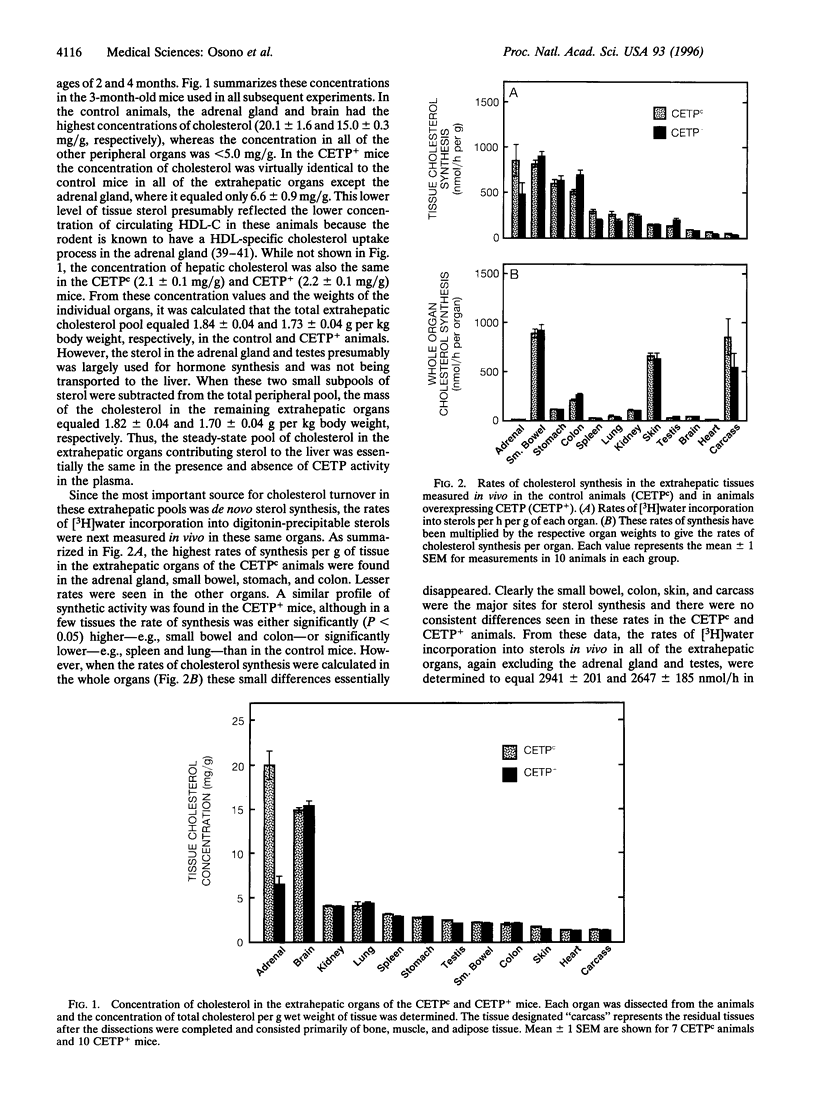
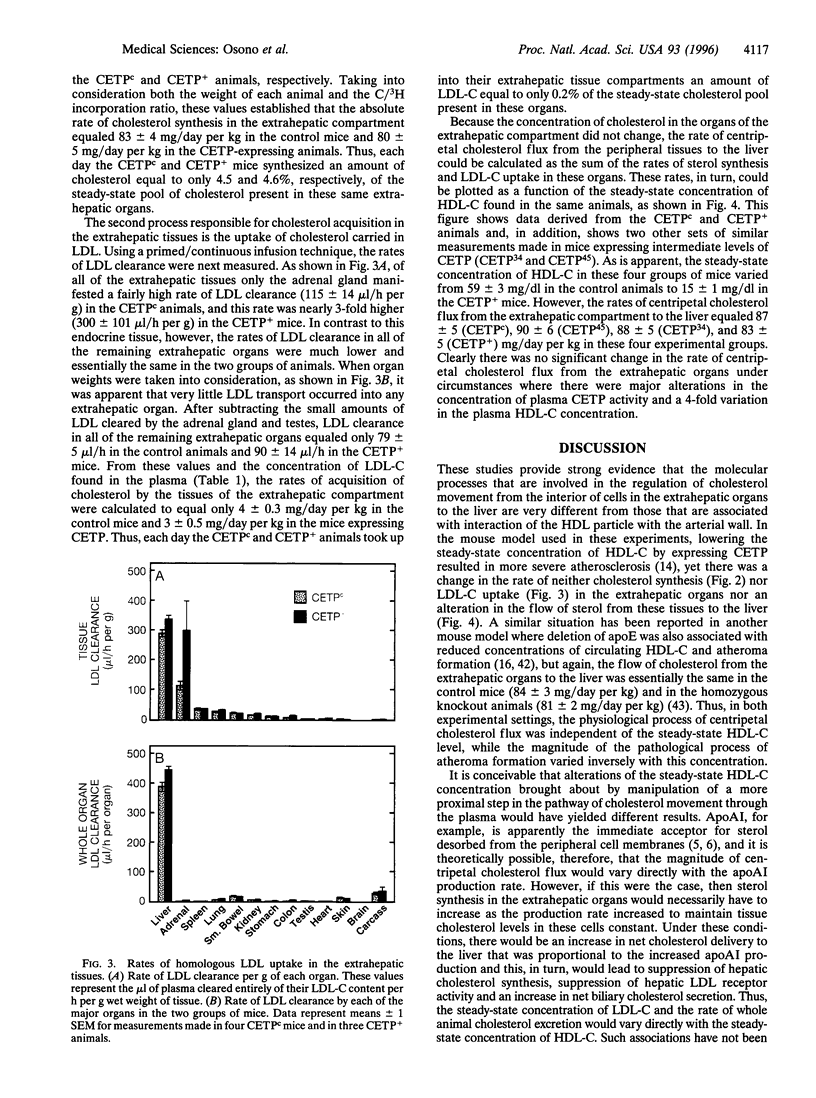
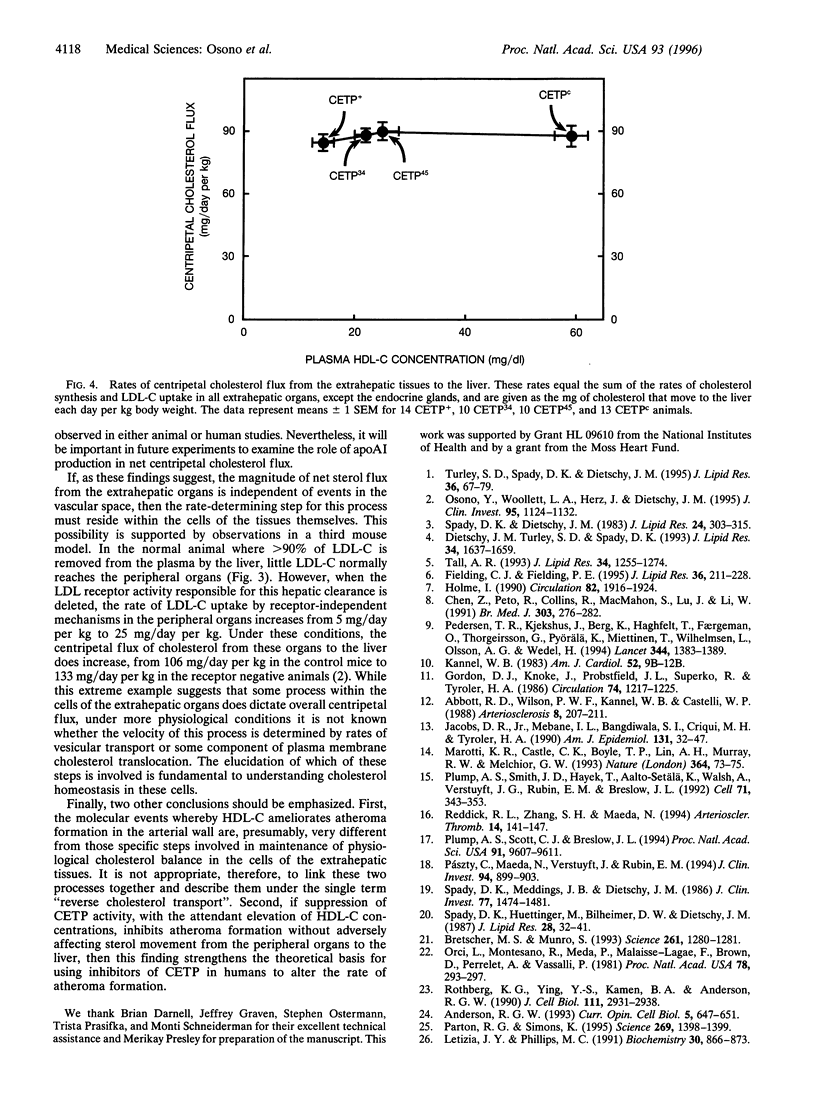
Abstract
High density lipoproteins (HDLs) play a role in two processes that include the amelioration of atheroma formation and the centripetal flow of cholesterol from the extrahepatic organs to the liver. This study tests the hypothesis that the flow of sterol from the peripheral organs to the liver is dependent upon circulating HDL concentrations. Transgenic C57BL/6 mice were used that expressed variable amounts of simian cholesteryl ester-transfer protein (CETP). The rate of centripetal cholesterol flux was quantitated as the sum of the rates of cholesterol synthesis and low density lipoprotein-cholesterol uptake in the extrahepatic tissues. Steady-state concentrations of cholesterol carried in HDL (HDL-C) varied from 59 to 15 mg/dl and those of apolipoprotein AI from 138 to 65 mg/dl between the control mice (CETPc) and those maximally expressing the transfer protein (CETP+). There was no difference in the size of the extrahepatic cholesterol pools in the CETPc and CETP+ animals. Similarly, the rates of cholesterol synthesis (83 and 80 mg/day per kg, respectively) and cholesterol carried in low density lipoprotein uptake (4 and 3 mg/day per kg, respectively) were virtually identical in the two groups. Thus, under circumstances where the steady-state concentration of HDL-C varied 4-fold, the centripetal flux of cholesterol from the peripheral organs to the liver was essentially constant at approximately 87 mg/day per kg. These studies demonstrate that neither the concentration of HDL-C or apolipoprotein AI nor the level of CETP activity dictates the magnitude of centripetal cholesterol flux from the extrahepatic organs to the liver, at least in the mouse.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. D., Wilson P. W., Kannel W. B., Castelli W. P. High density lipoprotein cholesterol, total cholesterol screening, and myocardial infarction. The Framingham Study. Arteriosclerosis. 1988 May-Jun;8(3):207–211. doi: 10.1161/01.atv.8.3.207. [DOI] [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Absolute rates of cholesterol synthesis in extrahepatic tissues measured with 3H-labeled water and 14C-labeled substrates. J Lipid Res. 1979 Aug;20(6):740–752. [PubMed] [Google Scholar]
