Abstract
Breast cancer and osteoporosis are common diagnoses in women. Breast cancer survival has improved due to earlier detection and improved treatments. As most breast cancers are estrogen receptor positive, treatment is often aimed at altering the hormonal environment. Both pre and postmenopausal women undergoing these therapies are at risk for bone loss. The patient's health care team ought to have an awareness of the potential for breast cancer treatments to accelerate bone loss. Women with early stage breast cancer are treated with curative intent and, therefore, maintaining bone health is important and is part of the survivorship care to ensure an optimal quality of life.
Keywords: Breast cancer, Osteoporosis, Survivorship, Aromatase inhibitors, Selective estrogen receptor modulators, Bisphosphonates, Denosumab
Introduction
Breast cancer and osteoporosis are common diagnoses in women and are frequently intertwined. In 2012, over 200, 000 US women were diagnosed with breast cancer, making it the most common type of cancer affecting women [1]. In 2008, US breast cancer survivors numbered 3 million [2]. Based on National Health and Nutrition Examination Survey data, 10 % of women or 4.5 million women over the age of 50 have osteoporosis, making both breast cancer and osteoporosis public health concerns [3].
Osteoporosis/osteopenia may be a comorbid condition in a patient at the time of breast cancer diagnosis, may develop during cancer treatment or during cancer-free survival. Breast cancer treatment itself may increase the risk of bone loss, primarily occurring due to the hypogonadal state induced by treatments. Due to early detection, improved methods for local control and the use of adjuvant therapy, breast cancer patients are experiencing increased cure rates and are living to a greater advanced age. The 10-year cancer free survival rate for stage I–III is 80 % [2]. Hence, maintenance of bone health is a concern in breast cancer survivorship.
The U.S. Preventive Services Task Force (USPSTF) recommends screening for osteoporosis in women aged 65 years or older and in younger women whose fracture risk is equal to or greater than that of a 65-year-old white woman [4]. For many women, breast cancer therapy constitutes a risk factor for osteoporosis and therefore screening is indicated. This article will review the effects of breast cancer therapy on bone health. The focus is on early stage breast cancer in the adjuvant setting when treatment is administered with curative intent. Prevention and treatment strategies for osteoporosis in the setting of breast cancer will be discussed as well as new therapies and directions for future research.
Breast and Bone: Estrogen Sensitive Organs
Breast and bone are both estrogen sensitive organs. A prolonged lifetime exposure to estrogen through early menarche, late menopause, and use of postmenopausal hormone therapy are factors known to reduce the risk of osteoporotic fractures [5, 6].
However, these same factors are associated with an increased risk of developing breast cancer due to the increased estrogen exposure. Endogenous estradiol has not been shown to be directly related to breast density [7]. Nonetheless, breast density and circulating estrogen levels are independently associated with breast cancer risk. Breast cancer risk was 2.4 % – 4.2 % higher in women with very high breast density particularly in women who use estrogen plus progestin [8]. Women with the highest levels of breast density have been found to have a 4- to 6-fold increased risk of breast cancer compared with women with less dense breasts [9].
Epidemiologic data suggests that higher bone mineral density (BMD) is also associated with a higher risk of breast cancer. Zhang et al. studied BMD in postmenopausal women and after adjusting for age, found those in the top quartile of BMD had a 3.5 times higher risk ratio of developing breast cancer than women in the lowest quartile of BMD [10]. This association was confirmed in a meta-analysis of 70,878 post-menopausal women from 10 studies; in which 1,889 cases of breast cancer followed for mean of 6 years showed that higher BMD was associated with a significantly higher risk of breast cancer [11]. In this meta-analysis, women with the highest hip or spine BMD had a 62 % and 82 % higher risk, respectively, of developing breast cancer than women in the lowest BMD categories [11]. For each increased standard deviation in BMD at the hip or spine, the risk for developing breast cancer increased by 20 % and 26 %, respectively [11]. Therefore higher estrogen levels are associated with higher bone density as well as a higher risk of breast cancer.
In estrogen deficient states such as menopause or those induced by breast cancer treatment, RANKL expression and the ratio of RANKL to OPG is increased, allowing increased differentiation of osteoclasts [12]. As estrogen stimulates osteoclast apoptosis, the estrogen deficient states allows prolonged survival of osteoclasts [13] that tilts the balance of bone formation and bone resorption toward resorption, ultimately leading to bone loss [12, 14].
Effects of Breast Cancer Therapy on Bone
Radiation
Radiation therapy to the breast or chest wall region is used in early stage breast cancer as part of breast conserving therapy or when there is substantial tumor bulk. In combination with surgery, use of either brachytherapy or whole breast irradiation (WBI) improves control of the tumor and decreases the risk of local recurrence from 24 % to 8.5 % in patients with early stage breast cancer [15]. However, due to either anatomical limitations of planning the treatment field or due to beam scatter, radiation therapy can negatively affect local bone health. Higher risk of rib fractures was seen in those treated with a larger dose of radiation [16]. A prospective study evaluating the combination of surgery and external beam radiation therapy found a radiation dose related increase in the risk of rib fracture. In this cohort, women who were treated with lower doses of radiation (less than 50 Gy vs greater than 50 Gy) had a significantly lower incidence of rib fractures (1.4 % vs 5.7 %, respectively) [16]. Typical adjuvant breast cancer radiation doses fall within the range of 50 Gy although higher doses are not uncommon when a boost is given after lumpectomy [17•].
Similarly, when radiation was combined with chemotherapy, women who received both had an increased rate of rib fracture over those receiving radiation less than 50 Gy alone (2.3 % vs 0.5 %, respectively) [15]. Both brachytherapy and WBI were associated with increased risk of rib fracture over the 5 years after treatment; however the risk with brachytherapy was higher at 4.5 % vs 3.6 % in women who underwent WBI [18].
