertility) and fertile (about 100% fertility) groups in the three populations, spikelet fertility presenting with normal distribution tended to be fertile in population 2004H3E244 (qSS4) and 2004H3E188 (qSS12), but spikelet fertility of 2004H3E142 (qSS1) showed bimodal distribution (Fig. 1).
Fig. 1.
Distribution of pollen grain and spikelet fertility in three BC7F1 mapping populations, 2004H3E142 (IRGC101854/Dianjingyou 1), 2004H3E244 (IRGC102528/Dianjingyou 1) and 2004H3E188 (IRGC103466/Dianjingyou 1), respectively. The data were collected from 2004 Winter Crop Season in Sanya, Hainan, P. R. China.
Mapping of sterile QTLs in BC7F1 populations
Three target SSR markers, RM562 on chromosome 1 linked to qSS1, RM518 on chromosome 4 linked to qSS4, RM5568 on chromosome 12 linked to qSS12 and their linked polymorphic markers were used to survey the genotypes of individuals in population 2004H3E142 (qSS1), 2004H3E244 (qSS4) and 2004H3E188 (qSS12) for fine mapping of the target gene, respectively.
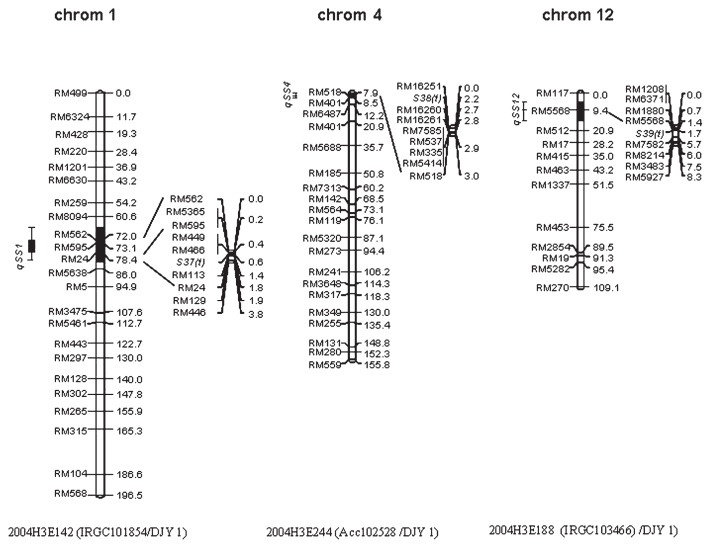
In population 2004H3E142 (qSS1), the introgression segment was about 3.8 cM between RM562 and RM446 and nine polymorphic SSR markers in the introgression region were used for mapping the target gene. The sterile gene was restricted to a 1.0 cM region flanked by RM449 and RM113 on the long arm of chromosome 1. In population 2004H3E244 (qSS4), the introgression segment was about 3.0 cM between RM16251 and RM518 and eight polymorphic SSR markers were used to locate the target gene. The sterile gene was mapped to a 2.7 cM region flanked by RM16251 and RM16260 on the short arm of chromosome 4. In population 2004H3E188 (qSS12), the introgression segment was about 8.3 cM between RM1208 and RM5927 and eight polymorphic SSR markers were used for mapping the target gene. The sterile gene was located in a 4.3 cM region flanked by RM5568 and RM7582 on the short arm of chromosome 12. Since sterile genes were not reported in these regions between O. sativa and O. glaberrima in previous studies, new genes, S37(t), S38(t), S39(t) on chromosome 1, 4 and 12, respectively, were named tentatively (Fig. 2).
Fig. 2.
The positions of QTLs in BC6F1 and segmental linkage maps of gene mapped in BC7F1 mapping populations for hybrid sterility (black bar represents QTL regions of hybrid sterility deduced with the probability of heterozygote genotypes in BC6F1 population. The small-scale linkage map shows the identified sterile loci in BC7F1 populations. DJY 1: Dianjingyou 1).