- Anderson R. G. Plasmalemmal caveolae and GPI-anchored membrane proteins. Curr Opin Cell Biol. 1993 Aug;5(4):647–652. doi: 10.1016/0955-0674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Azhar S., Stewart D., Reaven E. Utilization of cholesterol-rich lipoproteins by perfused rat adrenals. J Lipid Res. 1989 Nov;30(11):1799–1810. [PubMed] [Google Scholar]
- Bretscher M. S., Munro S. Cholesterol and the Golgi apparatus. Science. 1993 Sep 3;261(5126):1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- Chen Z., Peto R., Collins R., MacMahon S., Lu J., Li W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ. 1991 Aug 3;303(6797):276–282. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Spady D. K. Measurement of rates of cholesterol synthesis using tritiated water. J Lipid Res. 1984 Dec 15;25(13):1469–1476. [PubMed] [Google Scholar]
- Dietschy J. M., Turley S. D., Spady D. K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993 Oct;34(10):1637–1659. [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995 Feb;36(2):211–228. [PubMed] [Google Scholar]
- Glass C. K., Pittman R. C., Keller G. A., Steinberg D. Tissue sites of degradation of apoprotein A-I in the rat. J Biol Chem. 1983 Jun 10;258(11):7161–7167. [PubMed] [Google Scholar]
- Gordon D. J., Knoke J., Probstfield J. L., Superko R., Tyroler H. A. High-density lipoprotein cholesterol and coronary heart disease in hypercholesterolemic men: the Lipid Research Clinics Coronary Primary Prevention Trial. Circulation. 1986 Dec;74(6):1217–1225. doi: 10.1161/01.cir.74.6.1217. [DOI] [PubMed] [Google Scholar]
- Gwynne J. T., Mahaffee D., Brewer H. B., Jr, Ney R. L. Adrenal cholesterol uptake from plasma lipoproteins: regulation by corticotropin. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4329–4333. doi: 10.1073/pnas.73.12.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme I. An analysis of randomized trials evaluating the effect of cholesterol reduction on total mortality and coronary heart disease incidence. Circulation. 1990 Dec;82(6):1916–1924. doi: 10.1161/01.cir.82.6.1916. [DOI] [PubMed] [Google Scholar]