Chemotherapy
Chemotherapeutic agents such as cyclophosphamide and methotrexate have direct effects on the bone in preclinical models [19, 20]. Clinically, it is difficult to isolate the bone changes due to chemotherapy alone from effects of supportive medications, changes in physical activity and nutrition associated with breast cancer treatment. Yet chemotherapy regimens may have distinctive effects on bone.
Chemotherapeutic agents, particularly cyclophosphamide, may render younger breast cancer patients amenorrheic, often referred to as chemotherapy induced ovarian failure (CIOF) [21]. This premature ovarian dysfunctional state has variable definitions in the literature as the onset and duration of the amenorrheic state differs across studies. The decrease of circulating estradiol that occurs with the cessation of menses increases bone resorption (Fig. 1) [14]. The incidence of CIOF is age and treatment-related, occurring in greater than 50 % of premenopausal women within the first year of treatment [14]. The majority of the older premenopausal women remain amenorrheic after the first year [22]. Women under 40 receiving the chemotherapy regimen consisting of cyclophosphamide, methotrexate, and 5-Fluorouricil (CMF) have a 30 % – 40 % risk of CIOF; while women over 40 who are still menstruating at the onset of treatment have a risk between 80 % and 96 % [23]. Premature menopause has a negative impact on BMD [24, 25].
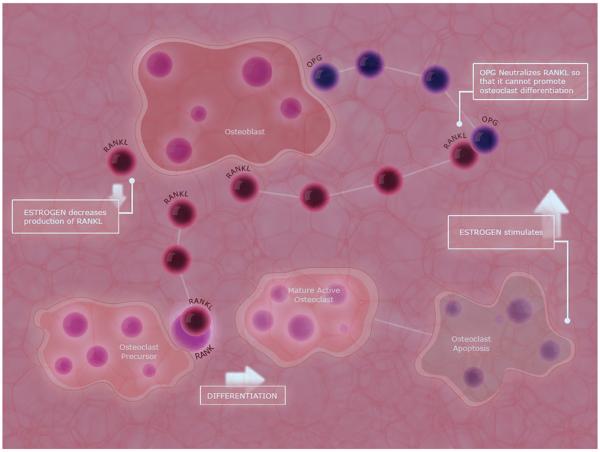
Fig. 1.
Action of estrogen on osteoblasts and osteoclasts. The principal activator of osteoclast-mediated bone resorption is the ligand of the receptor associated with nuclear factor kappa-B (RANK-L), which is expressed on the surface of osteoblasts [12]. Binding of RANKL to the RANK receptor on osteoclasts stimulates osteoclast differentiation [12]. Osteoprotegerin (OPG) is an endogenous inhibitor of the RANK/RANKL pathway and is secreted by osteoblasts. OPG binds to RANKL preventing its attachment to the RANK receptors. The presence of estrogen decreases the expression of RANKL on osteoblast cells, as well as increases the expression of OPG, allowing increased neutralization of RANKL, and stimulates apoptosis of osteoclasts [12]
With natural menopause there is a gradual decline in estrogen level. In contrast, there is a rapid decline in the estrogen levels following CIOF. Women with CIOF were found to have lumbar spine BMD loss of 7.7 % at 1 year compared with a 2.0 % loss in women in early natural menopause, and a loss of 1.0 % in late natural menopausal women [26]. A decline in BMD is seen as early as 6 months at the vertebral and femoral sites [24]. Thus, chemotherapeutic agents (described above) may induce a hypogonadal state that is associated with rapid loss of BMD. The impact on long term fracture risk in this patient population has not been defined.
Adjuvant Endocrine Therapy
Approximately 75 % of breast cancers express either estrogen receptor or progesterone receptors [27] and thus therapy with anti-estrogen agents is routinely used as part of breast cancer treatment. Tamoxifen is one of several medications known as Selective Estrogen Receptor Modulators (SERMs); it binds the estrogen receptors in hormone sensitive breast cancer tissue. Aromatase inhibitors (AIs), suppress peripheral estrogen production by blocking aromatization of androgens to estrogens. These drugs are the first choice of treatment for breast cancer in post-menopausal women as their use is associated with reduced risk of recurrence and improved disease free survival [28, 29].
Because of the integral role of estrogen signaling in bone health, AIs and SERMS exert an effect on bones. Tamoxifen and raloxifene are both SERMS that are FDA approved for preventing breast cancer. However, only tamoxifen is FDA approved for treatment of breast cancer and only raloxifene is FDA approved for the prevention and treatment of osteoporosis. In post-menopausal women, tamoxifen acts as a partial estrogen agonist on bone, particularly the trabecular bone. Postmenopausal women treated with tamoxifen for 5 years saw a positive effect on lumbar spine BMD (0.8 %) compared with placebo (−0.7) [30]. This positive effect on BMD is reversed upon drug discontinuation [14]. Conversely, in premenopausal women, tamoxifen is associated with a loss of BMD [31].
The third generation non-steroidal AIs are potent inhibitors of estrogen (>96 % inhibition) thus depriving tumor cells of the growth promoting effects of estrogen [32–34]. The Arimidex, Tamoxifen, Alone or in Combination trial (ATAC) a large double blinded phase III trial (N =9,000) with a bone sub-protocol (n =308) comparing anastrozole, tamoxifen, and a combination of both drugs in postmenopausal women with hormone receptor positive breast cancer demonstrated a greater loss in BMD in anastrozole-treated patients when compared with tamoxifen; at 5 years the loss in BMD at the lumbar spine was 6.08 % and total hip was 7.24 %. In contrast, tamoxifen showed an increase in BMD of 2.88 % and 0.74 % respectively at these sites [35]. Forty-three percent of women on anastrozole who had normal bone density at the beginning of treatment developed osteopenia compared with 9 % on tamoxifen [35]. Women with underlying osteopenia are at a higher risk of developing osteoporosis while on AI compared with tamoxifen [35]. Bone loss was accelerated in the first 2 years whereas the rate of change in BMD decreased over years 2–5 [35]. Following discontinuation of anastrozole, bone density increased at the lumbar spine and further loss was not seen at the hip during the off treatment follow-up period [36•].
