1 Current Knowledge about the Pathogen
In 1937 in Queensland Australia, Derrick described a febrile disease, which he called ‘query fever’, in 20 of 800 workers of a meat factory in Brisbane [1]. He used the word ‘query’ because of the inexplicable nature of the disease. The pathogenic agent was isolated from the blood and urine of the patients in Australia by Burnet and Freeman and was called Rickettsia (R. burnetii) [2]. At the same time, the pathogen was isolated from ticks in Montana, USA, by Davis and Cox [3], and called R. diaporica, and, later on, renamed to Coxiella burnetii to honor both research groups.
The World Health Organization (WHO), the United Nations, and the Australian Group have classified C. burnetii as dangerous pathogenic agent [4]. The pathogenic agent was also an integral part of the biological arms program of the USA and the former Soviet Union, and is classified as Category B of pathogens with a potential for biological weapons by the Centers for Disease Control and Prevention (CDC) [5].
C. burnetii has a worldwide geographical distribution and can be transmitted to humans as zoonotic pathogenic agent [6]. According to the current state of knowledge, sheep, goats, and cattle are the main source of infection for man. In addition, cats, dogs, rabbits, wild animals, and ducks, as well as ticks and the feces of ticks, have been identified as sources of infection. The pathogen causes acute and chronic infections and is transmitted by contact or inhalation of dust aerosols and droplets, but can also be transmitted by consumption of raw milk and raw milk products [7,8].
More than 30 genotypes of C. burnetii have been described, which can be distinguished by genome analysis [9]. Many of the genotypes have a worldwide geographical distribution, others are limited to a particular region. It has been established by means of pulsed field gel electrophoresis that some European and North American strains differ from each other [10], but that there are also strains which can be differentiated into regional groups such as German and Russian. The length of the genome of the bacterium is approximately 5 million base pairs (bp) [11].
1.1 Characteristics of the Pathogen
1.1.1 Structure
C. burnetii is a member of the family of the Coxiellaceae bacteria and replicates intracellularly in cells of different species. Phylogenetically related bacteria include Legionellaceae, Francisellaceae, Pseudomonaceae, and other Gammaproteobacteria. Coxiella are small Gram-negative, pleomorphic, coccoid bacteria with a size of 0.2-1.0 μm. They occur in 3 different forms: small cells (small cell variant, SCV) which are highly infectious, large cells (large cell variant, LCV) which develop also in cell culture, as well as spore-like particles (SLP) which are infectious and very robust to environmental conditions. Dependent on the host system, Coxiella undergoes a phase variation during growth [12]. In mammalian cells, bacteria grow as LCV, and form spore-like particles and 2 different antigenic forms described as Phase I and II.
Phase I
When C. burnetii replicates in cells of immunocompetent hosts, bacterial lipopolysaccharide (LPS) is synthesized in its full length, and additionally the cell wall antigens. C. burnetii is incorporated passively by the cells through phagocytosis and survives in the phagolysosome, also called parasitophorous vacuole, only at a low pH level, which is vital for the metabolic activity of the bacterium. Phase I Coxiella is extremely contagious for humans. Between 1 and 10 Coxiella form 1 human infectious dose (HID) and are sufficient for transmission of the infection [13]. The LPS is an essential virulence factor (cf. Section 1.2.). LPS is the main component on the surface of the external wall of the bacterium and covers the proteins. LPS thus blocks a rapid reaction of the immune system against the proteins of the bacterial wall. Coxiella LPS of the smooth type entails poor binding and activation of the components of the complement system, thus delaying or preventing lysis of bacterial cells [14]. The historical serologic distinction between the different Coxiella strains is based in the reactivity of LPS.
Phase II
When C. burnetii is grown in culture cells or in cells of a non-immunocompetent host system (e.g. embryonated chicken eggs), LPS is usually only incompletely synthesized by repression of the genes for LPS synthesis (raw type LPS of Coxiella); additionally the production of some cell wall antigens is suppressed. Many cell wall proteins thus remain easily accessible for the reaction of the immune system and characterization of Coxiella and for diagnostic purposes. Phase II Coxiella show low virulence in animal models and are considered as of little virulence for humans, since they are rapidly inactivated via the complement system. An intermediate form occurs with only incompletely synthesized LPS. Phase I and II forms cannot be distinguished morphologically, but they present different colors when stained using alkaline fuchsin and hematoxylin.
Spore-Like Particles
The term ‘spore’ derives from environmental resistance. SLP was adopted from the term by morphologic similarities in electron microscopy with endospores of other bacteria species, and is purely descriptive. The environmental resistance of Coxiella is not comparable with that of bacillus or clostridium spores. By the formation of SLPs, C. burnetii is able to remain infectious for more than 40 months even under very unfavorable external conditions. An overview of the different forms of C. burnetii can be found in the papers by Coleman et al. [15] and Angelakis und Raoult [11].
Plasmids
Four different plasmid types or chromosomally integrated plasmid-homologous sequences with a length of 36-56 kilo bp can be found in Coxiella of all phases [16]. Each bacterial cell has only 1 of the 4 plasmids, which contains around 2% of the genetic information [11,17].
Genotypes/Genogroups
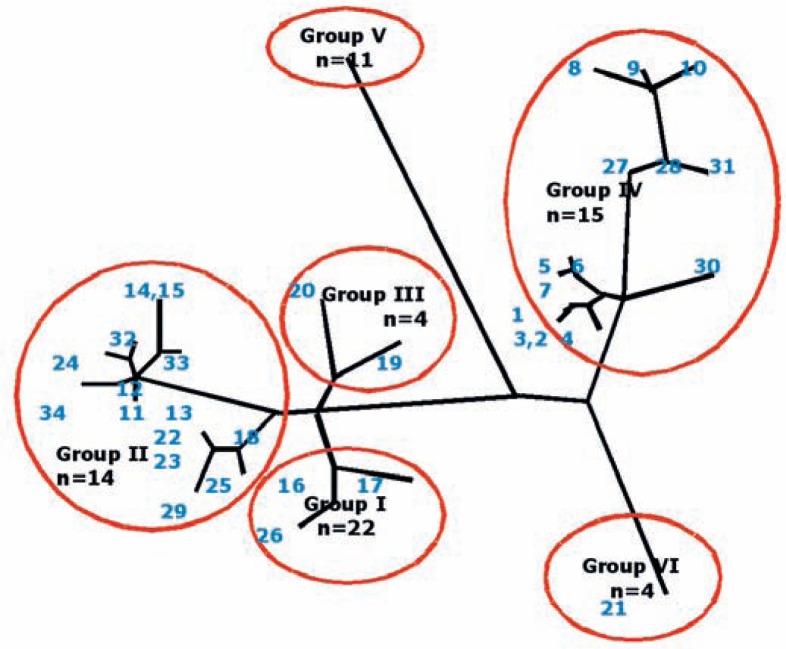
Based on the nucleic acid sequence analysis of different gene regions including the plasmids, a classification into genotypes or, if several genotypes were grouped together, genogroups, has been suggested by various authors. Individual genotypes display only partial agreement, but this agreement is sufficient to characterize outbreaks of disease – as occurred in England and the Netherlands – under molecular epidemiological aspects. Based on 10 selected regions, 173 Coxiella isolates from France were classified into 30 MST genotypes using the multispacer sequence typing (MST) method [9]. During the Q fever outbreak in the Netherlands, Coxiella isolates from Europe and other continents were classified into genotypes starting with the letters A to Q using ‘multiple-locus variable tandem repeat analysis’ (MLVA). I and J represented the dominant genotypes in cow's milk and milk products [18]. I and J corresponded to the genotype MST-20 by Glazunova et al., 2005 [9] and were found in all goats, while earlier outbreaks in France and Germany were caused by MST-33 [19]. The latest outbreak in England was also caused by MST-20 [20]. Another study in the Netherlands which used the MVLA method found the A genotype as the predominant strain in 38 of 45 selected goat farms [21]. Finally, the MST grouping by Glazunova et al. [9] was extended by single nucleotide polymorphism (SNP) analysis by means of real-time polymerase chain reaction (PCR) and melting point analysis considering insertions and deletions by bi-allelic and tri-allelic SNP [22]. By statistical analysis and creation of a phylogenetic tree, the Coxiella isolates could be classified into genogroups I-VI with individual groups containing between 1 and 15 MST (fig. 1). Part of the diversity was regarded as being due to mutations occurring in co-circulating Coxiella strains. MST genotypes 14 and 15 by Glazunova et al. [9] were found to be identical in the genome analysis by Hornstra et al. [22]. Most of the MST genotypes have a worldwide geographical distribution. MST 8 causes chronic infections in humans, and frequently endocarditis. Goats act as the reservoir [22]. Pathogenicity and virulence vary between the different genogroups [23].
Fig. 1.
The 6 genogroups of C. burnetii and the MST genotypes assigned to these groups. Genogroup VI contains the so-called Dugway strain. The phylogenetic tree shows the close relationship of individual MST genotypes which have partly developed by convergent evolution. In this analysis, MST 14 and 15 are identical in Group II. Graphic modelled on Hornstra et al., 2011 [22] (L. Guertler).
1.1.2 Replication
In the infected organism, C. burnetii always replicates intracellularly, an extracellular replication in mammals has so far not been described. Host cells are cells of poikilothermic animals such as arthropods and fish, and cells of homeotherms such as birds, rodents, marsupials, and, in particular, domestic and livestock animals [24]. In placental cells of sheep, a pathogen number of 109/g placental tissue can be reached [7]. C. burnetii can survive in fresh water amoeba for several weeks [25]. The doubling period in mammalian cells is 20-45 h [11].
After transmission, C. burnetii is phagocytosed by monocytes and macrophages in humans, and transported into the phagolysosome. There, a low pH level is created by Coxiella, thus preventing the decomposition of the bacterium; subsequently the replication process is started. Besides the normal bacterium, the SLP is generated with a thickened wall which is metabolically inactive [12] and is thus not affected by antibiotic treatment (cf. Section 3.4.).
C. burnetii intracellularly suppresses the production of oxygen derivatives and NO, synthesis of interferon gamma, and the formation of suppressor T-lymphocytes. The migration of immune defense cells to the replication sites during the acute phase causes the granulomas that are typical for the chronic disease [26] (cf. Section 1.2.2.).
In the past few years, studies have been performed on the replication of C. burnetii in cell-free cultures. The pathogenic agent was considered as prototype of a bacterium with the requirement of intracellular growth in eukaryotic cells. In infected cells, C. burnetii replicates in the acidic environment of the parasitophorous vacuole. More recent studies show that C. burnetii can replicate in special media in cell-free cultures [27,28]. This observation opens new pathways for a targeted investigation of pathogenicity mechanisms and the development of therapeutic intervention strategies.