Genetic pattern and action of new sterile loci
Correlation analysis between pollen grain fertility and spikelet fertility of BC7F1 population 2004H3E142 showed that a significant correlation was found for S37(t) (r = 0.780, p < 0.0001), but for S38(t) and S39(t), the correlation between pollen grain fertility and spikelet fertility was not significant (r = 0.0097 and r = 0.0092, respectively), indicating that hybrid sterile gene S37(t) may synchronously control male and female gamete sterility, or S37(t) is a tightly linked pollen and spikelet sterile locus. However, S38(t) and S39(t) only affect pollen sterility.
Based on the “one-locus allelic interaction model”, the semi-sterile phenotype was caused by the allelic interaction between alleles derived from O. glaberrima and O. sativa. In the backcross population, for a single pollen killer locus, the distribution of semi-sterile/fertile plants should meet a 1 : 1 segregation ratio and for a gamete eliminator locus, all individuals should be semi-sterile. In fact, in population 2004H3E244 (qSS4) and 2004H3E188 (qSS-12), semi-sterile individuals were significantly fewer than fertile plants, and population 2004H3E142 (qSS1) gave some fertile plants (Table 3), indicating that O. sativa alleles at these loci are not completely lethal, or sterility was affected by the interaction between detected loci and other unknown loci and further investigations are needed to understand the mechanism.
Table 3.
Significant test of sterile plants: normal plants ratio by the chi-square test in three mapping populations in 2004 Winter Crop Season in Sanya, Hainan, P. R. China
| BC7F1 population | Donor | Population size | No. of individuals | X2 (1 : 1) | P | |
|---|---|---|---|---|---|---|
|
| ||||||
| Semisterile | Normal | |||||
| 2004H3E142 | IRGC101854 | 91 | 70 | 21 | 25.32** | X2 (0.05, 1) = 3.841, X2 (0.01, 1) = 6.635 |
| 2004H3E244 | IRGC102528 | 882 | 398 | 484 | 8.39** | |
| 2004H3E188 | IRGC103466 | 904 | 420 | 484 | 4.53* | |
Pollen and spikelet fertility of NILs and test cross F1
The closest SSR markers RM449 and RM113 for S37(t), RM16251 and RM16260 for S38(t), RM5568 and RM7582 for S39(t) were used to select homozygous individuals derived from corresponding selfing progenies of BC7F1 heterozygous individuals. NIL-S37(t), NIL-S38(t), NIL-S39( t) were raised, which carried 1.0 cM, 2.7 cM, 4.3 cM homozygous introgression segments from O. glaberrima, respectively. Then NILs were crossed with their recurrent parent DJY1 and BC8F1 were obtained. All three NILs showed fertile pollen grains and spikelets as the recurrent parent of DJY 1. The test cross F1, (BC8F1), which was obtained by using DJY1 to cross NIL-S37(t), NIL-S38(t) and NIL-S39(t), respectively, showed semi-sterile pollen grains, while the test cross F1 of NIL-S38(t)/DJY1 and NIL-S39(t)/DJY1 showed normal spikelet fertility; however, NIL-S37( t)/DJY1 exhibited semi-sterile spikelet fertility (Table 4). These results were consistent with the phenotype in BC7F1 mapping populations.
Table 4.