Within both groups (anastrozole and tamoxifen) the greatest loss of bone mass was seen in women who were <4 years from their last menstrual cycle. On anastrozole, women who were less than 4 years since menopause lost 11.3 % BMD at the lumbar spine and 7.54 % at the total hip at the end of 5 years compared with those women who were over 4 years since menopause (−5.4 % and −7.24 %, respectively) [35]. Although the bone loss ceases and is partially reversible following discontinuation of aromatase inhibitors, it never returns to baseline [35, 36•, 37].
Concurrent Medications
Medications given to support patients through the side effects of cancer treatment can interrupt healthy bone remodeling. Glucocorticoids may be used as an anti-emetic or to decrease the risk of hypersensitivity reactions. Glucocorticoids increase bone resorption by inhibiting osteoblasts, increasing the lifespan of osteoclasts, and increasing the apoptotic rate of osteocytes [38]. In addition, steroids may cause myopathy and thereby an increased propensity to falls and possibly fractures [39]. Furthermore, unfractionated heparin, proton pump inhibitors, and selective serotonin reuptake inhibitors, all used for treatment of comorbidities in breast cancer and its treatment, negatively affect BMD [39].
Impact on Fractures
Breast cancer and osteoporosis both have an effect on mortality. Lower levels of estrogen have been shown to increase the relative risk of fractures [40]. Breast cancer therapies lower circulating estrogen levels either by inducing amenorrhea or suppressing peripheral estrogen production. Hence, breast cancer therapies can be associated with an increased risk of fracture. The Women's Health Initiative study has shown that in postmenopausal women, diagnosis of breast cancer resulted in a higher risk of hip fractures (HR=1.55) [41]. While hip fractures occurred with greater frequency in women with breast cancer, there was no increase risk seen with forearm fractures and while the clinical vertebral fractures risk was higher the difference was not statistically significant [41].
Fractures are associated with significant morbidity and mortality and any osteoporotic fracture increases the risk of death by 42 % [42]. In addition, the mortality risk remains high for 10 years following a hip fracture [42].
Overall, breast cancer survivors are estimated to experience 68.6 excess fractures per 10,000 person-years that essentially translates to an increased risk of 6.86 % per breast cancer survivor per decade of survivorship [43]. WHI found that vertebral fractures were higher in women diagnosed with breast cancer before age 55 but that this risk was not elevated for women diagnosed older than 55. The difference is attributed to the accelerated drop in estrogen levels due to CIOF in premenopausal women [43]. It is estimated that women with CIOF may experience fractures up to 10 years earlier than women without breast cancer [44]. The impact of premature cessation of menses on the risk of fractures has not been well characterized.
Although AIs reduce breast cancer recurrence, both steroidal AIs (exemestane) and non-steroidal AIs (anastrozole and letrozole) have been associated with higher bone turnover and higher rates of fractures [39]. In the ATAC trial, breast cancer patients treated with anastrozole alone had an increase in the yearly fracture rates (2.93 % vs 1.90 %) in the 5 years of treatment than patients treated with tamoxifen alone [45]. In a recent study comparing exemestane and anastrozole, self-reported new diagnosis of osteoporosis was lower (31 % vs 35 %) in the exemestane group but there was no difference noted in the number of new clinical fractures or fractures at fragility sites [46••]. When 3 aromatase inhibitors (anastrozole, exemestane, and letrozole) were compared head to head in postmenopausal women without cancer no difference was noted in the biochemical markers for bone metabolism [47].
The 2003 American Society of Clinical Oncology (ASCO) guidelines identify the use of adjuvant AIs as a risk factor for osteoporosis [48]. For this group, initial dual-energy X-ray absorptiometry (DXA) screening followed by annual assessment is recommended. The more recent UK Expert Group proposed 2 algorithms for the management of bone loss in early breast cancer; (A) Premenopausal women who experience premature menopause due to chemotherapy or ovarian suppression, ablation, or removal, and (B) postmenopausal women receiving an AI [49]. For postmenopausal women over the age of 75 with 1 or more risk factors, treatment is recommended. For postmenopausal women <75 years if T scores are −1.0 to −2.0 and annual bone loss occurs at a rate >4 %, therapy is recommended. The group recommends screening for secondary causes of osteoporosis before treatment is initiated [49]. Note that the ASCO guidelines do not support the use of an AI in the setting of CIOF. Consideration for an AI may be given if there are measures taken (surgery or chemical ovarian ablation) to assure the postmenopausal state [28].
The FRAX scoring tool developed by the World Health Organization helps in estimating the 10-year probability of hip or major osteoporotic fractures using clinical risk factors. The FRAX tool may be used to estimate fracture risk without use of BMD data [50]. This useful tool is available on line without cost [51], however, FRAX does not account for cancer specific risks to bone health and therefore cannot adequately estimate fracture risk.
Prevention and Treatment of Osteoporosis
Women diagnosed with breast cancer should be counseled by their oncology and primary care providers on basic lifestyle practices to minimize risk of bone loss and fractures associated with their cancer care.
Lifestyle Choices and Prevention
Large well-designed controlled trials evaluating the effect of exercise on fractures is lacking; however, several meta-analyses performed on studies with exercise as an intervention showed beneficial effects on BMD (spine and hip) in premenopausal and postmenopausal women [52–54]. Aerobic exercise (low to medium impact) for 15–60 min 3 times a week with strength training is recommended for optimizing bone health [52–54]. Weight bearing exercises were required for at least 1 year to see appreciable benefits [55]. In addition to the effect on BMD, muscle strengthening exercises also reduce falls and improves balance but their effectiveness in preventing fractures is less evident [56, 57].