- Huang Y., von Eckardstein A., Wu S., Maeda N., Assmann G. A plasma lipoprotein containing only apolipoprotein E and with gamma mobility on electrophoresis releases cholesterol from cells. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1834–1838. doi: 10.1073/pnas.91.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D. R., Jr, Mebane I. L., Bangdiwala S. I., Criqui M. H., Tyroler H. A. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow-up study of the Lipid Research Clinics Prevalence Study. Am J Epidemiol. 1990 Jan;131(1):32–47. doi: 10.1093/oxfordjournals.aje.a115483. [DOI] [PubMed] [Google Scholar]
- Kannel W. B. High-density lipoproteins: epidemiologic profile and risks of coronary artery disease. Am J Cardiol. 1983 Aug 22;52(4):9B–12B. doi: 10.1016/0002-9149(83)90649-5. [DOI] [PubMed] [Google Scholar]
- Koelz H. R., Sherrill B. C., Turley S. D., Dietschy J. M. Correlation of low and high density lipoprotein binding in vivo with rates of lipoprotein degradation in the rat. A comparison of lipoproteins of rat and human origin. J Biol Chem. 1982 Jul 25;257(14):8061–8072. [PubMed] [Google Scholar]
- Letizia J. Y., Phillips M. C. Effects of apolipoproteins on the kinetics of cholesterol exchange. Biochemistry. 1991 Jan 22;30(3):866–873. doi: 10.1021/bi00217a041. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H. Canine lipoproteins and atherosclerosis. I. Isolation and characterization of plasma lipoproteins from control dogs. Circ Res. 1974 Nov;35(5):713–721. doi: 10.1161/01.res.35.5.713. [DOI] [PubMed] [Google Scholar]
- Marotti K. R., Castle C. K., Boyle T. P., Lin A. H., Murray R. W., Melchior G. W. Severe atherosclerosis in transgenic mice expressing simian cholesteryl ester transfer protein. Nature. 1993 Jul 1;364(6432):73–75. doi: 10.1038/364073a0. [DOI] [PubMed] [Google Scholar]
- Marotti K. R., Castle C. K., Murray R. W., Rehberg E. F., Polites H. G., Melchior G. W. The role of cholesteryl ester transfer protein in primate apolipoprotein A-I metabolism. Insights from studies with transgenic mice. Arterioscler Thromb. 1992 Jun;12(6):736–744. doi: 10.1161/01.atv.12.6.736. [DOI] [PubMed] [Google Scholar]