1.1.3. Susceptibility to Inactivation and Stability under Environmental Conditions
C. burnetii SCV is very stable to environmental conditions and can remain infectious for many months [29] (cf. Section 1.1.1.). Phase I and II forms are destroyed by 2% formaldehyde, however, infection-competent C. burnetii could be extracted after 4-5 months from formalin-fixed tissue. Additionally, replication-competent C. burnetii was claimed to be isolated from paraffin-treated tissue. Likewise, the sterilization process can be inefficient using gaseous formaldehyde [29]. Effective inactivation of Coxiella is achieved by 1% phenol, 5% hydrogen peroxide, 5% chloroform, 0.5% hypochlorite, and exposure to heat at 65 °C and above for 1 h [30,31]. Irradiation with 10 kGy gamma rays inactivates Coxiella completely, while its antigenicity is preserved [32]. Disinfection is possible using 70% ethanol for 20 min. Autoclaving at 131 °C for 15 min inactivates C. burnetii; as well treatment using 5% formaldehyde for 5 min. The bacterium survives as spore stage (SLP) on wool of sheep for 7-10 months at 15-20 °C, for more than 1 month at 4 °C on fresh meat, and for more than 40 months in dry milk powder at room temperature [33,34].
1.2 Infection and Infectious Disease
As mentioned above, C. burnetii is extremely infectious for humans, 1-10 viable organisms suffice to induce an infection via the aerogenic route [35]. In hospitalized patients in France, the mortality rate was 2.4% [31].
Coxiella LPS is not toxic for hen embryos, even in very high doses [36]. Infected animals such as sheep and goats show no signs of disease [37]. Pregnant domestic animals may abort following an infection with C. burnetii[38]. In humans, distinctions are made between acute and chronic Q fever, and depending on the symptoms and clinical signs, between pneumonia, endocarditis, hepatitis, and neurological manifestations.
1.2.1 Acute Disease
At least 50% of the human infections with C. burnetii are asymptomatic. After an incubation period of 3-30 days, un-specific symptoms such as fever, sweating, nausea, vomiting, and diarrhea occur, and extreme fatigue in 5-20% of the patients. Frequently headache may occur which does not improve after administration of analgesics. The bacterium can be present in the blood during this acute stage. The disease is often self-limiting [39]. In endemic regions such as southern Spain, the Basque region and France, fever lasting more than 1 and less than 3 weeks is typical for Q fever [40].
Pneumonia occurs in 1-2% of acutely affected patients. The most frequent form of the disease is lobar pneumonia with high fever. Furthermore, the disease manifests itself as atypical pneumonia and rapidly progressing pneumonia. This form of pneumonia often occurs in connection with sharply delimited lung infiltrates on X-ray and consolidation of the alveolar cavity. Infiltrates can be present segmentally and non-segmentally. The rapidly progressing pneumonia clinically resembles the pneumonia caused by Legionella pneumophila. Atelectasis and swelling of hilar lymph nodes can occur. X-ray shows the resulting granulomas as multiple round opacities. Acute pneumonia may progress to the chronic form [41]. 5% of affected individuals have concurrent splenomegaly, and, during this period, C. burnetii can also be isolated from the cerebrospinal fluid [42].
1.2.2 Chronic Disease
Around 5-15% of acute cases become chronic. Manifestations include endocarditis, vasculitis, osteomyelitis, hepatitis, interstitial lung fibrosis, and long-lasting or recurring fever. An analysis of the > 4,000 cases of Q fever in the Netherlands showed the following risk factors for chronic course: preliminary cardiac valve surgery, blood vessel prosthesis implants, aneurysm, renal insufficiency, and advanced age [43].
Endocarditis
The entire vascular system can be affected, above all, artificial valves and the walls of aneurysms, on which C. burnetii adheres as biofilm. Endocarditis is the major manifestation of Q fever and comprises around 10% of all cases of endocarditis in England and Wales [44]. Endocarditis patients frequently present with splenomegaly; arterial embolism can also occur. Endocarditis is associated with anemia and hematuria. Endocarditis caused by Coxiella very rarely occurs in children. In 53% of the cases of endocarditis caused by C. burnetii, the bacterium can be detected in blood. The bacterial load rapidly decreases if doxycycline and chloroquine are administered. If antibiotic treatment is transiently interrupted, Coxiella resistant strains are selected within 3 years and lead to relapses of endocarditis [45,46]. Coxiella survives for years in granulomas which are formed in various organs, not only restricted to heart and cardiac valves tissues [46].
Hepatitis
While hepatitis caused by C. burnetii is very rare in the USA and in England, it is the most frequent manifestation of chronic C. burnetii infection in France and Spain in regions of intensive sheep breeding [31]. Consumption of raw milk and cheese from raw milk seems to be associated with hepatitis development. Hepatitis can be confirmed clinically and by laboratory tests (e.g. elevation of alanine transaminase) with formation of granulomas in the liver biopsy sample histology. The granuloma has a dense fibrin ring [47]. Hepatitis can be an additional manifestation found in patients with Q fever pneumonia, or Coxiella may damage only the liver. Hepatitis caused by Coxiella with elevation of transaminases may present without an increase in inflammatory parameters such as C-reactive protein [48].
Neurological Manifestation
Symptoms caused by Q fever include severe headache, aseptic meningitis, and encephalitis. In the cerebrospinal fluid partly mononuclear cells are found; the protein content is increased, and C. burnetii may be isolated. Meningism, reduced vision, paresthesia, and impaired sensory abilities can occur. Guillain-Barré syndrome caused by C. burnetii has been described in the literature [7]. In the eye, Coxiella can cause uveitis and retinal detachment [49]. 20-30% of affected individuals complain of chronic fatigue syndrome as post-fever manifestation. During this period, Coxiella can be identified partially, or periodically, via the DNA-NAT (nucleic acid amplification test) in blood and bone marrow [13,50,51].
Infection in Domestic Animals
Infections with C. burnetii in domestic animals are usually asymptomatic. During the acute phase, the pathogen can be detected in blood and in organs such as lung, liver, and spleen. In the chronic phase, C. burnetii is excreted in the feces and urine but also in the milk. Pregnant animals may suffer miscarriages (sheep and goat) and produce offspring with low birth weight (cattle). High amounts of Coxiella can be found in birth products such as the placenta and in the newborn animal [52,53].
1.3 Epidemiology
Q fever caused by C. burnetii is a zoonotic worldwide distributed infectious disease. In Europe, it reaches its seasonal peak in spring and early summer [54]. Ticks infected with C. burnetii seem to play an essential role in maintaining the transmission cycle in the natural environment of wild-type animal species. Ticks excrete high quantities of infectious pathogens in the feces, thus being able to spread Coxiella. During the outbreaks in livestock, the close contact among the animals seems to play the most essential role in the transmission of the pathogenic agent [11].
Direct and indirect detection of infection with C. burnetii is notifiable giving the name of the infected person pursuant to the German Infection Protection Act (Infektionsschutz-Gesetz (IfSG) Section 7, (9)). The annual incidence in Germany is around 1-5 cases per 106 inhabitants. In the period from 2000 to 2011, between 200 and 500 infections were reported to the Robert Koch-Institute annually. The majority of the infections occurred regionally in clusters and was related to farming or having contact with sheep [55], particularly during lamming [54]. A major outbreak of Q fever could be related to lamming on a farm market at which 300 persons developed the disease [56].
During the epidemic in the Netherlands of 2007-2009, C. burnetii was above all transmitted by goats and goat dung [57]. At the center of the epidemic (Hertogenbosch), 10.7% (84 of 785) of the persons tested in the screening program had Phase II IgG antibodies, resulting in an estimate of around 40,600 exposed persons for the entire region [58]. In Europe, outbreaks of Q fever in sheep herds occurred in the German Federal states of Baden-Württemberg [59], Bavaria [60] and Thuringia [61], as well as in France [62], Cheltenham in England [63], and Bosnia [64]. C. burnetii infected individuals were also identified among soldiers returning from Kuwait [65], Iraq [66], and Afghanistan [67].
Animals infected by C. burnetii endemically without any signs of disease include ruminants such as sheep, goats and cattle, but also dogs, rabbits, cats and birds [11] (cf. Section 1.). The reservoir for renewed infestation of endemic areas after culling potentially infected livestock (goats and cattle) and sterilization of contaminated manure in the Netherlands was most probably rats of the Rattus norvegicus and Rattus rattus species, in which a prevalence of Coxiella DNA in the spleen of 3-5% and an antibody prevalence in the serum of 14-50% was found [68].
Transmission Routes of C. burnetii
Aerogenic: inhalation of contaminated aerosol or dust [63,65,69,70,71,72]. This is the main transmission pathway for humans [73]. The risk of Coxiella transmission by dust persists even years after the sheep herds have left the region [74].
Contact: Human to human, animal to human, tick feces to human; direct and indirect contact with contaminated tissue, such as sheep placenta or goat manure [75]. Tick feces can be highly infectious, since one gram of tick feces contains around 109 Coxiella. After lamming, infectious Coxiella can be detected in the soil for up to 150 days [33]. C. burnetii can be transmitted by exposure to contaminated straw, enclosures, or dust [76,77], but also by contact with contaminated laundry [78]. C. burnetii can also be transmitted to humans by pets such as rabbits and cats. In sheep, Coxiella infection can be reactivated during pregnancy.
Oral: Consumption of contaminated milk or milk products [71,79].
Transcutaneous/percutaneous: Transmission of C. burnetii by arthropods (especially by ticks of species such as Ixodes, Dermatocenter, Hyalomna) between domestic animals has been reported. Transmission of C. burnetii to humans by ticks, however, seems to be a rare event [80]. In the Netherlands and Southern Germany, the prevalence of infected ticks in endemic areas was low [81,82].
Sexual: During the early phase of infection, transmission between man and woman may occur, as was reported from Australia [83], and from a soldier of the US Army returning from Iraq, who transmitted the infection to his wife [84]. C. burnetii has been detected in bull ejaculate [85], which provides proof of the penetration of this bacterium into the sexual compartments of mammals.
Cofactors Influencing Progression to a Prolonged or Chronic Course of Infection
Depending on the entry of infection, the primary manifestation of the disease can be pneumonia, hepatitis, or endocarditis (cf. Section 1.2.2.). C. burnetii is prevalent more frequently and causes chronic infections more often in immuno-suppressed individuals [11,75].
In London, pregnant women have been examined after frequent miscarriages. A Coxiella prevalence of 4.6% was found in these women, whereas a prevalence of 0.15-2% was found in the control population [86]. In an endemic region in Canada, a seroprevalence for C. burnetii of 3.8% was found in pregnant women [87]. The bacterium was detected in breast milk and post-partum placenta [34,88]. Possibly C. burnetii may also be reactivated in humans during pregnancy.
In children, the infection is normally asymptomatic, chronic courses have not been described [11]. Complications more frequently occur at an advanced age. Men are affected twice as often as women [89].
Direct non-sexual transmission between humans has so far not been established [90]. However, a possible transmission between humans who are members of the same household has been discussed [91]. C. burnetii has been transmitted during autopsy. Only 1 transmission by blood transfusion is known [92,93,94].