Alcohol has direct toxic effects on osteoblasts. Light alcohol consumption may have a beneficial effect on BMD and is not associated with an increased risk of fracture, however, heavy alcohol intake and binge drinking is associated with decreased BMD in men [58, 59]. Smoking is also known to have adverse effects on bone with an increased risk of fractures [60]. Therefore, counseling for smoking cessation and avoidance of excessive alcohol is critical to prevention of osteoporosis.
Prior to 1990 the mainstay of the treatment of osteoporosis was calcium and vitamin D. Calcium supplements in healthy older women have shown to reduce bone turnover by 20 % and thereby slow bone loss [61]. In this study, the effect on fracture was uncertain but the study was limited by poor long-term compliance [61]. A meta-analysis done by Tang et al. suggested that fractures were decreased by 10 % in calcium users [62]. Side effects of calcium therapy include increased GI discomfort and renal calculi. The recent Auckland calcium study raised concerns over the increased occurrence of cardiovascular disease with calcium supplements [63]. These results were duplicated in the WHI and other meta-analysis [63, 64••]. Debate on whether calcium supplements constitute a cardiovascular risk continues. Food is the best source of calcium supplements and if diet alone is insufficient, calcium supplements may be added. Similarly, vitamin D supplementation is generally recommended, although the data on its ability to reduce fractures is limited. Therefore adequate intake of calcium (1,000–1,200 mg/d) and vitamin D (600–800 units/d) is recommended by the Institute of Medicine and is supported by the America Society for Bone and Mineral Research (ASBMR) [65, 66]. These recommendations are not universally accepted. The Endocrine Society practice guidelines recommend higher daily intakes of Vitamin D in adults [67]. The optimal level of vitamin D, defining the population at risk and adequate dosing of daily vitamin D continue to remain a matter of further research. In addition, USPSTF states that there is not enough evidence on how supplements affect bone health in men and premenopausal women and evidence on calcium and vitamin D supplements to prevent fractures is insufficient.
Pharmacologic Interventions
Currently available drugs for the prevention and treatment of osteoporosis include anti-resorptive and anabolic agents. However, not all available medications are considered appropriate for women with a history of breast cancer. Bisphosphonates and denosumab (an inhibitor of RANKL) are considered the mainstay of pharmacologic interventions for managing bone health in women with a history of breast cancer and may be used to manage the comorbid condition of low bone mass, or to manage cancer therapy induced bone loss (CTIBL) [68–74, 75••, 76]. The anabolic agent, teriparatide is contraindicated in anyone with history of bone tumors or prior radiation therapy to bone. As radiation to the chest wall is frequently part of the adjuvant treatment for breast cancer, and due to the concern of occult micro-metastases to bone, the anabolic agent teriparatide is not generally considered an appropriate option for women with a history of breast cancer and concurrent osteoporosis [77].
Bisphosphonates, available as oral and IV preparations, are FDA approved for the prevention and treatment of osteoporosis. The bisphosphonates have been shown to be effective in reducing vertebral as well as hip fractures in postmenopausal women [78]. Studies of bisphosphonates in women with breast cancer demonstrate a decrease in the rate of bone loss and increases in BMD [79]. Denosumab inhibits RANKL and thereby the activation of osteoclasts, reducing bone turnover. Data from a large phase III trial comparing denosumab with placebo, showed a reduction in vertebral, non-vertebral as well as hip fractures in postmenopausal women with osteoporosis treated with denosumab for a period of 3 years [80]. Similarly, in women with non-metastatic breast cancer on an AI, 2-year treatment with denosumab resulted in significant increases in BMD at the lumbar spine, femoral neck, and radius [81]. Oral and intravenous bisphosphonates, as well as denosumab have been associated with osteonecrosis of the jaw and atypical subtrochanteric femoral fractures although these events are uncommon [82, 83].
Key studies on bisphosphonates and denosumab in CTIBL are illustrated in Table 1. The dose and schedule of drug administration in CTIBL is similar to that used in postmenopausal osteoporosis although for many of the zoledronic acid studies 4 mg every 6 months was used in the setting of normal or osteopenic BMD. Note that FDA indications for zoledronic acid in the management of osteopenia is dosing of 5 mg every 2 years [84]. Currently, clinical trials with bisphosphonates and denosumab have not shown fracture prevention in the setting of breast cancer and AI therapy.
Table 1.
