Abstract
Brassica napus is a leading oilseed crop throughout many parts of the world. It is well adapted to long day photoperiods, however, it does not adapt well to short day subtropical regions. Short duration B. napus plants were resynthesized through ovary culture from interspecific crosses in which B. rapa cultivars were reciprocally crossed with B. oleracea. From five different combinations, 17 hybrid plants were obtained in both directions. By self-pollinating the F1 hybrids or introgressing them with cultivated B. napus, resynthesized (RS) F3 and semi-resynthesized (SRS) F2 generations were produced, respectively. In field trial in Bangladesh, the RS B. napus plants demonstrated variation in days to first flowering ranging from 29 to 73 days; some of which were similar to cultivated short duration B. napus, but not cultivated short duration B. rapa. The RS and SRS B. napus lines produced 2–4.6 and 1.6–3.7 times higher yields, respectively, as compared to cultivated short duration B. napus. Our developed RS lines may be useful for rapeseed breeding not only for subtropical regions, but also for areas such as Canada and Europe where spring rapeseed production can suffer from late spring frosts. Yield and earliness in RS lines are discussed.
Keywords: Brassica napus, interspecific hybridization, ovary culture, resynthesis, semi-resynthesis, short duration
Introduction
Rapeseed, Brassica napus, is an important oleiferous crop. The major producers of rapeseed are Canada, China, India and EU countries. Amphidiploid B. napus (AACC, 2n = 38) resulted from spontaneous hybridization between its diploid progenitors, B. rapa (AA, 2n = 20) and B. oleracea (CC, 2n = 18) (U 1935). In South Asian countries, oils from mustard seed (B. rapa, B. juncea and B. carinata) are important sources of vegetable fat. In Bangladesh, B. rapa is the main crop producing species of Brassica, but its yield is the lowest in the world (FAOSTAT 2013). Low yields are the result of a short growth period that is characteristic in the mustard-rice cropping pattern, as well as insufficient agricultural practices, and use of low yield cultivars. In Bangladesh, mustard (B. rapa) is well suited as a catch crop in cropping patterns with rice var. Aman (autumn)–Mustard–rice var. Boro (winter). The growth period of mustard must be shorter to include Boro rice in the cropping pattern. At present, the leading genotype, Tori-7 (B. rapa), is preferred by mustard growers, yet it suffers low yield due to the reasons mentioned above.
Rapeseed (B. napus) produces a higher yield than B. rapa because of a higher photosynthetic rate compared to the two parental species and vigorous growth owing to fix heterosis effect (Tsunoda 1980). Introduction of spring type B. napus to the subtropical region is a conceivable choice. However, ordinary spring type B. napus cultivars, e.g., Swedish cultivar ‘Olga’, do not flower or need an extremely long time to flower in subtropical regions because of their strict requirement for long days prior to flowering. This cannot be satisfied in Bangladesh, where day length is 11 h–12 h (Akbar 1987, Zaman 1989). In order to produce short duration B. napus cultivars, two different approaches have been undertaken at the Oilseed Research Centre, Bangladesh Agricultural Research Institute (BARI). One of those approaches is resynthesis of B. napus. Interspecific crossing between early flowering variants of B. rapa and B. oleracea has proven to be a source of earliness, in short duration B. napus genotypes that were successfully resynthesized (Akbar 1987, 1989). The other approach involves introgression of earliness from B. rapa and B. oleracea into B. napus (Zaman 1989). As a result, introgression of earliness from both A and C genomes was highly effective. Finally, BARI sarisha-7 (BARIS-7) and BARI sarisha-8 (BARIS-8) were developed through resynthesis and introgression programs respectively (Akbar et al. 2009). Short duration rapeseed cultivars are also important in northern rapeseed growing areas in Sweden and Canada where early frost can damage rapeseed crops; short duration turnip rape (B. rapa) is the primary crop grown in these production areas (Falk 2009, Rahman et al. 2011).
There are few studies reporting resynthesis of short duration RS B. napus despite the agricultural importance of short duration B. napus. Although BARIS-7 and BARIS-8 were selected for earliness in BARI, their degree of earliness was not equal to the extreme earliness seen in existing B. rapa cultivars such as Tori-7. One of the reasons for this is the limited number of resources for resynthesis of B. napus (Akbar 1987, 1989). Therefore, in the present study we sought to improve yield as well as earliness in B. napus adapted to short day length conditions. To do this, we attempted to resynthesize B. napus by crossing short duration B. rapa with newer cross parents such as rapid cycling B. oleracea and yellow seeded B. rapa.
Materials and Methods
Plant materials
Parental materials were Tori-7 (toria) and BARI Sarisha 14 (yellow sarson), two short duration B. rapa cultivars, a Chinese genotype of B. oleracea var. alboglabra (B. alboglabra) and a rapid cycling line CrGC3-1 of B. oleracea. Tori-7 and BARIS-14 were provided by the BARI Seed Bank, Bangladesh. CrGC3-1 was obtained from the Crucifer Genetic Cooperative (CrGC), University of Wisconsin, Madison, WI, USA. Cultivated B. napus, BARIS-7 and BARIS-8 were collected from BARI. Rapid cycling B. napus CrGC 5-1 was collected from CrGC. Kirariboshi is a winter type B. napus which was obtained from the National Agriculture and Food Research Organization/NARO Tohoku Agricultural Research Center, Japan. Tori-7 was partially self-compatible and the remaining A and C genome parents were self-compatible.