Laboratory infections with C. burnetii have occurred repeatedly [30,95], therefore safety level 3 conditions are required for the replication of Coxiella. Laboratory infections with C. burnetii rank 4th of all lab infections in England. In conformity with the current regulations governing biological materials, Coxiella is classified as biosafety level 3.
Cattle, sheep, and goats are regarded as the main source of infection with C. burnetii for humans. In the past few decades, studies have been conducted on the distribution of C. burnetii infections in livestock. Guatteo et al. [96] reported a prevalence of C. burnetii infections in dairy farm animals of 20-38% in cows and 15-25% in sheep and goats. These studies were typically conducted in restricted regions only and on a relatively small number of animals, so that the figures provide only a rough estimate of the prevalence in the various regions. For Germany, more recent studies are available from the federal states of Saxony and Thuringia, which show that antibodies against Coxiella can be detected in some herds [61,97].
1.4 Detection Methods
1.4.1 Serological Detection Methods
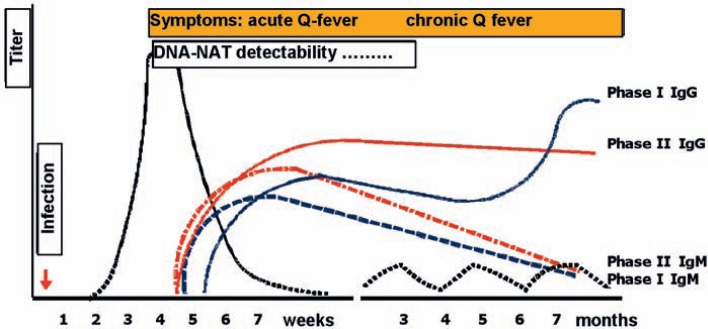
Antibodies: Besides IgM and IgG, also IgA antibodies against C. burnetii can be detected. Antibodies become detectable 2-3 weeks after occurrence of clinical symptoms. They then usually increase for some months and persist for years (fig. 2). According to some authors [98], the cross-reactivity of C. burnetii antibodies with other bacteria is low, however, it cannot be ruled out with some antigens of legionella, bartonella, and pseudomonas [99,100,101]. High antibody titers against Phase I, which barely decrease over a period of 600 days, point to a chronic course [45,102,103]. IgM Phase II is still detectable 12 months after transmission of Coxiella in 62-83% of the blood samples [104].
Fig. 2.
Temporal course of C. burnetii infection and serological response, detectable through Phase I and II antibodies. In the chronic course, DNA can be detected periodically years after acute infection, and antibody titers remain high. Diagram based on Van Wijk et al., 2011 [134] (L. Guertler).
Indirect immunofluorescence assay: The micro-immunofluorescence assay is considered as the reference standard. Its set-up was described as early as 1983 [105]. The assay becomes positive around 1-2 weeks after appearance of the symptoms, additionally IgM antibodies can be detected using this method [106]. Typically, titers of ≥ 1:800 are considered positive for IgG for chronic Q fever [107], and titers of ≥ 1:50 for IgM and ≥ 1:200 for IgG [108]. The evaluation should be performed according to the titer values provided by the manufacturer of the test. As usual, seroconversion or titer levels that increase 4-fold are regarded as positive for the detection of an acute or reactivated infection.
ELISA, agglutination test, and complement-fixation assay (CFA): All 3 tests have been developed for routine diagnostics, CFA was most frequently used for early prevalence studies [109]. CFA is regarded as positive if titers against Phase I are > 1:200 during chronic Q fever. Antibodies against Phase II antigens are considered as positive at ≥ 1:40 during acute Q fever. Because of the high workload and the non-detectability of IgG2, IgG4 and IgA, as well as lacking standardization, CFA is nowadays replaced by ELISA or micro-immunofluorescence tests. Only a few manufacturers offer ELISA for the detection of C. burnetii antibodies. Phase I and II, and IgM and IgG are determined in separate assays. ELISA was used for the identification of infected blood donors in the Netherlands, and reactive samples were further analyzed by PCR [102,104].
Western blot: 15 antigens are present on the strip for C. burnetii grown in Phase 1, with a molecular weight of 20-160 kD. 7-10 different antigens may be stained with sera from the acute infection, especially the bands with 50, 80 and 160 kD; while 12-15 antigens may be stained with sera from the chronic phase [110,111].
If the C. burnetii infection is treated with antibiotics, a slow decrease in antibody titers takes place. IgM antibodies can be detectable over a period of 670 days [112]. In another study, IgM antibodies could still be detected in 3% of 162 individuals after 1 year. [113]. IgA antibodies are detectable in higher titers in chronically C. burnetii-infected individuals [106].
1.4.2 Direct Detection of Coxiella burnetii1.4.2.1 Culture
The detection of C. burnetii in cell culture is performed on L929 fibroblasts or other cells such as vero cells [27,114,115]. For analysis, samples are extracted after filtration using 0.45 µm filters. Suitable for analysis are extracts from tissue samples and blood before the initiation of antibiotic treatment [116]. Culturing C. burnetii must be performed under safety level 3 conditions to avoid the risk of laboratory contamination [95]. More recent studies show that Coxiella can be cultured in acidified citrate cysteine (ACC) medium as Phase II Coxiella after an incubation period of around 6 days [27,117]. The bacteria grew by up to 3 log10 and were infectious [27]. Since only few laboratories have an authorization for handling replication-competent biosafety level 3 pathogens, detection and quantification of Coxiella, if required, is usually performed by PCR. If SCID mice (combined immunodeficiency disease) are inoculated with Coxiella-contaminated material, 1-10 bacteria can be detected [115,117].
1.4.2.2. NAT – Nucleic Acid Amplification Test
Conventional PCR, nested PCR, or real-time PCR are used for the detection of C. burnetii nucleic acid [118]. Primers used are derived from the superoxide dismutase gene [119], plasmid or other gene segments [9,120]. In addition, Coxiella DNA can be successfully detected in paraffin-embedded tissue. Real-time PCR with a sensitivity of 1 copy DNA has been developed [121]. Coxiella can be detected in the blood by PCR 2 weeks after transmission [104]. Further frequently used primer-binding sites for PCR include genome regions with repetitive elements and the outer membrane protein OMP [122,123,124]. Reliable PCR detection methods for Coxiella have been used for genotyping (see Section 1.1.) [9,18,22]. A non-symmetrical PCR with hybridizing and analysis of the products in solution, which simultaneously detects further bacteria, has been developed in the Netherlands [125]. The most extensive characterization of Coxiella strains was performed by amplification and sequencing of 139 chromosomal and plasmidic open reading frames (ORFs), including the genes for LPS biosynthesis [120]. This method was used for characterization of the strains of the outbreak in the Netherlands [20] and the strains in Spain [126].
2 Blood and Plasma Donors
2.1 Prevalence and Incidence in Donor Collectives
Up to now, no transfusion-associated C. burnetii transmission has been reported in Germany. Table 1 lists the prevalences of Coxiella antibody in blood donors of several countries [14]. Blood donations in Germany are currently not tested for the presence of C. burnetii, or antibodies against C. burnetii. C. burnetii is present also in Germany in areas with a high density of sheep. How efficient infected donors are excluded from donating blood based on febrile symptoms and the general fatigue associated with the infection depends on the symptoms present at the time of donation, the recording of anamnesis, and on whether or not local outbreaks become known in time. 60% of primary infections in adults show an asymptomatic course [11]; according to data from the Netherlands [127], the bacteremia which occurs during this phase is in the range of a few per mille [128]. Generally it should be assumed that asymptomatic individuals will donate blood, partially during the acute, and partially during the chronic phase of the disease [93]. Since C. burnetii grows in macrophages and monocytes, the bacterium can rapidly be spread by the bloodstream of the body in these cells. In persons with chronic infestation of the blood vessel walls, it should be expected that C. burnetii is temporarily released. In persons with acute infection, C. burnetii is spread via the blood and lymph system so that the bacterium can be detected in cerebrospinal fluid and urine [132]. The detectability of Coxiella in various body fluids supports the data that C. burnetii can cause temporary bacteremia.
Table 1.
Seroprevalence of C. burnetii among blood donors
| Country | Year | Seroprevalence, % | Author [ref.] |
|---|---|---|---|
| The Netherlands | 1987 | 45 | Richardus et al. [127] |
| France | 1992 | 4 | Tissot-Dupont et al. [31] |
| Newfoundland | 2001 | 8 | Hatchette et al. [129] |
| Germany | 2001 | 15–22 | Hellenbrand et al. [54] |
| Spain | 2007 | 23 (IgG), 0.3 (IgM) | Bartolomé et al. [131] |
| Turkey | 2008 | 32 (IgG), 3 (IgM) | Kilic et al. [130] |
| The Netherlands | 2012 | 12 (IgG) | Hogema et al.a [128] |
False-positive reactions in the test collectives cannot be ruled out.
2.2 Definition of Exclusion Criteria
General exclusion criteria, which might indicate an acute or chronic infection, apply to the deferral of blood donors possibly infected with C. burnetii, such as elevated body temperature, weakness, fatigue, diarrhea within 1 week before the donation, and anemia. Concerning regional outbreaks, known risk factors include contact with sheep, proximity and duration of stay at a potential transmission site, as well as exposure to contaminated animal material and dust within a radius of 5 km (cf. Section 1.3). Also short episodes of fever after contact with a Q fever sufferer are a possible exclusion criterion. The final deferral from the donation is decided dependent on the epidemiological situation and the individual risk assessment by the doctor. When asymptomatic individuals might have been exposed to C. burnetii, a lag period of 4 weeks should pass before the next donation. If a Q fever infection had happened that was cured with or without treatment with doxycycline plus chloroquine, the donor can be readmitted to donation 4 months after the symptoms have disappeared [133]. If blood was donated during the incubation period of C. burnetii, the blood components prepared from that donation must be recalled according to the known risk situation; these blood components should not be used for transfusion (exceptions, see Section 3.6). If the blood product was already transfused, treatment of the recipient with doxycycline plus chloroquine or fluoroquinolone should be considered depending on the clinical symptoms of the recipient. Further follow-up activities analogous to the look-back procedure for viral infections (Vote 34 of the German Advisory Committee ‘Blood’ (Arbeitskreis Blut)) are not required.
2.3 Donor Testing and Significance
2.3.. Detection of Antibodies
Detection of C. burnetii antibodies in blood donors is not performed; when needed for technical reasons it is possible only by using ELISA [128]. Routine antibody testing is not considered necessary based on the current epidemiological data in Germany. The detection of Coxiella antibody in general indicates immunity, not infectivity. However, increased IgM and IgG titers were found in 38 of 942 blood donors tested in the South of France; 2 of these 38 donors (total 2/942, 0.2%) developed acute Q fever which was undetectable at the time of the donation [31].