- Melchior G. W., Castle C. K., Murray R. W., Blake W. L., Dinh D. M., Marotti K. R. Apolipoprotein A-I metabolism in cholesteryl ester transfer protein transgenic mice. Insights into the mechanisms responsible for low plasma high density lipoprotein levels. J Biol Chem. 1994 Mar 18;269(11):8044–8051. [PubMed] [Google Scholar]
- Orci L., Montesano R., Meda P., Malaisse-Lagae F., Brown D., Perrelet A., Vassalli P. Heterogeneous distribution of filipin--cholesterol complexes across the cisternae of the Golgi apparatus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):293–297. doi: 10.1073/pnas.78.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osono Y., Woollett L. A., Herz J., Dietschy J. M. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J Clin Invest. 1995 Mar;95(3):1124–1132. doi: 10.1172/JCI117760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape M. E., Rehberg E. F., Marotti K. R., Melchior G. W. Molecular cloning, sequence, and expression of cynomolgus monkey cholesteryl ester transfer protein. Inverse correlation between hepatic cholesteryl ester transfer protein mRNA levels and plasma high density lipoprotein levels. Arterioscler Thromb. 1991 Nov-Dec;11(6):1759–1771. doi: 10.1161/01.atv.11.6.1759. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Simons K. Digging into caveolae. Science. 1995 Sep 8;269(5229):1398–1399. doi: 10.1126/science.7660120. [DOI] [PubMed] [Google Scholar]
- Plump A. S., Scott C. J., Breslow J. L. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Pászty C., Maeda N., Verstuyft J., Rubin E. M. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994 Aug;94(2):899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994 Nov 19;344(8934):1383–1389. [PubMed] [Google Scholar]
- Reaven E., Spicher M., Azhar S. Microvillar channels: a unique plasma membrane compartment for concentrating lipoproteins on the surface of rat adrenal cortical cells. J Lipid Res. 1989 Oct;30(10):1551–1560. [PubMed] [Google Scholar]