Key studies on bisphosphonates and denosumab in cancer treatment induced bone loss
| Drug | Treatment | Control group | Follow up duration (months) | Mean BMD changes from baseline |
Ref | |
|---|---|---|---|---|---|---|
| Lumbar spine | Total hip | |||||
| Risedronate 35 mg/wk | 37 | 37 | 24 | 5.7 % vs −1.5 % | 1.6 % vs −3.9 % | [72] |
| Risedronate 35 mg/wk | 43 | 44 | 24 | 0.4 % vs 1.2 % | 0.9 % vs −1.6 % | [68] |
| Risedronate 35 mg/w | 15 | 103 | 12 | 4.1 % vs −3.3 % | 1.8 % vs −2.8 % | [74] |
| Risedronate 35 mg/wk | 77 | 77 | 24 | 2.4 % vs −1.8 % | 1.8 % vs −1.1 % | [76] |
| Ibandronate 150 mg q28d | 25 | 25 | 24 | 3.0 % vs −3.2 % | 0.6 % vs. −3.9 % | [73] |
| Zoledronic acid 4 mg, q6m | upfront 301 | delayed 301 | 61 | 6.2 % vs −2.4 % | 2.5 % vs −4.1 % | [75••] |
| Zoledronic acid 4 mg, q6m | upfront 532 | delayed 533 | 60 | 4.3 % vs −5.4 % | 1.6 % vs. −4.2 % | [71] |
| Zoledronic acid 4 mg, q6m | 263 | 264 | 36 | 2.72 % vs −2.71 % | 1.72 % vs −1.59 % | [70] |
| Zoledronic acid 4 mg, q6m | upfront 274 | delayed 277 | 24 | 4.94 % vs −2.28 % | 1.22 % vs −3.34 % | [69] |
| Denosumab 60 mg q6m | 127 | 125 | 24 | 6.2 % vs −1.4 % | 3.7 % vs −1.0 % | [81] |
Bone Health in Breast Cancer Survivorship
Breast cancer survival has improved related to earlier detection as well as more effective treatment [85]. Attention to bone health should be part of survivorship care. As we have discussed above, both pre- and post-menopausal women undergoing adjuvant endocrine therapy are at risk for bone related toxicities of breast cancer therapy. In addition, supportive medications used in the survivorship period may also contribute to decline in bone health [39, 86]. Despite the known adverse effects of breast cancer treatments, bone health in survivors is not well addressed [87, 88]. Out of 500 oncologists surveyed in the UK in 2004, about one-third had not requested a DXA scan in their patients. About two-thirds of the oncologists did not address bone health in patients on an AI. Although, more than half of the oncologists agreed that it was their responsibility for treating bone health issues [89]. While goals of breast cancer therapy need to be acutely addressed, the long-term implication of cancer treatment, including bone health, must be taken into consideration in order to mitigate treatment toxicities.
Future Directions
Osteoclast inhibitors, specifically high dose bisphosphonates or denosumab, are being investigated for their potential to reduce the risk of breast cancer recurrence. A series of phase III studies have been reported with mixed results [90–93]. To date, none of the osteoclast inhibiting regimens studied for this effect has been FDA approved for that indication. Additional phase III studies are ongoing [94, 95]. Of note, fracture is not being evaluated in these cancer treatment focused studies, and the dose and interval of osteoclast inhibitors far exceeds that used in osteoporosis.
Cathepsin K, a cysteine protease produced by osteoclasts plays a key role in osteoclast mediated bone degradation. The cathepsin K inhibitor, odanacatib, is in phase III clinical trial for postmenopausal women with low bone mass [96]. Although if FDA approved, odanacatib may be appropriate for postmenopausal women with breast cancer therapy induced bone loss; however, odanacatib is presently not being investigated in this patient population. Another area of active research includes study of vitamin D, which may have a therapeutic impact on the risk of osteoporosis as well as may improve cancer outcomes [97, 98].
There is concern over use of bone related anabolic agents in women with a history of breast cancer since the risk of breast cancer recurrence generally persists through the lifespan. In women with breast cancer, there may be occult tumor cells present in the bone marrow years after completion of therapy. Curiously, these disseminated tumor cells are uniformly associated with breast cancer relapse [99, 100], even if found years after initial diagnosis and completion of adjuvant treatment [101, 102]. The balance between tumor dormancy and progression is not well understood. The novel monoclonal antibody targeting sclerostin (AMG 785, romosozumab), like the FDA approved parathyroid hormone analog, teriparatide, may act as an anabolic agent, and there are theoretical risks for promoting the outgrowth of dormant, occult, disseminated breast cancer cells, particularly from within the bone of women with a history of breast cancer. Improved understanding of the cell signaling between bone and micrometastatic tumor cells will aid in evaluating the safety of osteo-anabolic therapies, followed by clinical trials verifying safety.
Conclusions
Women with early stage breast cancer are treated with curative intent and maintaining bone health is an important component of survivorship care. Regardless of their age and menopausal status, an individual's history of breast cancer should be taken into consideration when evaluating risks for osteoporotic fractures. Osteoporosis, left undiagnosed and untreated, will contribute to morbidity in women for whom curative therapy is successful and add to lower quality of life, morbidity, and mortality. To date, clinical trials to investigate means to mitigate breast cancer therapy induced bone loss have suggested that established FDA approved drug doses and schedules of osteoclast inhibition are sufficient to preserve bone mass. However, there is a paucity of data addressing fractures and additional studies are indicated.
Acknowledgments
Catherine Van Poznak is supported by the NIDCR grant 5K23DE020197. The authors would like to thank Alonso Molina-Gomez for his help with the Figure on estrogen mechanism.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest P Choksi declares that she has no conflicts of interest.
M Williams declares that she has no conflicts of interest.
PM Clark declares that she has no conflicts of interest.
C Van Poznak declares that she has no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.US National Institutes of Health [Accessed July 29, 2013];Surveillance Epidemiology and End Results Cancer Statistics Review: lifetime risk. Available at: http://seer.cancer.gov/csr/1975_2009_pops09/ Lifetime Risk.
- 2.Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Res. 2008;100:1179–83. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center for Disease Control FastStats [Accessed Aug 26 2013];Osteoporosis. http://www.cdc. gov/nchs/fastats/osteoporosis.htm.