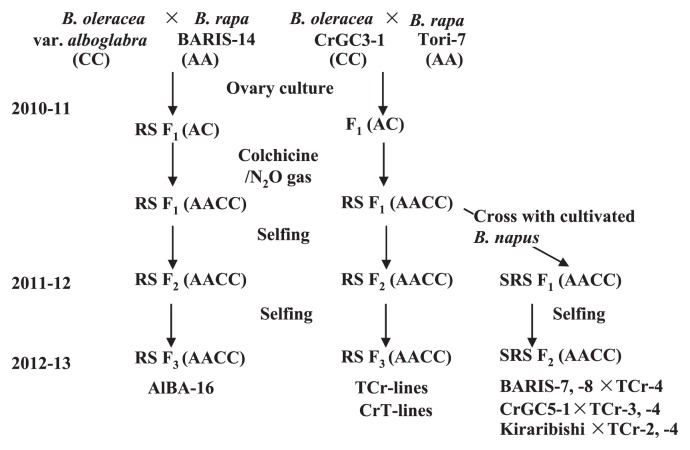
The RS F1 plants were self-pollinated to produce subsequent progenies, F2 and F3. At the same time, RS F1 plants were crossed with cultivated B. napus to obtain progeny SRS F1. The SRS F1 plants were then self-pollinated to obtain their corresponding F2 Progeny (Fig. 1).
Fig. 1.
Schematic diagram showing the development process of short-duration resynthesized (RS) and semi-resynthesized (SRS) B. napus (AACC).
Crossing, embryo rescue and chromosome doubling
Seeds were sown in 42 cell plastic trays (cell size: 3.5 × 3 cm) using vegetable soil (Yasaibaido No. 1, Honen Agri Co. Ltd., Japan). 10-day old seedlings were then transferred to plastic pots (12 cm). Seedlings and plants were grown in a greenhouse at Niigata University from 2010 to 2012. Crossing work was carried out in three seasons (except summer) under greenhouse conditions where the lowest temperature was approximately 15°C. Emasculation was conducted on floral buds one day prior to flowering. The emasculated buds were immediately dusted with fresh pollen grains collected from male parents. Pollinated flowers were isolated in thin paper bags. Ovaries bearing ovules were collected 16–20 d after pollination (DAP) for F1 embryo rescue.
Ovary culture was carried out according to the method reported by Inomata (1977). The harvested ovaries were surface-sterilized with a 70% ethanol for 3 min and subsequently treated with calcium hypochlorite solution containing approximately 1% chlorine for 15 min and rinsed twice with double sterile water. The ovaries were placed on MS (Murashige and Skoog 1962) medium aseptically supplied with 5% sucrose and 0.8% agar, adjusted to pH 5.8. Plastic petri dishes 90 × 15 mm were used for the cultures and placed in a growth chamber maintained at 24°C with a 12 h photoperiod. Ovaries were kept on the medium for 2–6 months until embryos were fully germinated and rooted. The seedlings were transplanted into six-inch pots containing vermiculite soil : regular soil (1 : 1) and covered with transparent polyethylene sheet. The plants were then placed in a growth chamber maintained at 24°C with a 12 h photoperiod. At two weeks, after proper hardening, the plants were transferred to a greenhouse. In order to restore seed fertility in the F1 hybrids, colchicine solution 0.05% was applied on leaf axils of each hybrid to double the chromosome number as per previous report (Chen et al. 1988). As an alternative method for chromosome doubling, nitrous oxide gas treatment for 30–48 h at 6 atm was also applied to F1 hybrids which were undergoing meiosis (Nukui et al. 2011). For chromosome observation, root tips from the RS F2 young seedlings were fixed in acetic alcohol (1 : 3) for 2 h at room temperature. The tips were hydrolyzed in 1N HCl for 30 sec at 60°C and stained with 1% acetocarmine solution, then squashed in 45% acetic acid. For measurement of pollen fertility, pollen grains of F2 plants and their parents were stained with 1% acetocarmine solution and fully stained pollen grains were scored as fertile; unstained pollen grains were scored as sterile.
Different trait evaluation
The open-field trial was laid out in a completely randomized block design with three replications (plots), from November 1, 2012 to March 30, 2013 at BARI, Gazipur, Bangladesh. Unit plot size for each experiment was 10 m × 2.5 m. The distances between plant-plant, line-line and plot-plot were 10, 40 and 100 cm, respectively. The recommended agricultural practices and pest control measures were applied when necessary for normal growth of the plants. Data were recorded for 18 traits related to flowering, morphology, yield components and oil content. Days to 1st flowering were recorded when the first plant flowered in each plot; 50% and 100% flowering dates were recorded when 50% of plants and 100% of plants in each plot flowered, respectively. Days to maturity were recorded at the 70–80% siliqua ripening stage. 10–50 plants per line in each plot were grown for phenotypic evaluation. Data for morphology and yield components were taken from 10 randomly selected RS F3 and SRS F2 plants per plot along with parents. For quantification of oil content, oil was extracted from rapeseeds with petroleum-ether by the soxhlet method. Identification and quantitation of fatty acid compounds was accomplished using capillary gas chromatography with flame ionization detection (GC-FID). Basic descriptive statistics including mean, standard deviation, LSD and correlation efficiency were performed using Microsoft Excel 2003 and statistical add-in software (Excel Toukei 2010 for Windows, Social Survey Research Information Co., Ltd., Tokyo, Japan).
In the greenhouse trials, seeds were germinated in cell-pots and transferred to 12-cm-diameter or 24-cm-diameter plastic pots. For phenotypic evaluation, 5 plants per line were grown in greenhouses where the lowest temperature was 15°C in March-May, 2013 at Niigata University.