2.3.2 Detection of Coxiella burnetii
The very time-consuming culture under biosafety level 3 conditions in the shell-vial test (e.g. 48-well microtiter plates) is unsuitable as a screening test. Detection via DNA as NAT, e.g. as PCR, is not routinely performed and, considering the current epidemiological situation, not necessary. In blood donors in the Netherlands, 1,004 donors in the area with the highest C. burnetii incidence were tested using PCR, and 3 (0.3%) were identified as being genome-positive [134]. The strategy shows that NAT can be introduced if required. It is unclear to what extent PCR-positive onors can infect recipients, since up to now only 1 Coxiella transmission by blood transfusion has been reported worldwide [93]. In human blood, leucocyte-depleted blood, red blood cell concentrate, and plasma spiked with C. burnetii the pathogenic agent remained infective for more than 6 weeks [135]. The bacterium could only be removed from rat serum spiked with C. burnetii by filtration with 0.1 µm filters, while heat treatment at 56 °C for 30 min was ineffective [136]. For Germany, there is a need for research to elucidate the importance of exposure to bacteremia, the type and duration of infectious chains, and analysis of the circulation of various Coxiella strains.
2.4 Donor Interview
Interviewing donors specifically concerning the possible risk of acquiring C. burnetii infection seems to be indicated only if a Q fever outbreak has been announced. Usually animals infected with C. burnetii show no symptoms of the disease, however, may excrete the pathogen in feces, urine, vaginal secretion, and milk over longer periods, so that a donor interview regarding contact with domestic animals or livestock is indicated only when an animal infection with C. burnetii has been confirmed. Clinical symptoms of acute Coxiella infection, e.g. flu-like symptoms, or of a chronic infection, e.g. relapsing endocarditis, are covered in the general donor interview, and individuals with a suspected infection are excluded from blood donation.
2.5 Donor Information and Counselling
Information and counselling specifically focused on C. burnetii will become necessary at a regional level if an outbreak has become known. The risk of transmitting an infection is imminent predominantly at farm markets during lamming, at animal fairs, after close contact with manure, during and after passing by sheep herds, after inhaling contaminated dust, and during processing natural wool. Since transmission of C. burnetii can occur by dust and wind, the regional influence of weather conditions must also be considered when assessing the infection risk of donors during local outbreaks [63,65,73]. Q fever can also be acquired by travelling to endemic countries as was shown in the case of a traveler returning from Australia to the USA and 5 travelers returning from Africa, South America, and the Philippines to Spain [137,138]. When needed, donor counselling and interviewing is carried out focusing on the specific symptoms of Q fever (as described in Section 1.2).
3 Recipients
3.1 Prevalence and Incidence of Blood-Associated Infection and Infectious Disease in Recipient Populations
Few data are available on assessing the Coxiella prevalence and incidence of recipients in Germany. It should be assumed that immunity differs depending on the region. For rural areas, Coxiella antibody prevalences of up to 7% were reported from France [101], 11-17% from Switzerland [69], and 4% among non-exposed and 15% among exposed individuals in England [139]. Around 12% of the population of Montana, USA, have C. burnetii antibodies [140]. A Coxiella antibody prevalence of 38% (162/424) was found among veterinarians in Germany [60].
3.2 Immune Status (Resistance, Existing Immunity, Immune Response, Age, Exogenous Factors)
A natural resistance against C. burnetii does not exist in humans. A small number of bacteria can be inactivated by complement lysis. Immunity is built up after recovery from acute infection; however, some infections proceed to assume a chronic course as is evident in patients with endocarditis who partially develop insufficient immunity and thus persistent infection (see Section 1.2.2.). An immune response can be detected using specific antibody tests 2-3 weeks after infection (see Section 1.4.1.). At a more advanced age, the proportion of occurring complications following acute C. burnetii infection is increased. Factors that aid chronic C. burnetii infection include immune deficiency, e.g. caused by infections such as HIV [101] or immunosuppressive treatment. Chronic C. burnetii infections are found at a higher rate in immunocompromised individuals [100].
3.2.1 Vaccination
A vaccine against C. burnetii has been produced by growing of the bacterium on hen's egg yolk sac, enrichment, and subsequent formalin inactivation [141]. However, up to 1994, this vaccine was not used extensively in both risk groups with contact to sheep and in sheep livestock, even though none of the vaccinated individuals in Australia developed Q fever [142]. An additional vaccination program was developed successfully in Australia in 2008, designed to reduce the risk of Q fever infection in persons with an infection risk such as workers in abattoirs or sheep farms [143]. In the Netherlands, health authorities also recommended vaccinating individuals at risk of severe illness following C. burnetii infection [57,144]. It remains unclear whether vaccination against Q fever in Germany is useful for individuals at risk of developing a severe C. burnetii infection or at high transmission risk. The answer to this question also depends on the future epidemiology of C. burnetii.
A commercial inactivated Coxiella Phase I vaccine manufactured in Hungary has been available for immunizing animals since 2010. Used in sheep, the vaccination initially did not prevent infection but lead to a reduction in the excretion of Coxiella by 92-98% [37]. The vaccination was most effective if administered during an animal's first pregnancy [57]. After vaccination, Coxiella DNA of the vaccine was found in the milk of vaccinated goats within a few hours to 9 days [79]. 2 years after vaccination, the sheep and their offspring no longer excreted Coxiella, however, even after 4 years, Coxiella was still detectable in the animals' environment [145], which underlines the significance of animal reservoirs such as rats [68]. It is yet unclear, according to current knowledge, to what extent vaccinating livestock such as cattle, sheep, and goats influences the epidemiology of C. burnetii[146]. Although vaccinating sheep and goat herds was introduced in the Netherlands during the C. burnetii epidemic in 2009, the extent to which this measure has contributed to reducing the incidence of the disease remains to be seen [144].
3.3 Severity and Course of the Disease
The majority of all Coxiella infections (> 60%) with or without fever are self-limiting [39]. In the event of clinical manifestation, a 2% death rate should be expected [31] depending on the time of initiation of antibiotic treatment, age, and immune status of the infected individual. As described in Section 1.2, infection with C. burnetii can be asymptomatic, or acute, or progress to a chronic outcome.
3.4 Therapy and Prophylaxis
Doxycycline is the preferable treatment. Other effective treatments include ampicillin, chloramphenicol, fluoroquinolones, and, in some instances rifampicin in combination with doxycycline or ciprofloxacin [147]. Antibiotics such as erythromycin, gentamycin, penicillin G, and streptomycin are not effective [148]. Acute infections should be treated for at least 2 weeks. Chronic infection is treated until symptoms subside, or for 3 years preferably with doxycycline in combination with hydroxychloroquine, in order to alkalize the contents of the phagosome, or with ampicillin. Some authors recommend lifelong treatment in the case of endocarditis [112] since repeated reactivations (relapses) have been observed during treatment interruptions [45]. More recent alternatives for antibiotic treatment include tigecycline and clarithromycin [114].
3.5 Transmissibility
As described in Section 1.3., C. burnetii is highly contagious; 1-10 bacteria suffice to infect a human. Transmission occurs most frequently via inhaled or ingested contaminated material. In placental tissue of infected animals, concentrations of up to 109 bacteria per gram of tissue can be reached [7]. Intrauterine transmission has been described in humans [88]. The spore-like particles as permanent forms of C. burnetii remain infectious in the environment for many months, so that the end of transmissibility after an outbreak of the disease is difficult to predict, and transmissibility can last several years [145]. Transmission by blood is possible theoretically, however, only 1 case has as yet been described [93]. A further possible case has been discussed in the Netherlands, however, the recipient originated from a region which was highly affected by the Dutch Q fever epidemic [128,144]. Transmissions from human to human can occur within families by contact with contaminated laundry [78]. During patient treatment, no nosocomial transmission was observed [13]. Sexual transmission during the acute infection phase is described in Section 1.3 [83,84].
3.6 Frequency of Administration, Type, and Amount of Blood Products
Transmission of C. burnetii by blood and blood products has so far not been reported in Germany. Coxiella transmission by fresh frozen plasma is theoretically possible but has not yet been reported. The risk of a transmission by plasma products can be ruled out since bacteria are removed during fractionation and filtration, and are inactivated or destroyed during heat or solvent-detergent treatment of the products.
4 Blood Products
4.1 Infectious Load of the Starting Material and Test Methods
The infectious load in blood and plasma is unknown in Germany but must be considered as being very low. In 863 blood donors in Albacete, South-East Spain, the seroprevalence of anti-phase II IgG was 23.1%, and that of IgM, indicating a possible acute infection, 0.3% [131]. Among 1,004 blood donor samples in the Netherlands in 2009, 3 (0.3%) contained Coxiella DNA, while Coxiella IgG could be detected in 66 of 543 (12.2%) of the donors [128]. 10 donors seroconverted during the investigation period, which corresponds to an incidence of 5.7% per year [128]. C. burnetii cannot be removed from blood and cellular blood components since the bacterium is present intracellularly, i.e. it is cell-associated and also grows in monocytes. During acute infection, C. burnetii is also present in granulocytes, however, cannot be removed totally by leucocyte depletion. During the Q fever outbreak in the Netherlands, a specific donor selection strategy was developed to reduce efficiently the risk of Coxiella transmission: Exclusion of all donors who have a professional risk of exposure, all donors who live within a radius of 5 km from the center of the outbreak, and all donors who show flulike symptoms or had such symptoms within the past 2 weeks; and additional testing of all donations with a phase I IgM ELISA, even though a high false-positive rate was expected using this test [134]. Testing blood donors for phase I IgG has a 100% sensitivity only if the titer is above 1,024 [144]. By use of this exclusion strategy, antibody test, and donor selection, no evidence for Coxiella transmission by transfusion was found in the Netherlands [144,149].
4.2 Methods of Removal and Inactivation of the Infectious Agent
4.2.1 Removal
Derichment of C. burnetii can be performed by leukocyte depletion, however, this procedure will be incomplete. Derichment from plasma can be performed by high-speed centrifugation or filtration through a 0.2-µm filter. Small particles of C. burnetii can, however, also pass through the membrane of 0.2 µm filters.
4.2.2 Inactivation
Studies on the inactivation of C. burnetii by heat treatment (pasteurization) are available only for milk products [150]. It can be assumed that inactivation of the large cell variant by plasma pasteurization (60 °C, 10 h) is possible, and the small cell variant should also be inactivated in a time-dependent manner. Trials for the thermo-inactivation of the variants have not yet been published.
4.3 Feasibility and Validation of Procedures for Removal/Inactivation of the Infectious Agent
C. burnetii can be replicated in cell culture at high concentrations. Culturing requires biosafety level 3 conditions. Theoretically, the efficiency of pathogen reduction and inactivation can be tested by spiking. However, it is not necessary to validate the inactivation as long as the epidemiological situation in Germany remains constant, since until now no C. burnetii infections by blood and plasma products have occurred in Germany.
5 Assessment
C. burnetii is a bacterium which occurs in many animal species worldwide. C. burnetii can remain infectious in soil and environment as spore-like particles for many months. Various C. burnetii strains show different degrees of pathogenicity. The hazard of the infection is its chronic course with endocardial and vascular lesions, granulomatous hepatitis, and pulmonary fibrosis. Intrauterine transmission can also occur in humans.