- 4.Nordin C. Screening for osteoporosis: U.S. Preventive Services Task Force recommendation statement. Ann. Intern Med. 2011;155:276–7. doi: 10.7326/0003-4819-155-4-201108160-00021. [DOI] [PubMed] [Google Scholar]
- 5.Ribot C, Pouilles J, Bonneu M, Tremollieres F. Assessment of the risk of post-menopausal osteoporosis using clinical factors. Clin Endocrinol. 1992;36(3):225–8. doi: 10.1111/j.1365-2265.1992.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 6.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the women's health initiative randomized trial. JAMA. 2003;290(13):1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 7.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in post-menopausal women. J Natl Cancer Inst. 2007;99:1178–87. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 8.Kerlikowske K, Cook AJ, Buist DS, Cummings SR, Vachon C, Vacek P, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–7. doi: 10.1200/JCO.2009.26.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society . Breast cancer facts & figures 2011–2012. American Cancer Society; Atlanta: 2011. [Google Scholar]
- 10.Zhang Y, Kiel DP, Kreger BE, Cupples LA, Ellison RC, Dorgan JF, et al. Bone mass and the risk of breast cancer among postmenopausal women. N Engl J Med. 1997;336:611–7. doi: 10.1056/NEJM199702273360903. [DOI] [PubMed] [Google Scholar]
- 11.Qu X, Zhang X, Qin A, Liu G, Zhai Z, Hao Y, et al. Bone mineral density and risk of breast cancer in postmenopausal women. Breast Cancer Res Treat. 2013;138:261–71. doi: 10.1007/s10549-013-2431-3. [DOI] [PubMed] [Google Scholar]
- 12.Hofbauer LC, Schoppet MS. Clinical implications of the Osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–5. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 13.Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 14.Michaud LB, Goodin S. Cancer-treatment-induced bone loss, parts 1 and 2. Am J Health Syst Pharm. 2006;63:419–30. 534–46. doi: 10.2146/ajhp050045.p1. [DOI] [PubMed] [Google Scholar]
- 15.Violet JA, Harmer C. Breast cancer: improving outcome following adjuvant radiotherapy. Br J Radiol. 2004;77:811–20. doi: 10.1259/bjr/44576710. [DOI] [PubMed] [Google Scholar]
- 16.Pierce SM, Recht A, Lingos TI, Abner A, Vicini F, Silver B, et al. Long term radiation complications following conservative surgery and radiation therapy in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:915–23. doi: 10.1016/0360-3016(92)90895-o. [DOI] [PubMed] [Google Scholar]
- 17•.Bellon JR, Harris EE, Arthur DW, Bailey L, Carey L, Goyal S, et al. American College of Radiology Appropriateness Criteria conservative surgery and radiation—stage I and II breast carcinoma: expert panel on radiation oncology: breast. Breast J. 2011;17:448–55. doi: 10.1111/j.1524-4741.2011.01132.x. [DOI] [PubMed] [Google Scholar]; This article discusses the effects of breast cancer radiation treatment and its effects on skeletal health.
- 18.Smith GL, Xu Y, Buchholz TA, Giordano SH, Jiang J, Shih YC, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307:1827–37. doi: 10.1001/jama.2012.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang TM, Shih C. Study of histomorphometric changes of the mandibular condyles in neonatal and juvenile rats after administration of cyclophosphamide. Acta Anat. 1986;127:93–9. doi: 10.1159/000146262. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler DL, Vander Griend RA, Wronski TJ, Miller GJ, Keith EE, Graves JE. The short- and long-term effects of methotrexate on the rat skeleton. Bone. 1995;16:215–21. doi: 10.1016/8756-3282(94)00032-u. [DOI] [PubMed] [Google Scholar]
- 21.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–29. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 22.Vehmanen L, Saarto T, Elomaa I, Makela P, Valimaki M, Blomqvist C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur J Cancer. 2001;37:2372–8. doi: 10.1016/s0959-8049(01)00317-3. [DOI] [PubMed] [Google Scholar]
- 23.Partridge AH, Burstein HF, Winer EP. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Nat Cancer Res Monogr. 2001;30:135–42. doi: 10.1093/oxfordjournals.jncimonographs.a003451. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19:3306–11. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- 25.Fuleihan GH, Salamoun M, Mourad YA, et al. Pamidronate in the prevention of chemotherapy-induced bone loss in premenopausal women with breast cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90:3209–14. doi: 10.1210/jc.2004-1444. [DOI] [PubMed] [Google Scholar]
- 26.Body J-J. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer. 2011;11:384–92. doi: 10.1186/1471-2407-11-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipton A, Smith MR, Ellis GK, Goessl C. Treatment-induced bone loss and fractures in cancer patients undergoing hormone ablation therapy: efficacy and safety of denosumab. Clin Med Insights Oncol. 2012;6:287–99. doi: 10.4137/CMO.S8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burstein GH, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, et al. American Society of Clinical Oncology Clinical Practice Guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report; 2004. J Clin Oncol. 2005;23:619–29. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 30.Love RR, Barden HS, Mazess RB, Epstein S, Chappell RJ. Effect of tamoxifen on lumbar spine bone mineral density in postmenopausal women after 5 years. Arch Intern Med. 1994;22:2585–8. [PubMed] [Google Scholar]
- 31.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 32.Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole (Femara) and anastrozole (Arimedex) on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, crossover-designed study. J Clin Oncol. 2002;20:751–7. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- 33.Geisler J, King N, Dowsett M, Ottestad L, Lundgren S, Walton P, et al. Influence of anastrazole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatization and plasma estrogen levels in postmenopausal women with breast cancer. Br J Cancer. 1996;74:1286–91. doi: 10.1038/bjc.1996.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geisler J, Ekse D, Duong NK, Evens DB, Nordbo Y, Aas T, et al. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4:2089–93. [PubMed] [Google Scholar]
- 35.Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26:1051–7. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 36•.Eastell R, Adams J, Clack G, Howell A, Cuzick J, Mackey J, et al. Long-term effects of anastrozole on bone mineral density: 7-year results from the ATAC trial. Ann Oncol. 2011;22:857–62. doi: 10.1093/annonc/mdq541. [DOI] [PubMed] [Google Scholar]; This study evaluated the effects of long term anastrazole on bone mineral density and improvement at the lumbar spine following discontinuation of anastrazole.