Results
Interspecific hybrid production
Ovaries were collected 16–20 DAP and cultured in MS medium. In the cultures, hybrid seedlings came directly from the ovaries. The cross set for Tori-7 (AA) × CrGC3-1 (CC) resulted in 191 ovaries out of 260 flowers pollinated for an ovary setting rate of 73.5%; 3 hybrid plants were obtained and named TCr-2, TCr-3 and TCr-4. In the reciprocal cross set of CrGC3-1 × Tori-7, 713 ovaries were obtained from 949 pollinated flowers and 13 hybrid plants were finally obtained and named CrT-1, CrT-5 to -15 and CrT-17. In the reciprocal crosses between BARIS-14 and B. alboglabra, only a single hybrid plant (AlBA-16) regenerated in ovary culture. Thus, 17 hybrid plants were obtained from a total of 1681 pollinations including the five crosses (Table 1). After the hybrid plants reached the 2–3 leaf stage, the regenerated plants were transferred to soil.
Table 1.
Crossability of interspecific crosses between Brassica rapa and B. oleracea
| Cross combination (♀ × ♂) | Flower Pollinated (a) | Ovary seta (rate, %) | Plantlets regenerated | Hybrids (b) | Cross ability (b/a, %) |
|---|---|---|---|---|---|
| Tori-7(AA) × CrGC3-1(CC) | 260 | 191 (73.5) | 3 | 3 | 1.2 |
| CrGC3-1 (CC) × Tori-7 (AA) | 949 | 713 (75.1) | 16 | 13 | 1.4 |
| BARI Sharisha-14 (AA) × B. albogabra (CC) | 40 | 2 (5.0) | 0 | 0 | 0.0 |
| B. albogabra (CC) × BARI Sharisha-14 (AA) | 294 | 74 (25.2) | 1 | 1 | 0.3 |
| CrGC3-1 (CC) × BARI Sharisha-14 (AA) | 138 | 108 (78.2) | 0 | 0 | 0.0 |
| Total | 1681 | 1088 (64.7) | 20 | 17 | 1.0 |
All ovaries set after pollination were used for culture.
Hybridity of F1 plants was confirmed by their morphological characteristics, such as floral petal color, leaf shapes, leaf color, plant height and vigorous growth. All F1 hybrids were pollen-sterile, indicating that the plants were true F1 hybrids and not false hybrids derived from female parents as matromorphous or by mere chance as a result of incomplete emasculation. Chromosome doubling of the axillary floral buds of the F1 hybrids induced fertile flowers and by self-pollination F2 seeds were successfully produced in TCr-2, TCr-3, TCr-4 and AlBA-16, as well as in the 7 plants of the CrT-lines. Chromosome counting confirmed that the three F2 plants in the TCr-lines were 2n = 38 chromosome species. The pollen fertilities of the confirmed F3 plants derived through resynthesis were found to be 81.1–97.4% which demonstrated similarity to the natural B. napus pollen fertility of 87.2–98.2% (Table 2). Nevertheless, seeds per pod in some of the F3 lines were few, especially in the CrT-lines. Due to low seed fertility, the CrT-lines, with the exception of CrT-14, were omitted in the field trial.
Table 2.
Characteristics of resynthesized (RS B. napus) F3 and semi-resynthesized (SRS B. napus) F2 along with their parents grown in the short-day climate of Bangladesh (Mean and ±SE)
| Parental species and resynthesized B. napus | Pollen viability (%) | Plant height (cm) | Pod/plant | Seed/pod | 1000 seeds (g) | Oil (%) | Erucic acid (%) |