Pollen and spikelet fertility of NILs and test cross BC8F1 between NILs and DJY 1 in 2009 Late Crop Season in Sanya, Hainan, P. R. China
| Materials | Donor | Generations | N | Pollen fertility | Spikelet fertility | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean (%) | STDEV | Mean (%) | STDEV | ||||
| DJY 1 | 5 | 98.73 | 0.26 | 98.09 | 1.13 | ||
| NIL-S37(t) | IRGC101854 | BC7F3 | 5 | 98.82 | 0.28 | 95.00 | 2.16 |
| NIL-S38(t) | IRGC102528 | BC7F3 | 5 | 98.82 | 0.84 | 94.51 | 2.24 |
| NIL-S39(t) | IRGC103466 | BC7F3 | 5 | 99.18 | 0.26 | 92.30 | 0.89 |
| NIL-S37(t)/DJY1 | BC8F1 | 5 | 55.75 | 2.30 | 44.12 | 1.32 | |
| NIL-S38(t)/DJY1 | BC8F1 | 5 | 46.24 | 1.42 | 92.21 | 0.91 | |
| NIL-S39(t)/DJY1 | BC8F1 | 5 | 47.91 | 2.43 | 93.27 | 1.47 | |
Discussion
Diversity of sterility genes and gene action in O. glaberrima
The purpose of this research was to understand the range of interspecific hybrid sterility by using multiple donors of O. glaberrima. In this study, we detected 8 sterile QTLs, among which 5 loci, qSS2, qSS3, qSS6, qSS7a and qSS7b, corresponded to previous sterile loci S29(t), S19, S1, S20 and S21 (Doi et al. 1999, Hu et al. 2006, Taguchi et al. 1999, Tao et al. 2003) and qSS1, qSS4 and qSS12, which were identified as 3 new loci, were named S37(t), S38(t) and S39(t), respectively. These results will help to further understand the complexes of interspecific hybrid sterility in O. glaberrima. However, the current method, in which only semi-sterile plants were chosen for analysis, led to sterile loci of S3 and S18, which were reported to have high sterility (Doi et al. 1998) and some possible recessive sterile loci of O. glaberrima based on the BDM model, could not be detected. For the morphology of sterile pollen grains, it was interesting that loci S19, S20, S21, S29(t), S38(t) and S39(t), which were related to male-only function, showed staining abortion with partial starch filling. S1 and S37(t), controlling both male and female gamete fertility, all showed semi-sterility of the spikelet; however, S1 showed shrunken aspherical pollen grains with typical abortion, whereas S37(t) produced nearly 50% small and spherical empty pollen grains and half-stained abortion. S1 confirmed that epistatic interaction with additional factors was the cause of the female sterility barrier between O. sativa and O. glaberrima (Garavito et al. 2010). S37(t) influenced both male and female gamete fertility, but the detailed molecular mechanism needs to be confirmed by further investigation.
Co-linear analysis of detected sterile genes among AA genome species in genus Oryza
Hybrid sterility is the most common sterility of postzygotic mechanisms between species or subspecies, provides an initial force to maintain the genome stability of species and plays an important role in maintaining species identity (Orr et al. 2000, Sano 1986). Several co-linear analyses of hybrid sterility loci between or among species or subspecies have been described using the reported hybrid sterile loci (Chen et al. 2009, Hu et al. 2006, Lin et al. 1993, Sano 1994, Zhao et al. 2012, Zhou et al. 2010). In this study, S39(t) was detected on the end of the short arm of chromosome 12 and comparative mapping indicated that the region of S39(t) was close to S25(t), which is also a pollen killer in O. sativa intersubspecies hybrid and is derived from indica IR24 (Kubo et al. 2001) and an interspecific sterile gene S36 was also reported on the end of the short arm of chromosome 12 between O. sativa ssp. japonica (Taichung 65) and O. nivara (IRGC105444). Comparison of map positions of S36 and S25 suggested that these two loci might be the same locus (Win et al. 2009). These examples of co-linearity among different sterility loci implied that orthologous loci of hybrid sterility might control the reproduction barrier among AA genome species of genus Oryza. Further identification and exploration of more hybrid sterile genes from other species of genus Oryza will contribute significantly to our understanding of the mechanism of speciation and identify methods of manipulating hybrid sterility between O. sativa and its relatives on the AA genome.
Overcoming hybrid sterility for interspecific hybrid between O. sativa and O. glaberrima
One purpose of this study was to overcome hybrid sterility in the process of interspecific hybridization breeding. Overcoming hybrid sterility in early generations will be propitious to introgress more and wider genetic variation. Raising bridge parents of O. sativa with hybrid sterility genes from O. glaberrima are a convenient tool to surmount or allay hybrid sterility (Tao et al. 2003, Xu et al. 2005). In our previous research, bridge parents harboring S1-g were applied in a breeding program, showing significantly increasing pollen fertility in BC1F1 progenies (Deng et al. 2010); however, it is still necessary to identify more sterile loci to elucidate their panoramic influence and mutual interaction for controlling sterility. To achieve this, NILs carrying different hybrid sterile locus alleles were considered as a favorable approach. In this research process, a series of NILs (BC7) carrying different hybrid sterility loci S1, S19, S20, S21, S29(t), S37(t), S38(t) and S39(t) in a Dianjingyou 1 background were raised, respectively, and these materials will be of benefit to reveal more genetic mechanisms of hybrid sterility.