The incidence of C. burnetii is related to exposure to contaminated dust and contact with infected animals. Outbreaks in rural regions in Germany and other European countries with intensive sheep and goat farming have so far not led to transmission of the bacterium by blood donation. The risk of transmission of C. burnetii by transfusion is thus very small. Likewise, no transmission of C. burnetii by plasma or plasma products has been reported.
A preventive deferral of donors originating or returning from regions with C. burnetii outbreaks for 4 weeks after the last human case has been reported is a suitable measure to reduce the infection risk [51,151,152]. The hygienic measures implemented in the Netherlands during the C. burnetii outbreak of 2007-2009 have contributed to preventing C. burnetii transmission by blood transfusion [128]. As a risk reduction measure, no arrangements for blood donations should be fixed during the recommended 4-week period in areas with increased risk of C. burnetii exposure. Local outbreaks should therefore be announced promptly. In the case of a massive local outbreak, during which donor deferral could lead to shortages in blood supply, NAT testing could alternatively be considered to reduce the risk of C. burnetii transmission.
The risk of C. burnetii transmission by blood components is extremely low so that initiating a look-back procedure as defined for viruses in Votum 34 of the Advisory Committee ‘Blood’ (Arbeitskreis Blut) following a local C. burnetii outbreak after the collection of blood donations does not seem beneficial.
References
- 1.Derrick EH. ‘Q’ fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Med J Aust. 1937;2:281–299. doi: 10.1093/clinids/5.4.790. [DOI] [PubMed] [Google Scholar]
- 2.Burnet FM, Freeman M. Experimental studies on the virus of ‘Q’ fever. Med J Aust. 1937;2:299–305. [Google Scholar]
- 3.Davis G, Cox HR. A filterpassing infectious agent isolated from ticks: isolation from Dermatocentor andersoni, reaction in animals, and filtration experiments. Public Health Rep. 1938;53:2259–2267. [Google Scholar]
- 4.WHO . Health Aspects of Chemical and Biological Weapons. 2nd ed. Geneva: World Health Organization; 2004. [Google Scholar]
- 5.Madariaga MG, Rezai K, Trenholme GM, Weinstein RA. Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3:709–721. doi: 10.1016/s1473-3099(03)00804-1. [DOI] [PubMed] [Google Scholar]
- 6.Süss J, Fingerle V, Hunfeld KP, Schrader C, Wilske B. Durch Zecken übertragbare humanpathogene und bisher als apathogen geltende Mikroorganismen in Europa. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2004;47:470–486. doi: 10.1007/s00103-004-0837-0. [DOI] [PubMed] [Google Scholar]
- 7.Marrie TJ. Mandell GL, Bennett JF, Dolin F. Principles and Practice of Infectious Diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. Coxiella burnetii (Q fever) pp. 2043–2050. [Google Scholar]
- 8.Walker D, Roault D, Dumler JS, Marrie T. Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL. Harrison's Principles of Internal Medicine. 15th ed. Philadelphia: McGraw Hill; 2001. Rickettsia, Mycoplasma and Chlamydia; pp. 1065–1073. [Google Scholar]
- 9.Glazunova O, Roux V, Freylikman O, Sekeyova Z, Fournous G, Tyczka J, Tokarevich N, Kovacova E, Marrie TJ, Raoult D. Coxiella burnetii genotyping. Emerg Infect Dis. 2005;11:1211–1217. doi: 10.3201/eid1108.041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiele D, Willems H, Kopf G, Krauss H. Polymorphism in DNA restriction patterns of Coxiella burnetii isolates investigated by pulse field gel electrophoresis and image analysis. Eur J Epidemiol. 1993;9:419–425. doi: 10.1007/BF00157400. [DOI] [PubMed] [Google Scholar]
- 11.Angelakis E, Raoult D. Q fever – review. Vet Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 12.McCaul TF, Williams JC. Development cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiation. J Bacteriol. 1981;147:1063–1076. doi: 10.1128/jb.147.3.1063-1076.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagawa FT, Wehner JH, Mohindra V. Q fever as a biological weapon. Sem Resp Infections. 2003;18:183–196. [PubMed] [Google Scholar]
- 14.Vishwanath S, Hackstadt T. Lipopolysaccharide phase variation determines the complement-mediated serum susceptibility of Coxiella burnetii. Infect Immun. 1988;56:40–44. doi: 10.1128/iai.56.1.40-44.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman SA, Fischer ER, Howe D, Mead DJ, Heinzen RA. Temporal analysis of Coxiella burnetii morphological differentiation. J Bacteriol. 2004;186:7344–7352. doi: 10.1128/JB.186.21.7344-7352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voth DE, Beare PA, Howe D, Sharma UM, Samoilis G, Cockrell DC, Omsland A, Heinzen RA. The Coxiella burnetii cryptic plasmid is enriched in genes encoding type IV secretion system substrates. J Bacteriol. 2011;193:1493–503. doi: 10.1128/JB.01359-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuel JE, Frazier ME, Mallavia LP. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect Immun. 1985;49:775–777. doi: 10.1128/iai.49.3.775-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilburg JHC, Roest HJLJ, Nabuurs-Franssen MH, Horrevorts AM, Klaassen CHW. Genotyping reveals the presence of a predominant genotype of Coxiella burnetii in consumer milk products. J Clin Microbiol. 2012;50:2156–2158. doi: 10.1128/JCM.06831-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tilburg JHC, Roest HJI, Buffet S, Nabuurs-Franssen MH, Horrevorts AM, Raoult D, Klaassen CHW. Epidemic genotype of Coxiella burnetii among goats, sheep, and humans in the Netherlands. Emerg Infect Dis. 2012;18:887–889. doi: 10.3201/eid1805.111907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichel R, Mearns R, Brunton L, Jones R, Horigan M, Vipond R, Vincent G, Evans S. Description of a Coxiella burnetii abortion outbreak in a dairy goat herd, and associated serology, PCR and genotyping results. Res Vet Sci. 2012;93:1217–1224. doi: 10.1016/j.rvsc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 21.De Bruin A, Van Alphen PTW, Van der Plaats RQJ, de Heer L, Reusken CBEM, Van Rotterdam BJ, Janse I. Molecular typing of Coxiella burnetii from animal and environmental matrices during Q fever epidemics in the Netherlands. BMC Veterinarian Res. 2012;8:165. doi: 10.1186/1746-6148-8-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornstra HM, Priestly RA, Georgia SM, Kachur S, Birdsell DN, Hilsabeck R, Gates LT, Samuel JE, Heinzen RA, Kersh GJ, Keim P, Massung RF, Pearson T. Rapid typing of Coxiella burnetii. PLoS One. 2011;6:e26201. doi: 10.1371/journal.pone.0026201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russel-Lodrigue KE, Andoh M, Poels MW, Shive HR, Weeks BR, Zhang GQ, Tersteeg C, Masegi T, Hotta A, Yamaguchi T, Fukushi H, Hirai K, Mc-Murray DN, Samuel JE. Coxiella burnetii isolates cause genogroupspecific virulence in mouse and guinea pig models of acute Q fever. Infect Immun. 2009;77:5640–5650. doi: 10.1128/IAI.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baca OG, Paretsky D. Q fever and Coxiella burnetii: a model for hostparasite interaction. Microbiol Rev. 1983;47:127–149. doi: 10.1128/mr.47.2.127-149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Scola B, Raoult D. Survival of Coxiella burnetii within freeliving amoeba Acanthamoeba castelanii. Clin Microbiol Infect. 2001;7:75–79. doi: 10.1046/j.1469-0691.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- 26.Mege JL, Maurin M, Capo C, Raoult D. Coxiella burnetii: The ‘query’ fever bacterium. A model of immune subversion by a strictly intracellular microorganism. FEMS Microbiol Rev. 1997;19:209–217. doi: 10.1111/j.1574-6976.1997.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 27.Omsland A, Cockrell DC, Howe D, Fischer ER, Virtaneva K, Sturdevant DE, Porcella SF, Heinzen RA. Host cellfree growth of Q fever bacterium Coxiella burnetii. Proc Natl Acad Sci USA. 2009;106:4430–4434. doi: 10.1073/pnas.0812074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omsland A, Heinzen RA. Life on the outside: the rescue of Coxiella burnetii from its host cell. Annu Rev Microbiol. 2011;65:111–128. doi: 10.1146/annurev-micro-090110-102927. [DOI] [PubMed] [Google Scholar]
- 29.Scott OH, Williams JC. Susceptibility of Coxiella burnetii to chemical disinfectants. Ann N Y Acad Sci. 1990;590:291–296. doi: 10.1111/j.1749-6632.1990.tb42235.x. [DOI] [PubMed] [Google Scholar]
- 30.Hall CJ, Richmond J, Caul EQ, Pearce NH, Silver IA. Laboratory outbreak of Q fever acquired from sheep. Lancet. 1982;1:1004–1006. doi: 10.1016/s0140-6736(82)92001-3. [DOI] [PubMed] [Google Scholar]
- 31.Tissot-Dupont H, Raoult D, Broqui P, Janbon F, Peyramond D, Weiller PJ, Chicheportiche C, Nezri M, Poirier R. Epidemiological features and clinical presentations of acute Q fever in hospitalized patients: 323 French cases. Am J Med. 1992;93:427–434. doi: 10.1016/0002-9343(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 32.Scott GH, McCaul TF, Williams JC. Inactivation of Coxiella burnetii by gamma irradiation. J Gen Microbiol. 1989;135:3263–3270. doi: 10.1099/00221287-135-12-3263. [DOI] [PubMed] [Google Scholar]
- 33.Welsh HH, Lenette EH, Abinanti FR, Winn JF, Kaplan W. Q fever studies XXI. The recovery of Coxiella burnetii from the soil and the surface water of premises habouring infected sheep. Am J Hygiene. 1959;70:14–20. [PubMed] [Google Scholar]
- 34.Kumar A, Yadav MP, Kakkar S. Human milk as a source of Q fever infection in breastfed babies. Indian J Med Res. 1981;73:510–512. [PubMed] [Google Scholar]
- 35.Sawyer LA, Fishbein DB, McDade JE. Q fever: current concepts. Rev Infect Dis. 1987;9:935–946. doi: 10.1093/clinids/9.5.935. [DOI] [PubMed] [Google Scholar]