|---|---|---|---|---|---|---|---|
| RS B. napus F3 | |||||||
| TCr-2a | 97.4 ± 1.2 | 139 ± 3.8 | 526 ± 75.6 | 11.9 ± 0.2 | 4.5 ± 0.1 | 42.1 | 40.4 |
| TCr-3 | 92.2 ± 4.8 | 104 ± 3.7 | 536 ± 109.0 | 13.0 ± 0.6 | 4.4 ± 0.1 | 41.8 | 43.6 |
| TCr-4 | 91.1 ± 1.7 | 121 ± 3.4 | 324 ± 93.0 | 11.8 ± 1.1 | 4.5 ± 0.2 | 42.0 | 47.8 |
| CrT-14 | 86.0 ± 1.5 | 104 ± 22.7 | 447 ± 203.1 | 9.9 ± 1.8 | 4.7 ± 0.0 | – | 43.4 |
| AlBA-16 | 81.1 ± 2.2 | 119 ± 8.9 | 611 ± 204.9 | 24.3 ± 1.5 | 3.9 ± 0.0 | 42.5 | 40.5 |
| SRS B. napus F2 | |||||||
| BARIS-7 × TCr-4 | 92.8 ± 2.1 | 94 ± 2.2 | 232 ± 26.1 | 18.0 ± 0.5 | 4.5 ± 0.1 | 42.4 | 47.9 |
| BARIS-8 × TCr-4 | 95.1 ± 1.8 | 135 ± 7.9 | 443 ± 176.1 | 21.0 ± 1.5 | 3.6 ± 0.3 | 41.7 | 47.0 |
| CrGC5-1 × TCr-3 | 87.0 ± 1.5 | 134 ± 4.1 | 550 ± 236.0 | 11.8 ± 1.9 | 3.6 ± 0.1 | 42.5 | 34.3 |
| CrGC5-1 × TCr-4 | 92.9 ± 1.5 | 144 ± 3.6 | 532 ± 121.1 | 12.9 ± 1.5 | 3.6 ± 0.2 | 42.9 | – |
| Kirariboshi × TCr-2 | 93.6 ± 2.5 | 147 ± 6.0 | 501 ± 84.7 | 12.5 ± 1.0 | 2.9 ± 0.1 | 42.4 | – |
| Kirariboshi × TCr-4 | 97.9 ± 1.2 | 134 ± 7.0 | 344 ± 33.0 | 13.8 ± 0.1 | 3.1 ± 0.2 | 42.8 | 27.2 |
| Parental species | |||||||
| Tori-7 (AA) | 96.1 ± 0.0 | 69 ± 1.9 | 201 ± 24.3 | 15.3 ± 0.3 | 2.2 ± 0.0 | 40.6 | 43.2 |
| BARIS-14 (AA) | 91.3 ± 1.4 | 73 ± 1.2 | 75 ± 2.0 | 26.0 ± 0.6 | 2.7 ± 0.0 | 42.8 | 52.8 |
| CrGC3-1 (CC) | 92.2 ± 1.5 | 55 ± 2.8 | 164 ± 8.3 | 8.9 ± 0.5 | 4.4 ± 0.1 | 41.3 | 53.3 |
| B. alboglabra (CC) | 99.0 ± 0.6 | 139 ± 3.2 | 267 ± 48.9 | 13.4 ± 4.9 | 4.3 ± 0.0 | 41.4 | 46.6 |
| CrGC5-1 (AACC) | 87.2 ± 0.3 | 139 ± 3.8 | 343 ± 59.3 | 17.0 ± 0.8 | 2.9 ± 0.1 | 42.3 | 33.6 |
| BARIS-7 (AACC) | 98.2 ± 0.5 | 88 ± 1.4 | 113 ± 11.3 | 21.1 ± 0.6 | 3.7 ± 0.1 | 41.6 | 40.2 |
| BARIS-8 (AACC) | 91.4 ± 1.2 | 93 ± 1.9 | 112 ± 2.8 | 23.3 ± 0.4 | 3.4 ± 0.2 | 40.5 | 51.2 |
| Kirariboshi (AACC) | 95.2 ± 4.8 | 96.4 ± 5.1 | 72 ± 26.7 | 5.0 ± 0.0 | 1.5 ± 0.0 | – | – |
| LSD (0.05)b | 6.1 | 19.0 | 318 | 4.3 | 0.4 | ||
TCr (Tori-7 × CrGC3-1), CrT-14 (CrGC3-1 × Tori-7), AlBA (B. alboglabra × BARIS-14), BARIS (BARI sarisha).
LSD (0.05); least significant difference at P = 0.05.
Flowering and maturity
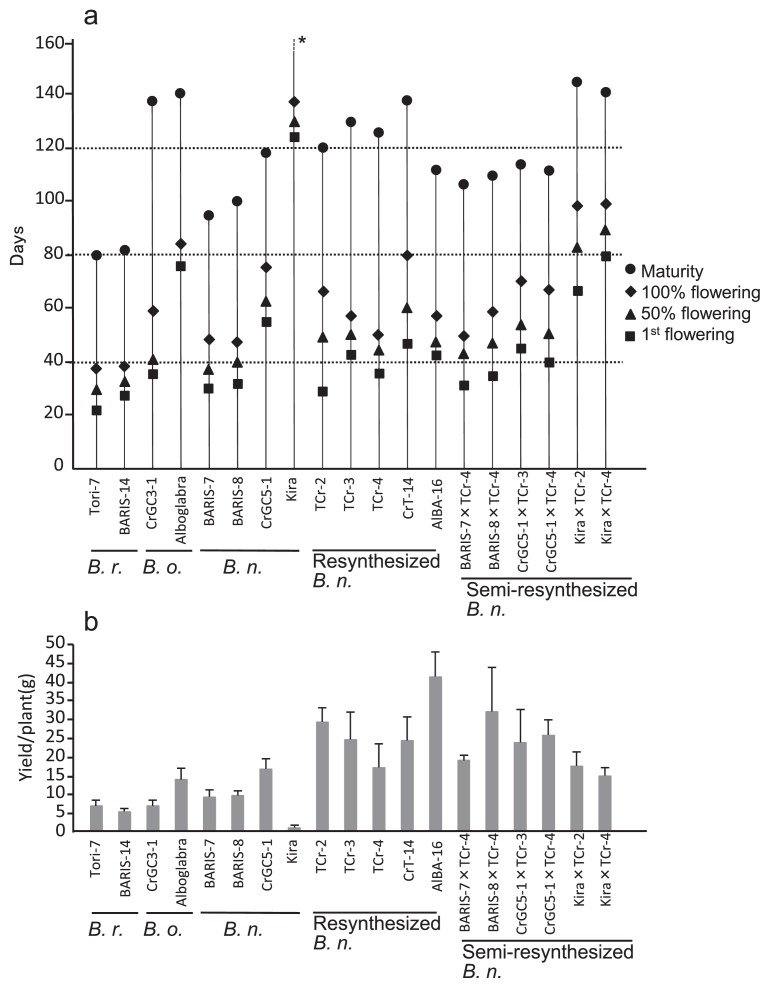
RS F3 plants, along with their parents and cultivated B. napus, were grown under short-day length (11–12 h) conditions in the cropping season in Bangladesh (Fig. 2a and Supplemental Table 1). The donors of the A genome, from Tori-7 and BARIS-14, were found to be earliest for 1st flowering at 22.3 d and 30.0 d respectively. Among with the C genome donor parents, CrGC3-1 of B. oleracea was found to be early at 37.3 d while B. alboglabra was the latest, requiring 79.5 d during the trial. Of the RS B. napus plants, TCr-2 was the earliest, requiring 29.3 d to 1st flowering. TCr-4 required 35.0 d and TCr-3 and AlBA-16 required 42.7 d and 41.3 d, respectively. The entry CrT-14 required the maximum duration of 47.5 d to 1st flowering.
Fig. 2.