- Reddick R. L., Zhang S. H., Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb. 1994 Jan;14(1):141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kamen B. A., Anderson R. G. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990 Dec;111(6 Pt 2):2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblat G. H., Mahlberg F. H., Johnson W. J., Phillips M. C. Apolipoproteins, membrane cholesterol domains, and the regulation of cholesterol efflux. J Lipid Res. 1992 Aug;33(8):1091–1097. [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res. 1983 Mar;24(3):303–315. [PubMed] [Google Scholar]
- Spady D. K., Huettinger M., Bilheimer D. W., Dietschy J. M. Role of receptor-independent low density lipoprotein transport in the maintenance of tissue cholesterol balance in the normal and WHHL rabbit. J Lipid Res. 1987 Jan;28(1):32–41. [PubMed] [Google Scholar]
- Spady D. K., Meddings J. B., Dietschy J. M. Kinetic constants for receptor-dependent and receptor-independent low density lipoprotein transport in the tissues of the rat and hamster. J Clin Invest. 1986 May;77(5):1474–1481. doi: 10.1172/JCI112460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall A. R. Plasma cholesteryl ester transfer protein. J Lipid Res. 1993 Aug;34(8):1255–1274. [PubMed] [Google Scholar]
- Turley S. D., Andersen J. M., Dietschy J. M. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. 1981 May;22(4):551–569. [PubMed] [Google Scholar]
- Turley S. D., Herndon M. W., Dietschy J. M. Reevaluation and application of the dual-isotope plasma ratio method for the measurement of intestinal cholesterol absorption in the hamster. J Lipid Res. 1994 Feb;35(2):328–339. [PubMed] [Google Scholar]
- Turley S. D., Spady D. K., Dietschy J. M. Role of liver in the synthesis of cholesterol and the clearance of low density lipoproteins in the cynomolgus monkey. J Lipid Res. 1995 Jan;36(1):67–79. [PubMed] [Google Scholar]
- Woollett L. A., Osono Y., Herz J., Dietschy J. M. Apolipoprotein E competitively inhibits receptor-dependent low density lipoprotein uptake by the liver but has no effect on cholesterol absorption or synthesis in the mouse. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12500–12504. doi: 10.1073/pnas.92.26.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]