- 37.Geisler J, Lønning PE, Krag LE, Løkkevik E, Risberg T, Hagen AI, et al. Changes in bone and lipid metabolism in postmenopausal women with early breast cancer after terminating 2-year treatment with exemestane: a randomized, placebo-controlled study. Eur J Cancer. 2006;42:2968–75. doi: 10.1016/j.ejca.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein RS, Jilka RL, Parfitt AM, et al. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of the deleterious effects on bone. J Clin Invest. 1998;102:274–82. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidge-Pitts CJ. Update on medications with adverse skeletal effects, a concise review for clinicians. Mayo Clin Proc. 2011;86:338–43. doi: 10.4065/mcp.2010.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733–8. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 41.Chen Z, Maricic M, Aragaki AK, Mouton C, Arendell L, Lopez AM, et al. Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: results from the Women's Health Initiative. Osteoporos Int. 2009;20:527–36. doi: 10.1007/s00198-008-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–21. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 43.Chen Z. Fracture risk among breast cancer survivors. Arch Intern Med. 2005;165:332–8. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 44.Bruning PF, Pit MJ, de Jong-Bakker M, van den Ende A, Hart A, van Enk A. Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br J Cancer. 1990;61:308–10. doi: 10.1038/bjc.1990.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists' Group, effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 46••.Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27—a randomized controlled phase III trial. J Clin Oncol. 2013;31:1398–404. doi: 10.1200/JCO.2012.44.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compares the two AIs exemestane and anastrazole and the self-reported new diagnoses of osteoporosis after treatment.
- 47.McCloskey EV, Hannon RA, Lakner G, Fraser WD, Clack G, Miyamoto A, et al. Effects of third generation aromatase inhibitors on bone health and other safety parameters: results of an open, randomized, multi-center study of letrozole, exemestane and anastrozole in healthy postmenopausal women. Eur J Cancer. 2007;43:2523–31. doi: 10.1016/j.ejca.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 48.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–57. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Reid DM, Doughty J, Eastell R, Heys SD, Howell A, McCloskey EV, et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(Suppl 1):S3–S18. doi: 10.1016/j.ctrv.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–46. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 51.Kanis J. [Accessed Sep 1 2013];FRAX WHO Fracture Risk Assessment Tool. http://www. shef.ac.uk/FRAX.
- 52.Kelley GA, Kelley KS, Tran ZV. Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. J Gerontol A Biol Sci Med Sci. 2002;57:M599–604. doi: 10.1093/gerona/57.9.m599. [DOI] [PubMed] [Google Scholar]
- 53.Kelley GA, Kelley KS. Efficacy of resistance exercise on lumbar spine and femoral neck bone mineral density in premenopausal women: a meta-analysis of individual patient data. J Women's Health. 2004;13:293–300. doi: 10.1089/154099904323016455. [DOI] [PubMed] [Google Scholar]
- 54.Wolff I, Croonenberg JJ, Kemper HC, Kostense PJ, Twisk JW. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int. 1999;9:1–12. doi: 10.1007/s001980050109. [DOI] [PubMed] [Google Scholar]
- 55.Kam D, Smulders E, Weerdesteyn V, Smits-Engelsman BC. Exercise interventions to reduce fall-related fractures and their risk factors in individuals with low bone density: a systematic review of randomized controlled trials. Osteoporos Int. 2009;20:2111–25. doi: 10.1007/s00198-009-0938-6. [DOI] [PubMed] [Google Scholar]
- 56.Kannus P, Sievanen H, Palvanen M, Jarvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366:1885–93. doi: 10.1016/S0140-6736(05)67604-0. [DOI] [PubMed] [Google Scholar]
- 57.Tinetti ME, Kumar C. The patient who falls: it's always a trade-off. JAMA. 2010;3:258–66. doi: 10.1001/jama.2009.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23:1–16. doi: 10.1007/s00198-011-1787-7. [DOI] [PubMed] [Google Scholar]
- 59.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–42. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 60.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–62. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 61.Reid IR, Mason B, Horne A, Ames R, Reid HE, Bava U, et al. Randomized controlled trial of calcium in healthy older women. Am J Med. 2006;119:777–85. doi: 10.1016/j.amjmed.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 62.Tang BMP, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370:657–66. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 63.Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R. Vascular events in healthy older women receiving calcium supplementation: randomized controlled trial. BMJ. 2008;336:262–6. doi: 10.1136/bmj.39440.525752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Bolland MJ, Grey A, Gamble GD. Calcium and vitamin D supplements and health outcomes: a re-analysis of the Women's Health Initiative limited-access dataset. Am J Clin Nutr. 2011;94:1144–9. doi: 10.3945/ajcn.111.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study evaluates the cardiovascular risk of calcium supplementation. The optimal vitamin D intake in adult is debated.
- 65.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.American Society for Bone and Mineral Research American Society for Bone and Mineral Research. [Accessed Aug 26 2013];New recommendations for calcium and vitamin D intake: Institute of Medicine Report includes significant evidence on bone health. http://www.asbmr.org/about/pressreleases/detail.aspx?cid=4a1e3f7d-9193-4c11-a2ba-61edb4c31432.