Flowering, maturity and yield per plant evaluated in field trial of RS F3 and SRS F2 B. napus lines and their parents grown in short-day winter in Bangladesh. (a) days to maturity and flowering, (b) yield. TCr (Tori-7 × CrGC3-1), CrT-14 (CrGC3-1 × Tori-7), AlBA (B. alboglabra × BARIS-14), BARIS (BARI sarisha), Kira (Kirariboshi). * indicates that Kira did not mature within the experiment duration. Yield data are indicated by the mean ± SE.
A total of 6 SRS F2 lines were obtained by crossing RS B. napus with cultivated genotypes BARIS-7 (30.3 d), BARIS-8 (33.7 d), CrGC5-1 (56.0 d) and Kirariboshi (125.3 d). The combination BARIS-7 × TCr-4 was the earliest to flower at 32.0 d and was followed by BARIS-8 × TCr-4 at 36.3 d. Both of the SRS lines required a duration almost equal to that of the donor B. napus parent for time to 1st flowering. The SRS F2 generation plants that evolved from the cross of CrGC5-1 as female required 40.3 d and 44.0 d; both being much earlier than the said female parent. During trials to determine days to 1st flowering, observations of early, medium early and late parental types used and their derived RS as well as SRS types obtained revealed a correspondence for 50% and 100% flowering time accordingly (Fig. 2a and Supplemental Table 1). When Kirariboshi was crossed with TCr-2 and TCr-4, the F3 lines required a longer duration for flowering than other F3 lines because Kirariboshi belongs to the winter type B. napus which requires exposure to lower temperatures prior to flower initiation. This result indicates that the warm winter in Bangladesh inhibited induction of flowering in the winter type B. napus, Kirariboshi.
Parental lines showed extensive variation in days to maturity—80–82 d in B. rapa, 95–99 d in BARIS-7 and -8, 137–143 d in B. oleracea and 159 d in B. napus Kirariboshi, indicating that B. rapa Tori-7 and BARIS-14 could be characterized as very early, whereas CrGC5-1 and B. alboglabra required a long time for ripening. Days to maturity in RS B. napus ranged from 112–138 d, and the time for SRS B. napus was shorter by comparison. In comparison with its cultivars, SRS B. napus had a delayed maturity of 9 d later than cultivated B. napus (BARIS-7, BARIS-8) and 25 d later than cultivated B. rapa (Toti-7 and BARI-14). Greenhouse trials for RS F3 and SRS F2 were also conducted in Japan. The flowering response in RS F3 lines grown during winter in Japan ranged from 35–42 d (Supplemental Fig. 1). This confirmed the earliness of the RS F3 lines under day length (12 h) conditions in Japan.
Yield
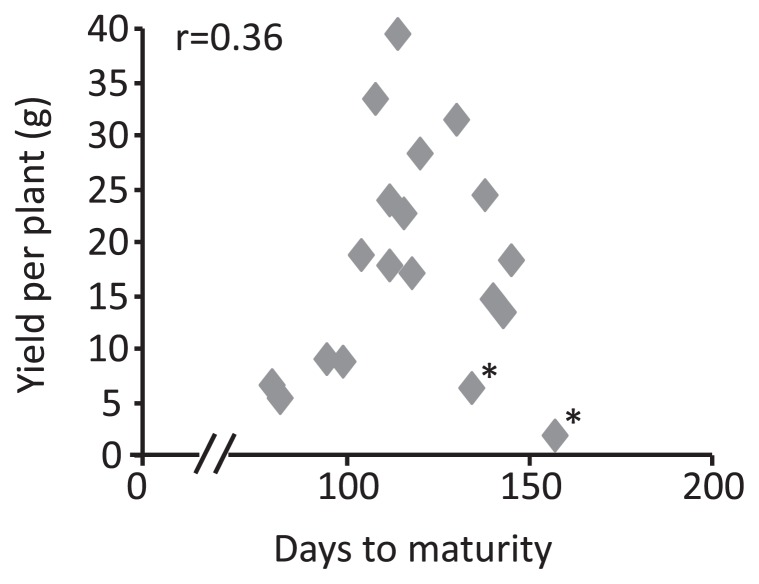
In B. rapa, seed yield per plant was 6.7 g and 5.3 g for Tori-7 and BARIS-14, respectively (Fig. 2b and Supplemental Table 1). In B. oleracea, CrGC3-1 and B. alboglabra produced 6.4 g and 13.5 g seed yield per plant, respectively. B. napus, BARIS-7 and BARIS-8, produced higher yields of 9.0 g and 8.9 g. The RS B. napus lines, with the exception of AlBA-16, had a lower number of seeds per siliqua, ranging from 9.9–13.0 (Supplemental Tables 1, 2). Nevertheless, the yield per plant was higher, due to an increased number of pods per plant. AlBA-16 showed the highest yield of 41.4 g due to an increase in seeds per pod (24.3) as well as increased pods per plant (611). The relatively higher yield was also achieved in remaining RS B. napus lines, TCr-2 (28.3), TCr-3 (31.4), TCr-4 (17.8) and CrT-14 (24.5), indicating that the four RS lines, with the exception of TCr-4, showed a 97–363% significantly higher yield compared to the mean yield of 9.0 g per plant seen in the check cultivars (BARIS-7 and BARIS-8). In SRS B. napus, the F2 lines of BARIS-8 × TCr-4 showed the highest yield at 33.4 g, followed by CrGC5-1 × TCr-4 (23.8 g); the lowest yield recorded was seen in the F3 lines of Kirariboshi × TCr-4 (14.7 g). SRS B. napus showed a 64–273% higher yield in comparison to the mean yield of the check cultivars. The late ripening RS lines tended to produce higher yields than the early ripening lines and there was a weak association between days to maturity and yield in the parents and the tested lines, although the correlation coefficient was not significant (r = 0.36) (Fig. 3). The two exceptional cultivars of CrGC 3-1 and Kirariboshi were omitted because they had low seed fertilities of 8.9 and 5.0 seeds/pod, respectively. Such low fertility was likely the result of undetermined environmental factors such as immaturity due to late flowering or genetically controlled sterility.