- 36.Hackstadt T, Peacock MG, Hitchcock PJ, Cole RL. Lipopolysaccharide variation in Coxiella burnetii: intrastrain heterogeneity in structure and antigenicity. Infec Immun. 1985;48:359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Cremoux R, Rousset E, Touratier A, Audusseau G, Nicollet P, Ribaud D, David V, Le Pape M. Assessment of vaccination by a phase I Coxiella burnetii-inactivated vaccine in goat herds in clinical Q fever situation. FEMS Immuno Med Microbiol. 2012;64:104–106. doi: 10.1111/j.1574-695X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 38.Stocker MGP, Marmion BP. The spread of Q fever from animals to man. The natural history of a rickettsial disease. Bull WHO. 1955;13:781–806. [PMC free article] [PubMed] [Google Scholar]
- 39.Clark WH, Romer MS, Holmes MA, Welsh HH, Lennette EH, Abinanti FR. Q fever in California VIII. An epidemic of Q fever in a small rural community in northern California. Am J Hygiene. 1951;54:25–34. [PubMed] [Google Scholar]
- 40.Villanueva AA, Viciana P, Lopez-Cortes L, Torronteras R, Bernabeu M, Cordero E, Pachon J. Q fever: epidemiology, clinical features and prognosis. A study from 1983 to 1999 in the south of Spain. J Infect. 2003;47:110–116. doi: 10.1016/s0163-4453(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 41.Millar JK. The chest fil findings in ‘Q’ fever – a series of 35 cases. Clin Radiol. 1978;329:371–375. doi: 10.1016/s0009-9260(78)80092-0. [DOI] [PubMed] [Google Scholar]
- 42.Robins FC. Q fever in the Mediterranean area: report on its occurrence in Allied Troops. Am J Hygiene. 1946;49:51–71. doi: 10.1093/oxfordjournals.aje.a119083. [DOI] [PubMed] [Google Scholar]
- 43.Kampschreur LM, Dekker S, Hagenaars JCJP, Lestrade PJ, Renders NHM, De Jager-Leclercq MGL, Hermans MHA, Groot CAR, Groenwold RHH, Hoepelman ALM, Wever PC, Oosterheert JJ. Identification of risk factors for chronic Q fever, the Netherlands. Emerg Infect Dis. 2012;18:563–570. doi: 10.3201/eid1804.111478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer SR, Young SEJ. Q fever endocarditis in England and Wales, 1975-1981. Lancet. 1982;2:1448–1449. doi: 10.1016/s0140-6736(82)91341-1. [DOI] [PubMed] [Google Scholar]
- 45.Morguet AJ, Jansen A, Raoult D, Schneider T. Late relapse of Q fever endocarditis. Clin Res Cardiol. 2007;96:519–521. doi: 10.1007/s00392-007-0522-z. [DOI] [PubMed] [Google Scholar]
- 46.Karakousis PC, Trucksis M, Dumler SJ. Chronic Q fever in United States. J Clin Microbiol. 2006;44:2283–2287. doi: 10.1128/JCM.02365-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tjwa M, Hertogh DG, Neuville B, Roskams T, Nevens F, Steenbergen VW. Hepatic fibrinring granulomas in granulomatous hepatitis: report of four cases and review of literature. Acta Clin Belg. 2001;56:341–348. doi: 10.1179/acb.2001.051. [DOI] [PubMed] [Google Scholar]
- 48.Boattini M, Almeida A, Moura RB, Abreu J, Santos AS, Rico MT. Cronic Q fever with no elevation of inflammatory markers. Case Rep Med. 2012;2012:249705. doi: 10.1155/2012/249705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Udaondo P, Garcia-Delpech S, Salom D, Garcia-Pous M, Diaz-Llopis M. Q fever: a new ocular manifestation. Clin Opthamol. 2011;5:1273–1275. doi: 10.2147/OPTH.S18771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris RJ, Storm PA, Lloyd A, Arens M, Marmion BP. Long-term persistence of Coxiella burnetii in the host after primary Q fever. Epidemiol Infect. 2000;124:543–549. doi: 10.1017/s0950268899003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fenollar F, Fournier PE, Raoult D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. J Clin Microbiol. 2004;42:4919–4924. doi: 10.1128/JCM.42.11.4919-4924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arricau-Bouvery N, Rodolakis A. Is Q fever an emerging or reemerging zoonosis? Vet Res. 2005;36:327–349. doi: 10.1051/vetres:2005010. [DOI] [PubMed] [Google Scholar]
- 53.Rodolakis A. Review: Q fever in dairy animals. Ann N Y Acad Sci. 2009;1166:90–93. doi: 10.1111/j.1749-6632.2009.04511.x. [DOI] [PubMed] [Google Scholar]
- 54.Hellenbrand W, Breuer T, Peterson L. Changing epidemiology of Q fever in Germany, 1947-1999. Emerg Infect Dis. 2001;7:789–796. doi: 10.3201/eid0705.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Editorial MMWR. Q fever outbreak – Switzerland. Mort Morb Weekly Rep. 1984;33:355–361. [PubMed] [Google Scholar]
- 56.Porten K, Rissland J, Tigges A, Broll S, Hopp W, Lunemann M, van Treeck U, Kimmig P, Brockmann SO, Wagner-Wiening C, Hellenbrand W, Buchholz U. A superspreading ewe infects hundreds with Q fever at a farmers' market in Germany. BMC Infect Dis. 2006;6:147. doi: 10.1186/1471-2334-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogerwerf L, Van den Brom R, Roest HIJ, Bouma A, Vellema P, Pieterse M, Dercksen D, Nielen M. Reduction of Coxiella burnetii prevalence by vaccination of goats and sheep, The Netherlands. Emerg Infect Dis. 2011;17:379–386. doi: 10.3201/eid1703.101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kampschreur LM, Hagenaars JCJP, Wielders CCH, Elsman P, Lestrade PJ, Koning OHJ, Oosterheert JJ, Renders NHM, Wever PC. Screening for Coxiella burnetii seroprevalence in chronic Q fever highrisk groups reveals the magnitude of the Dutch Q fever outbreak. Epidemiol Infect. 2012;13:1–5. doi: 10.1017/S0950268812001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hilbert A, Reith P, Brockmann SO, Tyczka J, Fischer SF, Piechotowski I, Wagner-Wiening C, Winter Ch, Bendak J, Meier C, Spengler D, Miller T, Kleine-Albers C, Renner C, Koepsel U, Hensler E, Henning K, Fröhlich A, Conraths FJ, Kramer M. Epidemiolocial inquiries in two Q fever outbreaks in a community of Baden-Württemberg during 2008 and 2009. Berl Munch Tierarztl Wochenschr. 2011;124:295–302. [PubMed] [Google Scholar]
- 60.Bernard H, Brockmann SO, Kleinkauf N, Klinc C, Wagner-Wiening C, Stark K, Jansen A. High seroprevalence of Coxiella burnetii antibodies in veterinarians associated with cattle obstretics, Bavaria, 2009. Vector Borne Zoonotic Dis. 2012;12:552–557. doi: 10.1089/vbz.2011.0879. [DOI] [PubMed] [Google Scholar]
- 61.Hilbert A, Schmoock G, Lenzko H, Moog U, Diller R, Fröhlich A, Hoffmann L, Horner S, Elschner M, Tomaso H, Henning K, Neubauer H, Sprague LD. Prevalence of Coxiella burnetii in clinically healthy German sheep flocks. BMC Res Notes. 2012;5:152. doi: 10.1186/1756-0500-5-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berri M, Crochet D, Santiago S, Rodolakis A. Spread of Coxiella burnetii infection in a flock of sheep after an episode of Q fever. Vet Res. 2005;157:737–740. doi: 10.1136/vr.157.23.737. [DOI] [PubMed] [Google Scholar]
- 63.Wallensten A, Moore P, Webster H, Johnson C, Van der Burg G, Pritchard G, EllisIversen J, Oliver I. Q fever outbreak in Cheltenhan, United Kingdom, in 2007 and the use of dispersion modeling to investigate the possibility of airborne spread. Euro Surveill. 2010;15 pii:19521. [PubMed] [Google Scholar]
- 64.Beslagic E, Hamzic S, Puvacic S, Cavaljuga-Hotic S. Q fever serologic diagnostics with inhabitants of Sarajevo 2001 year. Med Arh. 2003;57:71–74. [PubMed] [Google Scholar]
- 65.Leski TA, Malanoski AP, Gregory MJ, Lin B, Stenger DA. Application of a broad-range resequencing array for detection of pathogens in desert dust samples from Kuwait and Iraq. App Environm Microbiol. 2011;77:4285–4292. doi: 10.1128/AEM.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson AD, Baker TR, Litrell AC, Mott RL, Niebuhr DW, Smoak BL. Seroepidemiologic survey for Coxiella burnetii among hospitalized US troops deployed to Iraq. Zoonoses Public Health. 2011;58:276–283. doi: 10.1111/j.1863-2378.2010.01347.x. [DOI] [PubMed] [Google Scholar]
- 67.Bailey MS, Trinick TR, Dunbar JA, Hatch R, Osborne JC, Brooks TJ, Green AD. Undifferentiated febrile illnesses amongst British troops in Helmand, Afghanistan. J R Army Med Corps. 2011;157:150–155. doi: 10.1136/jramc-157-02-05. [DOI] [PubMed] [Google Scholar]
- 68.Reusken C, Van der Plaats R, Opsteegh M, De Bruin A, Swart A. Coxiella burnetii (Q fever) in Rattus norvegicus und Rattus rattus at lifestock farms and urban locations in the Netherlands; could Rattus spp represent reservoirs for (re)introduction? Prev Vet Med. 2011;101:124–130. doi: 10.1016/j.prevetmed.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Dupuis G, Peter O, Mottiez MC, Vouilloz M. Seroprevalence de la fièvre Q humaine en Suisse. Schweiz Med Wochensch. 1986;116:494–498. [PubMed] [Google Scholar]
- 70.Brouqui P, Sékéné B, Raoult D. Q fever outbreak in homeless shelter. Emerg Infect Dis. 2004;10:1297–1299. doi: 10.3201/eid1007.031020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bell JA, Beck MD, Huebner RJ. Epidemiologic studies of Q fever in Southern California. J Am Med Ass. 1950;142:868–872. doi: 10.1001/jama.1950.02910300006002. [DOI] [PubMed] [Google Scholar]
- 72.Hogerwerf L, Borlée F, Still K, Heederik D, Van Rotterdam B, De Bruin A, Nielen M, Wouters IM. Detection of Coxiella burnetii DNA in inhalable airborne dust samples in goat farms after mandatory culling. Appl Environm Microbiol. 2012;78:5410–5412. doi: 10.1128/AEM.00677-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.RKI Q-Fieber Ausbruch in Rollshausen, Hessen 1996. Epidemiol Bull. 1997;4:19–21. [Google Scholar]
- 74.Cone LA, Curry N, Shaver P, Brooks D, Deforge J, Potts BE. Q fever in the Southern California desert: epidemiology, clinical presentation and treatment. Am J Trop Med Hyg. 2006;75:29–32. doi: 10.4269/ajtmh.2006.75.1.0750029. [DOI] [PubMed] [Google Scholar]
- 75.Delsing CE, Kullberg BJ, Bleeker-Rovers CP. Q fever in the Netherlands from 2007-2010. Netherland J Med. 2010;68:382–387. [PubMed] [Google Scholar]