- 67.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 68.Greenspan SL, Brufsky A, Lembersky BC, et al. Risedronate prevents bone loss in breast cancer survivors: a 2-year, randomized, double-blind, placebo-controlled clinical trial. J Clin Oncol. 2008;28:967–75. doi: 10.1200/JCO.2007.15.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hines SL, Mincey B, Dentchev T, et al. Immediate versus delayed zoledronic acid for prevention of bone loss in postmenopausal women with breast cancer starting letrozole after tamoxifen-N03CC. Breast Cancer Res Treat. 2009;117:603–9. doi: 10.1007/s10549-009-0332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Llombart A, Frassoldati A, Paija O, et al. American Society of Clinical Oncology 2009 Breast Cancer Symposium. San Francisco, CA: 2009. Zoledronic acid prevents aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: E-ZO-FAST 36-month follow-up (Abstract 213) [Google Scholar]
- 71.Eidtmann H, de Boer R, Bundred N, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol. 2010;21:2188–94. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 72.Markopoulos C, Tzoracoleftherakis E, Polychronis A, et al. Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: results from the ARBI prospective clinical trial. Breast Cancer Res. 2010;12:R24. doi: 10.1186/bcr2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lester JE, Dodwell D, Purohit OP, et al. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Cancer Res. 2008;14:6336–42. doi: 10.1158/1078-0432.CCR-07-5101. [DOI] [PubMed] [Google Scholar]
- 74.Confavreux CB, Fontana A, Guastalla JP, et al. Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. Prevention with bisphosphonates. Bone. 2007;41:346–52. doi: 10.1016/j.bone.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 75••.Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118:1192–201. doi: 10.1002/cncr.26313. [DOI] [PubMed] [Google Scholar]; This study gives us the 5-year data on the effects of zoledronic acid in preserving bone mass in postmenopausal women on an AI.
- 76.Van Poznak C, Hannon RA, Mackey JR, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol. 2010;28:967–75. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 77.Van Poznak C, Souter SP. Clinical management of osteoporosis in women with a history of breast carcinoma. Cancer. 2005;104:443–56. doi: 10.1002/cncr.21201. [DOI] [PubMed] [Google Scholar]
- 78.Eastell R, Walsh JS, Watts NB, Siris E. Bisphosphonates for postmenopausal osteoporosis. Bone. 2011;49:82–8. doi: 10.1016/j.bone.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 79.Taxel P, Choksi P, Van Poznak C. The management of osteoporosis in breast cancer survivors. Maturitas. 2012;73:275–9. doi: 10.1016/j.maturitas.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 81.Ellis GK, Bone HJ, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–82. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 82.Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–94. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 83. [Accessed Aug 26 2013];Prolia (denosumab) product information. http://pi.amgen.com/united_states/prolia/prolia_pi.pdf.
- 84.Medication Instructions, Zometa. http://www.pharma.us.novartis.com/product/pi/pdf/Zometa.pdf.
- 85.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 86.National Osteoporosis Foundation [Accessed Aug 26, 2013];Medicines that May Cause Bone Loss. Available at: http://www.nof.org/articles/6.
- 87.Snyder CF, Frick KD, Kantsiper ME, Peairs KS, Herbert RJ, Blackford AL, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: changes from 1998 to 2002. J Clin Oncol. 2009;27:1054–61. doi: 10.1200/JCO.2008.18.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–51. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 89.Lester JE, Dodwell D, Horsman JM, Mori S, Coleman RE. Current management of treatment-induced bone loss in women with breast cancer treated in the United Kingdom. Br J Cancer. 2006;94:30–5. doi: 10.1038/sj.bjc.6602892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coleman RE, Marshall H, Cameron D, et al. Breast cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365:1396. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 91.Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62–month follow-up from the ABCSG-12 randomized trial 631. Lancet Oncol. 2011;12:631–41. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 92.Paterson AH, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project) protocol B-34; a multi-center, placebo-controlled, randomized trial. Lancet Oncol. 2012;13:734–42. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mobus V, Diel IJ, Elling D, et al. GAIN Study: a phase III trial to compare ETC vs EC-TX and ibandronate vs observation in patients with node-positive primary breast cancer—1st interim efficacy analysis, Abstract S2–S4. CTRC-AACR San Antonio Breast Cancer Symposium. 2011 [Google Scholar]
- 94.Southwest Oncology Group [Accessed 10/8/2013]; NCT00127205 S0307 Zoledronate, clodronate, or ibandronate in treating women who have undergone surgery for stage I, stage II, or stage III breast cancer. http://clinicaltrials.gov/ct2/show/NCT00127205?term=NCT00127205&rank=1.
- 95. [Accessed 10/8/2013];Study of Denosumab as Adjuvant Treatment for Women With High Risk Early Breast Cancer Receiving Neoadjuvant or Adjuvant Therapy (D-CARE) NCT01077154. http://clinicaltrials.gov/ct2/show/NCT01077154?term=NCT01077154.&rank=1.
- 96. [Accessed 10/8/2013];Efficacy and safety of odanocatinib in postmenopausal women previously treated with oral bisophosphonate NCT01803607. 2013 May; http://clinicaltrials.gov/ct2/show/NCT01803607?term=NCT01803607&rank=1.
- 97. [Accessed 10/8/2013];Vitamin D and Physical Activity on Bone Health NCT01419730. http://clinicaltrials.gov/ct2/show/NCT01419730?term=NCT01419730&rank=1.
- 98. [Accessed 10/8/2013];Cholecalciferol in Improving Survival in Patients With Newly Diagnosed Cancer With Vitamin D Insufficiency NCT01787409. http://clinicaltrials.gov/ct2/show/NCT01787409?term=NCT01787409.&rank=1.
- 99.Braun S, Pantel K, Muller P, Janni W, Hepp F, Kentenich CRM, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000;342:525–33. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 100.Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, et al. Circulating tumor cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13:688–95. doi: 10.1016/S1470-2045(12)70209-7. [DOI] [PubMed] [Google Scholar]
- 101.Wiedswang G, Borgen E, Kåresen R, Qvist H, Janbu J, Kvalheim G, et al. Isolated tumor cells in bone marrow three years after diagnosis in disease-free breast cancer patients predict unfavorable clinical outcome. Clin Cancer Res. 2004;10:5342–8. doi: 10.1158/1078-0432.CCR-04-0245. [DOI] [PubMed] [Google Scholar]
- 102.Janni W, Vogl FD, Wiedswang G, et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse—a European pooled analysis. Clin Cancer Res. 2011;17:2967–76. doi: 10.1158/1078-0432.CCR-10-2515. [DOI] [PubMed] [Google Scholar]