Fig. 3.
Relationship between yield per plant and days to maturity in RS, SRS B. napus and their parents. The correlation coefficient was calculated by omitting the exceptional data of two cultivars (*).
The oil content in seeds was approximately 40% in all tested parental and hybrid lines, which makes them sufficient for use in oil production (Table 2). The seeds contained a remarkable 27.2–53.3% erucic acid; other important characteristics are presented in Supplemental Table 3.
Discussion
Ovary culture
Embryo, ovule and ovary culture are used as major embryo rescue techniques in interspecific hybridization of Brassica species where hybrid embryos cease their growth due to lack of appropriate endosperm (Chen et al. 1988, Inomata 1993, Nishi et al. 1959, Olsson 1960, Takeshita et al. 1980). Previous studies reported that ovary culture was useless in crosses of B. oleracea (♀) × B. rapa (♂), but effective in the reciprocal cross (Hossain et al. 1989, Inomata 1977, 1978, Takeshita et al. 1980). However, Song et al. (1993) successfully resynthesized B. napus in both crossing directions by using B. oleracea CRGC 3-1 as a parent. In this study, we also confirmed that CrGC3-1 had higher crossability with B. rapa.
Earliness
Short duration rapeseed cultivars must possess not only no requirement for vernalization, but also short day adaptability. The trait requiring no vernalization could be easily incorporated in short duration rapeseed because selection for that characteristic is simple; i.e. evaluating flowering time in the spring to summer cropping; many spring cultivated rapeseed genotypes are also available as parents. In transferring short-day adaptability via introgession, Rahman et al. (2011) and Zaman (1989) demonstrated that the C genome of B. alboglabra carries early flowering allele(s) despite the fact that B. alboglabra is quite late flowering in comparison to B. rapa and B. napus. In this study also, B. alboglabra was late (79.5 d) to 1st flowering compared to the B. rapa parent BARIS-14 (30.0 d). However, the hybrid plant, AlBA-16, resulting from the cross of B. alboglabra × BARIS-14, flowered early (41.3 d), indicating that its late flowering nature was recessively inherited in AlBA-16 and earliness seen in AlBA-16 must have come from BARIS-14. Using RS B. napus in this study, we could not determine to what extent the C genome of B. alboglabra contributes to earliness in RS B. napus, however, this study could demonstrate that the C genome of B. alboglabra did not have any negative effect on earliness.
Akbar (1989) produced RS B. napus by crossing B. rapa Tori-7 (toria), Kalyania (brown sarson) and Sonali sarisha (yellow sarson) with B. alboglabra and cauliflower cultivars as B. oleracea parents. He carried out the field trial for earliness evaluation in Bangladesh and demonstrated early flowering in his developed RS lines, indicating that the parents used were able to transmit sufficient short day adaptability to their descendants. In the present study, yellow seeded B. rapa BARIS-14 and rapid cycling B. oleracea CrGC3-1 were used as parents. These two variants have never been used as parents to produce RS B. napus in previous studies. In the winter of 2012–2013 in Bangladesh, the days to 1st flowering (37.3 d) for CrGC3-1 was much earlier than that for B. alboglabra (79.5 d) and days to 1st flowering of the resultant hybrid plants (TCr-2, 3, 4. CrT-14) ranged from 29.3–47.5 days. Although there was a big difference in the flowering time of B. alboglabra and CrGC3-1, the hybrids derived from the crosses using CrGC3-1 had limited achievement of earliness. As a result, compared to cultivated BARIS-7, our RS and SRS lines showed a similar level of early flowering, but late maturity due to the extended period between flowering and maturity. The late maturity had a negative effect for earliness but contributed to the high yield by increasing yield components such as pods per plant and weight per 1000 seeds. BARIS-7, which was selected over seven years from the initial RS lines produced by Akbar (1989), showed earliness, but low yield, whereas our developed lines achieved moderate earliness and high yield. This difference may be due to differences in the varieties of parents used in each study. Akbar (1987) found that some of the reselected successive F3 lines exhibited transgressive earliness in comparison to F2 lines; this was consistent with our results (data not shown). Similarly, Rahman et al. (2011) reported that days to flowering progressively decreased with repeated selection for earliness across self-pollinated generations (F2 to F6). Successive selection in the next generation could reduce the number of days to maturity and ensure optimal high yield adaptability to existing ecological systems where the crop is produced.
Yield
Previous studies reported that artificial rutabaga hybrids derived from turnip × kohlrabi (Namai et al. 1980) were superior as a fodder crop due to their vigorous growth. On the other hand, artificial rapeseeds have been considered only as raw materials for further breeding because of low yields for RS rapeseeds (Kräling 1987, Olsson 1960, Seyis et al. 2010); one reason being the low fertility of RS B. napus, due to meiotic irregularities in genome stability in RS B. napus (Namai et al. 1980, Szadkowski et al. 2010). In this study, pollen fertility in F3 plants was relatively high (81–97%), yet seed fertility (seeds/pod) was low, especially for CrT-lines produced by using CrGC3-1 as the female parent. In order to eliminate meiotic irregularities in the RS lines, selection of lines showing stable seed fertility, as well as crossing RS lines with ordinary B. napus cultivars were found to be very effective (Namai et al. 1980). We therefore selected TCr-lines and AlBA-16 which had high seed fertility among their developed lines and crossed our RS lines with cultivated B. napus. Even so, several of the selected RS and SRS lines still had a low number of seeds/pod (Table 2), which is consistent with the results of Zhao et al. (2009) who reported that SRS lines derived from a cross between RS lines and cultivated B. napus had fewer seeds per pod, but many more pods per plant in comparison to rapeseed cultivars. This suggests that the seeds-per-pod trait is a key component for improving yield in rapeseed breeding using RS B. napus. In our developed RS lines, the drawback concerning fewer seeds per pod was counterbalanced by the setting of an increased number of pods per plant, resulting in a high yield. As a result, our developed RS lines revealed significant heterosis on yield, at least in comparison to Bangladeshi rapeseed cultivars, thus confirming the usefulness of our RS lines. This is in contrast to results showing that although RS lines have significant potential in heterosis as parents for production of F1 hybrid cultivars, raw amphidiploids derived from interspecific hybridization display low yield (Gehringer et al. 2007, Jesske et al. 2013, Seyis et al. 2006, Zou et al. 2010).