- 76.Salmon MM, Howells B, Glencross EJ, Evans AD, Palmer SR. Q fever in an urban area. Lancet. 1982;1:1002–1004. doi: 10.1016/s0140-6736(82)92000-1. [DOI] [PubMed] [Google Scholar]
- 77.Woerden HCV, Manson BW, Nehaul LK, Smith R, Salmon RL, Healy B, Valappil M, Westmoreland D, Martin SD, Evans MR, Lloyd G, Hamilton-Kirkwood M, Williams NS. Q fever outbreak in industrial setting. Emerg Infect Dis. 2004;10:1282–1289. doi: 10.3201/eid1007.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oliphant, JW, Gordon WA, Meis A, Parker RR. Q fever in laundry workers presumably transmitted from contaminated clothing. Am J Hygiene. 1949;46:76–82. doi: 10.1093/oxfordjournals.aje.a119261. [DOI] [PubMed] [Google Scholar]
- 79.Hermans MHA, Huijsmans CRJJ, Schellekens JJA, Savelkoul PHM, Wever PC. Coxiella burnetii DNA in goat milk after vaccination with Coxevac. Vaccine. 2011;29:2653–2656. doi: 10.1016/j.vaccine.2011.01.111. [DOI] [PubMed] [Google Scholar]
- 80.Rolain JM, Gouriet F, Brouqui P, Larrey D, Janbon F, Vene S, Jarnestrom V, Raoult D. Concomitant or consecutive infection with Coxiella burnetii and tickborne diseases. Clin Infect Dis. 2005;40:82–88. doi: 10.1086/426440. [DOI] [PubMed] [Google Scholar]
- 81.Pluta S, Hartelt K, Oehme R, Mackenstedt U, Kimmig P. Prevalence of Coxiella burnetii and Rickettsi spp in ticks and rodents in southern Germany. Ticks Tick Borne Dis. 2010;1:145–147. doi: 10.1016/j.ttbdis.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Sprong H, Tijsse-Klasen E, Langelaar M, De Bruin A, Fonville M, Gassner F, Takken W, Van Wieren S, Nijhof A, Jongejan F, Maassen CB, Scholte EJ, Hovius JVV, Emil-Hovius K, Spitalská E, Duynhoven YT. Prevalence of Coxiella burnetii in ticks after a large outbreak of Q fever. Zoonoses Public Health. 2012;59:69–75. doi: 10.1111/j.1863-2378.2011.01421.x. [DOI] [PubMed] [Google Scholar]
- 83.Milazzo A, Hall R, Storm PA, Harris RJ, Winslow W, Marmion BP. Sexually transmitted Q fever. Clin Infect Dis. 2001;33:399–402. doi: 10.1086/321878. [DOI] [PubMed] [Google Scholar]
- 84.Miceli M, Veryser AK, Anderson AD, Hofinger D, Lee SA, Tancik C. A case of perso-to-person transmission of Q fever from an active duty serviceman to his spouse. Vector Borne Zoonotic Dis. 2010;10:539–541. doi: 10.1089/vbz.2009.0101. [DOI] [PubMed] [Google Scholar]
- 85.Kruszewska D, Tylewska-Wierzbanowska ST. Isolation of Coxiella burnetii from bull semen. Res Vet Sci. 1997;62:299–300. doi: 10.1016/s0034-5288(97)90210-1. [DOI] [PubMed] [Google Scholar]
- 86.Baud D, Peter O, Langel C, Regan L, Greub G. Seroprevalence of Coxiella burnetii and Brucella abortus among pregnant women. Clin Microbiol Infect. 2009;15:499–501. doi: 10.1111/j.1469-0691.2009.02779.x. [DOI] [PubMed] [Google Scholar]
- 87.Langley JM, Marrie TJ, Leblanc JC, Almudevar A, Resch L, Raoult D. Coxiella burnetii seropositivity in parturient women is associated with adverse pregnancy outcomes. Am J Obstet Gynecol. 2003;189:228–232. doi: 10.1067/mob.2003.448. [DOI] [PubMed] [Google Scholar]
- 88.Syrucek L, Sobelavsky O, Gutvirth I. Isolation of Coxiella burnetii from human placentas. J Hyg Epidemiol Microbiol Immunol. 1958;2:29–35. [PubMed] [Google Scholar]
- 89.Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis. 2005;5:219–226. doi: 10.1016/S1473-3099(05)70052-9. [DOI] [PubMed] [Google Scholar]
- 90.Weber DJ, Ratain WA. Risks and prevention of nosocomial transmission of rare zoonotic diseases. Clin Infect Dis. 2001;32:446–456. doi: 10.1086/318509. [DOI] [PubMed] [Google Scholar]
- 91.Mann JS, Douglas JG, Inglis JN, Leitch AG. Q fever: person to person transmission within a family. Thorax. 1986;41:974–975. doi: 10.1136/thx.41.12.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harman JB. Q fever in Great Britain; clinical account of eight cases. Lancet. 1949;2:1028–1030. doi: 10.1016/s0140-6736(49)91600-1. [DOI] [PubMed] [Google Scholar]
- 93.Editorial comment on Q fever transmitted by blood transfusion – United States. Cur Dis Weekly Rep. 1977;3:210. [Google Scholar]
- 94.Goldberg JS, Perkins HA, Zapitz VM, Hurst BB, Suther D, Jensen F, Lenette EH, Damus K, Roberto RR. Q fever – California. Morb Mort Weekly Rep. 1977;26:86–87. [Google Scholar]
- 95.Johnson, JE, Kadull PJ. Laboratory acquired Q fever. A report of fifty cases. Am J Med. 1966;41:391–403. doi: 10.1016/0002-9343(66)90085-4. [DOI] [PubMed] [Google Scholar]
- 96.Guatteo R, Seegers H, Taurel AF, Joly A, Beaudeau F. Prevalence of Coxiella burnetii infection in domestic ruminants: a critical review. Vet Microbiol. 2011;149:1–16. doi: 10.1016/j.vetmic.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 97.Runge M, Binder A, Schotte U, Ganter M. Investigations concerning the prevalence of Coxiella burnetii and Chlamydia abortus in sheep in correlation with management systems and abortion rate in Lower Saxony in 2004. Berl Munch Tierarztl Wochenschr. 2012;125:138–143. [PubMed] [Google Scholar]
- 98.Peter O, Dupuis G, Peacock MG, Burgdorfer W. Comparison of enzyme linked immunosorbent assay and complement fixation and indirect fluorescent-antibody tests for detection of Coxiella burnetii antibody. J Clin Microbiol. 1987;25:1063–1067. doi: 10.1128/jcm.25.6.1063-1067.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fournier PE, Marrie TJ, Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834. doi: 10.1128/jcm.36.7.1823-1834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brouqui P, Dupont HT, Drancourt H, Berland Y, Etienne J, Leport C. Goldstein F, Massip P, Micoud M, Bertrand A. Chronic Q fever: ninetytwo cases from France, including 27 cases without endocarditis. Arch Intern Med. 1993;153:642–649. doi: 10.1001/archinte.153.5.642. [DOI] [PubMed] [Google Scholar]
- 101.Raoult D, Levy PY, Dupont HT, Chicheportiche C, Tamalet C, Gastaut JA, Salducci J. Q fever and HIV infection. AIDS. 1993;7:81–86. doi: 10.1097/00002030-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 102.Kampschreur LM, Oosterheert JJ, Koop AMC, Wegdam-Blans MCA, Delsing CE, Bleeker-Rovers CP, De Jager-Leclerq MGL, Groot CAR, Prong T, Nabuurs-Franssen MH, Benders NHM, Van Kasteren ME, Soethoudt Y, Blank SN, Pronk MJH, Groenwold RHH, Hoepelman AIM, Wever PC. Microbiological challenges in the diagnosis of chronic Q fever. Clin Vaccine Immunol. 2012;19:787–790. doi: 10.1128/CVI.05724-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teunis PFM, Schimmer B, Notermans DW, Leenders ACAP, Wever PC, Kretzschmar MEE, Schneeberger PM. Time-course of antibody responses against Coxiella burnetii following acute Q fever. Epidemiol Infect. 2013;141:62–730. doi: 10.1017/S0950268812000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wegdam-Blans MC, Wielders CC, Meekelenkamp J, Korbeeck JM, Herremans T, Tjhie HT, Bijlmer HA, Koopmans MP, Schneeberger PM. Evaluation of commonly used serological tests for the detection of Coxiella burnetii antibodies in well-defined acute and followup sera. Clin Vaccine Immunol. 2012;19:1110–1115. doi: 10.1128/CVI.05581-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Field PR, Hunt JG, Murphy AM. Detection and persistence of specific IgM antibodies to Coxiella burnetii by enzyme linked immunosorbent assay: a comparsion with immunofluorescence and complement fixation test. J Infect Dis. 1983;148:477–487. doi: 10.1093/infdis/148.3.477. [DOI] [PubMed] [Google Scholar]
- 106.Hunt JG, Field PR, Murphy AM. Immunglobulin responses to Coxiella burnetii (Q fever): single-serum diagnosis of acute infection using an immunofluorescence technique. Infect Immun. 1983;39:977–981. doi: 10.1128/iai.39.2.977-981.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fournier PE, Casalta JP, Habib G, Messana T, Raoult D. Modification of the diagnostic criteria proposed by the Duke Endocarditis Service to permit improved diagnosis of Q fever endocarditis. Am J Med. 1996;100:629–633. doi: 10.1016/s0002-9343(96)00040-x. [DOI] [PubMed] [Google Scholar]
- 108.Tissot-Dupont H, Thirion X, Raoult D. Q fever serology: cut off determination for microimmunofluorescence. Clin Diagn Lab Immunol. 1994;1:189–196. doi: 10.1128/cdli.1.2.189-196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murphy AM, Field PR. The persistence of complement fixing antibodies to Q fever (Coxiella burnetii) after infection. Med J Aust. 1970;1:1148–1150. doi: 10.5694/j.1326-5377.1970.tb84481.x. [DOI] [PubMed] [Google Scholar]
- 110.Blondeau JM, Williams JC, Marrie TJ. The immune response to phase 1 and phase 2 Coxiella burnetii antigens as measured by Western blotting. Ann NY Acd Sci. 1990;590:187–202. doi: 10.1111/j.1749-6632.1990.tb42220.x. [DOI] [PubMed] [Google Scholar]
- 111.Minnick MF, Heinzen RA, Reschke DK, Frazier ME, Mallavia LP. A plasmid encoded surface protein found in chronic disease isolates of Coxiella burnetii. Infect Immun. 1991;59:4735–4739. doi: 10.1128/iai.59.12.4735-4739.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Worswick D, Marmion BP. Antibody response in acute and chronic Q fever and in subjects vaccinated against Q fever. J Med Microbiol. 1985;119:281–296. doi: 10.1099/00222615-19-3-281. [DOI] [PubMed] [Google Scholar]
- 113.Dupuis G, Peter O, Peacock M, Burgdorfer W, Haller E. Immunglobulin responses in acute Q fever and in subjects vaccinated against Q fever. J Clin Microbiol. 1985;22:484–487. doi: 10.1128/jcm.22.4.484-487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spyridaki I, Psaroulaki A, Vranakis J, Tselentis Y, Gikas A. Bacteriostatic and bactericidal activities of tigecycline against Coxiella burnetii and comparison with those of six other antibiotics. Antimicrob Agents Chemother. 2009;53:2690–2692. doi: 10.1128/AAC.01424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lockhart M, Islam A, Graves S, Fenwick S, Stenos J. Detecting and measuring small numbers of viable Coxiella burnetii. FEMS Immunol Med Microbiol. 2012;64:61–65. doi: 10.1111/j.1574-695X.2011.00898.x. [DOI] [PubMed] [Google Scholar]