Acknowledgments
This research was funded partially by grants from the Ministry of Science and Technology (2006CB708207), Ministry of Agriculture (2009ZX08009-107B) and Yunnan Department of Science and Technology (2002C0009Z, 2003RC02, 2004PY01-21, 2006GP09), the People’s Republic of China.
Literature Cited
- Attere, A. and Fatokun, C. (1983) Reaction of Oryza glaberrima accessions to rice yellow mottle virus. Plant Dis. 67: 420–421 [Google Scholar]
- Bateson, W. (1909) Heredity and variation in modern lights. In: Seward, A.C. (ed.) Darwin and Modern Science, Cambridge University Press, Cambridge, pp. 81–101 [Google Scholar]
- Bernardo, R. (2004) What proportion of declared QTL in plants are false? Theor. Appl. Genet. 109: 419–424 [DOI] [PubMed] [Google Scholar]
- Chen, J.J., Ding, J.H., Ouyang, Y.D., Du, H.Y., Yang, J.Y., Cheng, K., Zhao, J., Qiu, S.Q., Zhang, X.L., Yao, J.L.et al. (2008) A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica–japonica hybrids in rice. Proc. Natl. Acad. Sci. USA 105: 11436–11441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z.W., Hu, F.Y., Xu, P., Li, J., Deng, X.N., Zhou, J.W., Li, F., Chen, S.N. and Tao, D.Y. (2009) QTL analysis for hybrid sterility and plant height in interspecific populations derived from a wild rice relative, Oryza longistaminata. Breed. Sci. 59: 441–445 [Google Scholar]
- Deng, X.N., Zhou, J.W., Xu, P., Li, J., Hu, F.Y. and Tao, D.Y. (2010) The role of S1-g allele from Oryza glaberrima in improving interspecific hybrid sterility between O. sativa and O. glaberrima. Breed. Sci. 60: 342–346 [Google Scholar]
- Dobzhansky, T. (1936) Studies on hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics 21: 113–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, K., Yoshimura, A. and Iwata, N. (1998) RFLP mapping and QTL analysis of heading date and pollen sterility using backcross population between Oryza sativa L. and Oryza glaberrima Steud. Breed. Sci. 48: 395–399 [Google Scholar]
- Doi, K., Taguchi, K. and Yoshimura, A. (1999) RFLP mapping of S20 and S21 for F1 pollen semi-sterility found in backcross progeny of Oryza sativa and O. glaberrima. Rice Genet. Newsl. 16: 65–68 [Google Scholar]
- Garavito, A., Guyot, R., Lozano, J., Gavory, F., Samain, S., Panaud, O., Tohme, J., Ghesquière, A. and Lorieux, M. (2010) A genetic model for the female sterility barrier between Asian and African cultivated rice species. Genetics 185: 1425–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghesquiere, A., Sequier, J., Second, G. and Lorieux, M. (1997) First steps towards a rational use of African rice, Oryza glaberrima, in rice breeding through a ‘contig line’ concept. Euphytica 96: 31–39 [Google Scholar]
- Heuer, S. and Miezan, K.M. (2003) Assessing hybrid sterility in Oryza glaberrima × O. sativa hybrid progenies by PCR marker analysis and crossing with wide compatibility varieties. Theor. Appl. Genet. 107: 902–909 [DOI] [PubMed] [Google Scholar]
- Hu, F.Y., Xu, P., Deng, X.N., Zhou, J.W., Li, J. and Tao, D.Y. (2006) Molecular mapping of a pollen killer gene S29(t) in Oryza glaberrima and co-linear analysis with S22 in O. glumaepatula. Euphytica 151: 273–278 [Google Scholar]
- Ikehashi, H. and Araki, H. (1986) Genetics of F1 sterility in remote crosses of rice. In: International Rice Research Institute-IRRI, Rice Genetics, IRRI, Manila, pp. 119–130 [Google Scholar]