It is reported that correlation between flowering time and yield was discovered in genetic analyses using the segregating population derived from spring type and winter type rapeseed cultivars and both negative and positive correlations were observed depending on population and cropping pattern (Butruille et al. 1999, Udall et al. 2006). For example, in a segregating population, early flowering plants normally set pods in moderate climate and, thereafter, late flowering plants encounter the hot summer which inhibits seed production. In such cases, flowering time and yield have been negatively correlated. In this study, the yield for the developed short duration B. napus, BARIS-7 and -8 was low, while late ripening RS lines tended to produce a higher yield than early ripening lines (Fig. 3). Although further studies are required to confirm this result, the positive relationship between high yields and late maturity was often found in spring rapeseeds (Butruille et al. 1999, Starmer et al. 1998) and winter rapeseeds (Habekotté 1997), thus benefitting from a longer growth period. If yield and days to maturity could be positively correlated, it would be difficult to combine short duration with high yield in rapeseed breeding; the successive selection for earliness in RS B. napus populations might sacrifice yield. Overcoming negative correlation between earliness and yield might be necessary to obtain high yield short duration B. napus.
At present, none of the B. napus cultivars performed in short duration and yield at a satisfactory level to replace B. rapa Tori-7. The artificial B. napus plants we developed achieved moderate earliness and higher yield in comparison with Bangladeshi rapeseed cultivars, BARIS-7 and -8. Our RS lines could potentially be used for short duration B. napus breeding not only for cultivars suited to subtropical regions like Bangladesh, but also for use in production areas suffering from late season frosts such as Canada and Northern Europe. In that regard, the successive selection with appropriate balance of the two important traits (earliness and yield) will be required in subsequent generations.
Acknowledgements
We thank Dr. M.A. Akbar, Principal Scientific Officer (Retd.), Oilseed Research Centre BARI, Gazipur, Bangladesh, for his critical reading and invaluable comments. The authors sincerely thank Dr. Y. Honda at the National Agriculture and Food Research Organization/NARO Tohoku Agricultural Research Center, Japan, for kindly providing a rapeseed cv. Kirariboshi. Thanks to all members of the Plant Breeding Laboratory, Niigata University, for their kind help and assistance during these experiments. The author is also deeply indebted to Honjo International Scholarship Foundation (HISF) for a scholarship grant. Field experiments and oilseed content measurement at the Oilseed Research Centre, Bangladesh Agricultural Research Institute, is gratefully acknowledged.
Literature Cited
- Akbar, M.A. (1987) Artificial Brassica napus flowering in Bangladesh. Theor. Appl. Genet. 73: 465–468 [DOI] [PubMed] [Google Scholar]
- Akbar, M.A. (1989) Resynthesis of Brassica napus aiming for improved earliness and carried out by different approaches. Hereditas 111: 239–246 [Google Scholar]
- Akbar, M.A., Mian, M.A.K. and Ali, M.M. (2009) Genetic enhancement and crop improvement by varietal development in Brassica spp through breeding in Bangladesh. Proceedings and Development of Oilseed Crops in Bangladesh and Future Challenges. Oilseed Research Center, Bangladesh Agricultural Research Institute, Gazipur, Bangladesh [Google Scholar]
- Butruille, D.V., Guriesb, R.P. and Osborn, T.C. (1999) Increasing yield of spring oilseed rape hybrids through introgression of winter germplasm. Crop Sci. 39: 1491–1496 [Google Scholar]
- Chen, B.Y., Heneen, W.K. and Jonsson, R. (1988) Resynthesis of Brassica napus L. through interspecific hybridization between B. aloboglabra Bailey and B. campestris L. with special emphasis on seed colour. Plant Breed. 101: 52–59 [Google Scholar]
- Falk, K.C. (2009) Developing high yielding Brassica rapa cultivars with resistance to brown girdling root rot, blackleg, white rust, and rlubroot. Agriculture and Agri-Food Canada, Saskatoon: Project Code: CGDP-SCDC 11/03-01. http://www.saskcanola.com/research/breeding.php?detail=65 [Google Scholar]
- Food and Agriculture Organization of the United Nations, FAOSTAT|© FAO Statistics Division 2013|28 February 2013. http://faostat3.fao.org/home/index
- Gehringer, A., Snowdon, R., Spiller, T., Basunanda, P. and Friedt, W. (2007) New oilseed rape (Brassica napus) hybrids with high levels of heterosis for seed yield under nutrient-poor conditions. Breed. Sci. 57: 315–320 [Google Scholar]
- Habekotté, B. (1997) Options for increasing seed yield of winter oilseed rape (Brassica napus L.): a simulation study. Field Crops Res. 54: 109–126 [Google Scholar]
- Hossain, M.M., Inden, H. and Asahira, T. (1989) Interspecific hybrids between Brassica campestris L. and B. oleracea L. through embryo and ovary culture. Mem. Coll. Agric., Kyoto Univ. 135: 21– 30 [Google Scholar]
- Inomata, N. (1977) Production of interspecific hybrids between Brassica campestris and Brassica oleracea by culture in vitro of excised ovaries. I. Effects of yeast extract and casein hydrolysate on the development of excised ovaries. Jpn. J. Breed. 27: 295–304 [Google Scholar]
- Inomata, N. (1978) Production of interspecific hybrids between Brassica campestris and Brassica oleracea by culture in vitro of excised ovaries. II. Effects of coconut milk and casein hydrolysate on the development of excised ovaries. Jpn. J. Genet. 53: 1–11 [Google Scholar]
- Inomata, N. (1993) Embryo rescue techniques for wide hybridization. In: Labana, K.S., Banga, S.S. and Banga, S.K. (eds.) Breeding Oilseed Brassicas. Springer, Berlin Heidelberg New York Tokyo, pp. 94–107 [Google Scholar]
- Jesske, T., Olberg, B., Schierholt, A. and Becker, H.C. (2013) Resynthesized lines from domesticated and wild Brassica taxa and their hybrids with B. napus L.: genetic diversity and hybrid yield. Theor. Appl. Genet. 126: 1053–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräling, K. (1987) Utilization of genetic variability of resynthesized rapeseed. Plant Breed. 99: 209–217 [Google Scholar]
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15: 473– 497 [Google Scholar]
- Namai, H., Sarashima, M. and Hosoda, T. (1980) Interspecific and intergeneric hybridization breeding in Japan. In: Brassica Crops and Wild Allies, Biology and Breeding, Jpn. Sci. Soc. Press, Tokyo, pp. 191–203 [Google Scholar]
- Nishi, S., Kawata, J. and Toda, M. (1959) In the breeding of interspecific hybrids between two genomes “C” and “A” of Brassica through the application of embryo culture techniques. Jpn. J. Breed. 8: 215–222 [Google Scholar]
- Nukui, S., Kitamura, S., Hioki, T., Ootsuka, H., Miyoshi, K., Satou, T., Takatori, Y., Oomiya, T. and Okazaki, K. (2011) N2O induces mitotic polyploidization in anther somatic cells and restores fertilityin sterile interspecific hybrid lilies. Breed. Sci. 61: 327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson, G. (1960) Species cross within the genus Brassica. II. Artificial Brassica napus L. Hereditas 46: 351–386 [Google Scholar]
- Rahman, M.H., Bennett, R.A., Yang, R.C., Kebede, B. and Mohan, R.T. (2011) Exploitation of the late flowering species Brassica oleracea L. for the improvement of earliness in B. napus L.: an untraditional approach. Euphytica 177: 365–374 [Google Scholar]
- Seyis, F., Friedt, W. and Lühs, W. (2006) Yield of Brassica napus L. hybrids developed using resynthesized rapeseed material sown at different locations. Field Crops Res. 96: 176–180 [Google Scholar]
- Seyis, F., Friedt, W. and Luhs, W. (2010) Yield of Brassica napus L. hybrids developed using resynthesised rapeseed material. Anadolu J. Agric. Sci. 25: 159–167 [Google Scholar]
- Song, K., Tang, K. and Osborn, T.C. (1993) Development of synthetic Brassica amphidiploids by reciprocal hybridization and comparison to natural amphidiploids. Theor. Appl. Genet. 86: 811–821 [DOI] [PubMed] [Google Scholar]
- Starmer, K.P., Brown, J. and Davis, J.B. (1998) Heterosis in spring canola hybrids grown in northern Idaho. Crop Sci. 38: 376–380 [Google Scholar]
- Szadkowski, E.E.F., Huteau, V., Lodé, M., Huneau, C., Belcram, H., Coriton, O., Manzanares-Dauleux, M.J., Delourme, R., King, G.J., Chalhoub, B.et al. (2010) The first meiosis of resynthesized Brassica napus, a genome blender. New Phytol. 186: 102–112 [DOI] [PubMed] [Google Scholar]
- Takeshita, M., Kato, M. and Tokumasu, S. (1980) Ovule culture in the production of intergeneric or interspecific hybrids in Brassica and Raphanus. Jpn. J. Genet. 55: 373–387 [Google Scholar]
- Tsunoda, S. (1980) Eco-physiology of wild and cultivated forms in Brassica and allied genera. In: Brassica Crops Wild Allies, Jpn. Sci. Soc. Press, Tokyo, pp. 109–119 [Google Scholar]
- U. N. (1935) Genome-analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn. J. Bot. 7: 390–452 [Google Scholar]
- Udall, J.A., Quijada, P.A., Lambert, B. and Osborn, T.C. (2006) Quantitative trait analysis of seed yield and other complex traits in hybrid spring rapeseed (Brassica napus L.): 2. Identification of alleles from unadapted germplasm. Theor. Appl. Genet. 113: 597–609 [DOI] [PubMed] [Google Scholar]
- Zaman, M.W. (1989) Introgression in Brassica napus for adaptation to the growing conditions in Bangladesh. Theor. Appl. Genet. 77: 721–728 [DOI] [PubMed] [Google Scholar]
- Zhao, Y., Xiao, L. and Lu, C. (2009) Genetic analysis of yield and its components of B. napus hybrids using resynthesized rapeseed lines. Agricultural Sciences in China 8: 1286–1292 [Google Scholar]
- Zou, J., Zhu, J., Huang, S., Tian, E., Xiao, Y., Fu, D., Tu, J., Fu, T. and Meng, J. (2010) Broadening the avenue of intersubgenomic heterosis in oilseed Brassica. Theor. Appl. Genet. 120: 283–290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.