- Jones, M.P., Mande, S. and Aluko, K. (1997) Diversity and potential of Oryza glaberrima Steud. in upland rice breeding. Breed. Sci. 47: 396–398 [Google Scholar]
- Khush, G.S. (1989) Multiple disease and insect resistance for increase yield stability in rice. In: International Rice Research Institute-IRRI, Progress in rice research, IRRI, Manila, pp. 79–92 [Google Scholar]
- Kubo, T., Eguchi, M. and Yoshimura, A. (2001) A new gene for F1 pollen sterility located on chromosome 12 in japonica/indica cross of rice. Rice Genet. Newsl. 18: 54–55 [Google Scholar]
- Lander, E.S., Green, P., Abrahamson, J., Barlow, A., Daly, M.J., Lincoln, S.E. and Newburg, L. (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1: 174–181 [DOI] [PubMed] [Google Scholar]
- Li, J., Xu, P., Deng, X.N., Zhou, J.W., Hu, F.Y., Wan, J.M. and Tao, D.Y. (2008) Identification of four genes for stable hybrid sterility and an epistatic QTL from a cross between Oryza sativa and Oryza glaberrima. Euphytica 164: 699–708 [Google Scholar]
- Li, J., Xu, P., Zhou, J.W., Hu, F.Y., Deng, X.N., Chen, Z.W. and Tao, D.Y. (2011) Molecular mapping of sterility QTLs qSS-3, qSS-6a and qSS-7 as single Mendelian factors via NIL strategy. Rice Sci. 18: 110–115 [Google Scholar]
- Li, Z.B. (1980) A preliminary discussion about the classification of male sterile lines of rice in China. Acta Agron. Sin. 6: 17–26 [Google Scholar]
- Lin, S.Y. and Ikehashi, H. (1993) A gamete abortion locus detected by segregation distortion of isozyme locus Est-9 in wide crosses of rice (Oryza sativa L.). Euphytica 67: 35–40 [Google Scholar]
- Long, Y.M., Zhao, L.F., Niu, B.X., Su, J., Wu, H., Chen, Y.L., Zhang, Q.Y., Guo, J.X., Zhuang, C.X., Mei, M.T.et al. (2008) Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc. Natl. Acad. Sci. USA 105: 18871–18876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorieux, M., Ndjiondjop, M. and Ghesquiere, A. (2000) A first interspecific Oryza sativa × Oryza glaberrima microsatellite based genetic linkage map. Theor. Appl. Genet. 100: 593–601 [Google Scholar]
- Muller, H.J. (1942) Isolating mechanisms, evolution and temperature. Biological Symposium 6: 71–125 [Google Scholar]
- Oka, H.I. (1974) Analysis of genes controlling F1 sterility in rice by the use of isogenic lines. Genetics 77: 521–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olga, F.L. (2002) African rice (Oryza glaberrima): History and future potential. Proc. Natl. Acad. Sci. USA 99: 16360–16365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, H.A. and Presgraves, D.C. (2000) Speciation by postzygotic isolation: forces, genes and molecules. BioRssays 22: 1085–1094 [DOI] [PubMed] [Google Scholar]
- Reversat, G. and Destombes, D. (1995) Resistance to Heterodera sacchari in rice. Nematologica 41: 333–334 [Google Scholar]
- Sano, Y. (1983) A new gene controlling sterility in F1 hybrids of two cultivated rice species. J. Hered. 74: 435–439 [Google Scholar]
- Sano, Y. (1986) Sterility barriers between Oryza sativa and O. glaberrima. In: International Rice Research Institute-IRRI (ed.) Rice Genetics, IRRI, Manila, pp. 109–118 [Google Scholar]
- Sano, Y. (1990) The genic nature of gamete eliminator in rice. Genetics 125: 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano, Y. (1994) Gamete eliminator adjacent to the wx locus as revealed by pollen analysis in rice. J. Hered. 85: 310–312 [Google Scholar]
- Sano, Y., Chu, Y. and Oka, H.I. (1979) Genetic studies of speciation in cultivated rice, 1. Genic analysis for the F1 sterility between O. sativa L. and O. glaberrima Steud. Jpn. J. Genet. 54: 121–132 [Google Scholar]
- Sano, Y., Chu, Y. and Morishima, H. (1984) Neighbor effects between two occurring rice species, Oryza sativa and O. glaberrima. Appl. Ecol. 21: 245–254 [Google Scholar]
- Semon, M., Nielsen, R., Jones, M.P. and McCouch, S.R. (2005) The population structure of African cultivated rice Oryza glaberrima (Steud.): Evidence for elevated levels of linkage disequilibrium caused by admixture with O. sativa and ecological adaptation. Genetics 169: 1639–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrid, H., Kouam, M. and Mi, Z. (2003) Assessing hybrid sterility in Oryza glaberrima × O. sativa hybrid progenies by PCR marker analysis and crossing with wide compatibility varieties. Theor. Appl. Genet. 107: 902–909 [DOI] [PubMed] [Google Scholar]
- Silue, D. and Notteghem, J. (1991) Resistance of 99 Oryza glaberrima varieties to blast. International Rice Research Newsl. 16: 13–14 [Google Scholar]
- Taguchi, K., Doi, K. and Yoshimura, A. (1999) RFLP mapping of S19, a gene for F1 pollen semi-sterility found in backcross progeny of Oryza sativa and O. glaberrima. Rice Genet. Newsl. 16: 70–71 [Google Scholar]
- Tanksley, S.D. and McCouch, S.R. (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277: 1063–1066 [DOI] [PubMed] [Google Scholar]
- Tao, D.Y., Xu, P., Li, J., Yang, Y.Q., Zhou, J.W., Hu, F.Y. and Jones, M.P. (2003) Studies on hybrid sterility inheritance and mapping of sterile genes among near-isogenic lines derived from interspecific hybrid between cultivated rice species Oryza sativa L. and O. glaberrima Steud. Chinese J. Rice Sci. 17: 11–15 [Google Scholar]
- Voorrips, R. (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J. Hered. 93: 77–78 [DOI] [PubMed] [Google Scholar]
- Win, K.T., Kubo, T., Miyazaki, Y., Doi, K., Yamagata, Y. and Yoshimura, A. (2009) Identification of two loci causing F1 pollen sterility in inter- and intraspecific crosses of rice. Breed. Sci. 59: 411–418 [Google Scholar]
- Xu, P., Tao, D.Y., Hu, F.Y., Zhou, J.W., Li, J. and Deng, X.N. (2005) Interspecific hybridization of cultivated rice for breeding japonica rice in Yunnan Province. Chinese J. Rice Sci. 19: 41–46 [Google Scholar]
- Yamagata, Y., Yamamoto, E., Aya, K., Win, K.T., Doi, K., Sobrizal, Ito, T., Kanamori, H., Wu, J.Z., Matsumoto, T.et al. (2010) Mitochondrial gene in the nuclear genome induces reproductive barrier in rice. Proc. Natl. Acad. Sci. USA 107: 1494–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J.Y., Zhao, X.B., Cheng, K., Du, H.Y., Ouyang, Y.D., Chen, J.J., Qiu, S.Q., Huang, J.Y., Jiang, Y.H., Jiang, L.W.et al. (2012) A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science 337: 1336–1340 [DOI] [PubMed] [Google Scholar]
- Zhao, J.Y., Li, J., Xu, P., Zhou, J.W., Hu, F.Y., Deng, X.N., Deng, W. and Tao, D.Y. (2012) A new gene controlling hybrid sterility between Oryza sativa and Oryza longistaminata. Euphytica 187: 339–344 [Google Scholar]
- Zhou, J.W., Xu, P., Deng, X.N., Li, J., Hu, F.Y., Ren, G.Y., Zhang, Z., Luan, Y.H., Deng, W., Zhao, Z.G.et al. (2010) Genetic dissection of a chromosomal region conferring hybrid sterility using multi-donors from Oryza glaberrima. Euphytica 175: 395–407 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.