- 116.Musso D, Raoult D. Coxiella burnetii blood cultures from acute and chronic Q fever patients. J Clin Microbiol. 1995;33:3129–3132. doi: 10.1128/jcm.33.12.3129-3132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Omsland A, Beare PA, Hill J, Cockrell DC, Howe D, Hansen B, Samuel JE, Heinzen RA. Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Appl Environ Microbiol. 2011;77:3720–3725. doi: 10.1128/AEM.02826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klee SR, Tyczka J, Ellerbrok H, Franz T, Linke S, Baljer G, Appel B. Highly sensitive realtime PCR for specific detection and quantification of Coxiella burnetii. BMC Microbiol. 2006;6:2. doi: 10.1186/1471-2180-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stein A, Raoult D. Detection of Coxiella burnetii by DNA amplification using polymerase chain reaction. J Clin Microbiol. 1992;30:2462–2466. doi: 10.1128/jcm.30.9.2462-2466.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beare PA, Samuel JF, Howe D, Virtaneva K, Porcella SF, Heinzen RA. Genetic diversity of Q fever agent, Coxiella burnetii, assessed by microarray-based wholegenome comparisons. J Bacteriol. 2006;188:2309–2324. doi: 10.1128/JB.188.7.2309-2324.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fournier PE, Raoult D. Comparison of PCR and serology assays for early diagnosis of acute Q fever. J Clin Microbiol. 2003;41:5094–5098. doi: 10.1128/JCM.41.11.5094-5098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kato K, Arashima Y, Asai S, Furuya Y, Yoshida Y, Murakami M, Takahashi Y, Hayashi K, Katayama T, Kumasaka K, Arakawa Y, Kawano K. Detection of Coxiella burnetii specific DNA in blood samples from Japanese patients with chronic nonspecific symptoms by nested polymerase chain reaction. FEMS Immunol Med Microbiol. 1998;21:139–144. doi: 10.1111/j.1574-695X.1998.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 123.Lorenz H, Jäger C, Willems H, Baljer G. PCR detection of Coxiella burnetii from different clinical specimens, especially bovine milk, on the basis of DNA preparation with a silica matrix. Appl Environ Microbiol. 1998;64:4234–4237. doi: 10.1128/aem.64.11.4234-4237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berri M, Arricau-Bouvery N, Rodolakis A. PCR-based detection of Coxiella burnetii from clinical samples. Meth Mol Biol. 2003;216:153–161. doi: 10.1385/1-59259-344-5:153. [DOI] [PubMed] [Google Scholar]
- 125.Janse I, Bok JM, Hamidjaja RA, Hodemaekers HM, Van Rotterdam BJ. Development and comparison of two assay formats for parallel detection of four biothreat pathogens by using suspension microarrays. PLoS One. 2012;7:e31958. doi: 10.1371/journal.pone.0031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jado I, Carranza-Rodriguez C, Barandika JF, Toledo A, Garcia-Amil C, Serrano B, Bolaños M, Gil H, Escudero R, Garcia-Pérez AL, Olmeda AS, Astobiza I, Lobo B, Rodriguez-Vargas M, Pérez-Arellano JL, López-Gatius F, Pascual-Velasco F, Cilla G, Rodriguez NF, Anda P. Molecular method for the characterization of Coxiella burnetii from clinical and environmental samples: variability of genotypes in Spain. BMC Microbiol. 2012;12:91 e22656068. doi: 10.1186/1471-2180-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Richardus JH, Donkers A, Dumas AM, Schaap GJ, Akkermans JP, Huisman J, Valkenburg HA. Q fever in the Netherlands: a sero-epidemiological survey among human population groups from 1968-1983. Epidemiol Infect. 1987;98:211–219. doi: 10.1017/s0950268800061938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hogema BM, Slot E, Molier M, Schneeberger PM, Hermans MH, Van Hannen EJ, Van der Hoek W, Cuijpers T, Zaaijer HL. Coxiella burnetii infection among blood donors during the 2009 Q fever outbreak in the Netherlands. Transfusion. 2012;52:144–150. doi: 10.1111/j.1537-2995.2011.03250.x. [DOI] [PubMed] [Google Scholar]
- 129.Hatchette TF, Hudson RC, Schlech WF, Campell NA, Hatchette JE, Ratnam S, Raoult D, Donovan C, Marrie T. Goat associated Q fever: a new disease in Newfoundland. Emerg Infect Dis. 2001;7:413–419. doi: 10.3201/eid0703.010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kilic S, Yilmaz GR, Komiya T, Kurtoglu Y, Karakoc EA. Prevalence of Coxiella burnetii antibodies in blood donors in Ankara, Central Anatolia, Turkey. New Microbiol. 2008;31:527–534. [PubMed] [Google Scholar]
- 131.Bartolomé J, Riquelme E, Hernández-Pérez N, Garcia-Ruiz S, Luján R, Lorento S, Medrano-Callejas R, Crespo MD. Seroepidemiology of Coxiella burnetii infection among blood donors in Albacete. Enferm Infecc Microbiol Clin. 2007;25:382–386. doi: 10.1157/13106963. [DOI] [PubMed] [Google Scholar]
- 132.Marrie TJ, Raoult D. Rickettsial infections of the central nervous system. Semin Neurol. 1992;12:213–224. doi: 10.1055/s-2008-1041178. [DOI] [PubMed] [Google Scholar]
- 133.AFSSPS – Agence francaise de sécurité sanitaires des produits de santé. Risque de transmission de la Fièvre Q (Coxiella burnetii) par les produits de santè, November 15 2002.
- 134.Van Wijk MJ, Hogema BM, Maas DW, Bokhorst AG. A Q fever outbreak in the Netherlands: consequences for tissue banking. Transfus Med Hemother. 2011;38:357–364. doi: 10.1159/000334599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kersh GJ, Priestley R, Massung RF. Stability of Coxiella burnetii in stored human blood. Transfusion. 2013;53:1493–1496. doi: 10.1111/j.1537-2995.2012.03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Posteegh M, de Heer L, Van den Berg H, Van der Giessen J. Inactivation or clearance of Coxiella burnetii in rat serum samples to enable safe serological testing. J Basic Microbiol. 2012;52:1–3. doi: 10.1002/jobm.201200211. [DOI] [PubMed] [Google Scholar]
- 137.Cohen NJ, Papernik M, Singleton J, Segreti J, Eremeeva ME. Q fever in an American tourist returned from Australia. Travel Med Infect Dis. 2007;5:194–195. doi: 10.1016/j.tmaid.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 138.Ta TH, Jiménez B, Navarro M, Meije Y, González FJ, Lopéz-Velez R. Q fever in returned febrile travellers. J Travel Med. 2008;15:126–129. doi: 10.1111/j.1708-8305.2008.00191.x. [DOI] [PubMed] [Google Scholar]
- 139.Davis TR, Edwards Y, Morgan A, Caul EO. Prevalence of Q fever in rural practice. J Public Health Med. 1997;19:324–327. doi: 10.1093/oxfordjournals.pubmed.a024638. [DOI] [PubMed] [Google Scholar]
- 140.Luoto L, Casey ML, Pickens EG. Q fever studies in Montana. Detection of asymptomatic infection among residents of infected diary premises. Am J Epidemiol. 1965;81:356–369. doi: 10.1093/oxfordjournals.aje.a120522. [DOI] [PubMed] [Google Scholar]
- 141.Ascher MS, Berman MA, Ruppaner R. Initial clinical and immunological evaluation of a new phase 1 Q fever vaccine and skin tests in humans. J Infect Dis. 1983;148:214–222. doi: 10.1093/infdis/148.2.214. [DOI] [PubMed] [Google Scholar]
- 142.Ackland JR, Worswick DA, Marmion BP. Accine prophylaxis of Q fever: a follow up study of the efficacy of Q-Vax (CSL) 1985-1990. Med J Aust. 1994;160:704–708. [PubMed] [Google Scholar]
- 143.Gidding HF, Wallace C, Lawrence GL, McIntyre PB. Australia's national Q fever vaccination program. Vaccine. 2009;27:2037–2041. doi: 10.1016/j.vaccine.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 144.Van der Hoek W, Wielders CCH, Schimmer B, WegdamBlans MCA, Meekenlenkamp J, Zaaijer HL, Schneeberger PM. Detection of phase I IgG antibodies to Coxiella burnetii with ELISA as a screening test for blood donations. Eur J Clin Microbiol Infect Dis. 2012;31:3207–3209. doi: 10.1007/s10096-012-1686-7. [DOI] [PubMed] [Google Scholar]
- 145.Astobiza I, Barandika JF, Ruiz-Fons F, Hurtado A, Povedano I, Juste RA, Garcia-Pérez AL. Four-year evaluation of the effect of vaccination against Coxiella burnetii on reduction of animal infection and environmental contamination in a naturally infected dairy sheep flock. Appl Environ Microbiol. 2011;77:7405–7407. doi: 10.1128/AEM.05530-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Taurel AF, Guatteo R, Joly A, Beaudeau F. Effectiveness of vaccination and antibiotics to control Coxiella burnetii shedding around calving in dairy cows. Vet Microbiol. 2012;159:432–437. doi: 10.1016/j.vetmic.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 147.Walker DH, Dumler JS, Marrie T. Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J. Harrison's Principles in Internal Medicine. 18th ed. New York: McGraw Hill; 2012. Rickettsial diseases, chapter 174; pp. 1414–1417. [Google Scholar]
- 148.Yeaman MR, Mitscher LA, Baca OG. In vitro susceptibility of Coxiella burnetii for antibiotics, including several quinolones. Antimicrob Agents Chemother. 1987;31:1079–1084. doi: 10.1128/aac.31.7.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Van Kraaij MGJ, Slot E, Hogema BM, Zaaijer HL. Lookback procedures after postdonation notifications during a Q fever outbreak in the Netherlands. Transfusion. 2013;53:716–721. doi: 10.1111/j.1537-2995.2012.03792.x. [DOI] [PubMed] [Google Scholar]
- 150.Cerf O, Condron R. Coxiella burnetii and milk pasteurization: an early application of the precautionary principle? Epidemiol Infect. 2006;134:946–951. doi: 10.1017/S0950268806005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wilson K, Ricketts MN. Transfusion transmission of vCJD: a crisis avoided? Lancet. 2004;364:477–479. doi: 10.1016/S0140-6736(04)16820-7. [DOI] [PubMed] [Google Scholar]
- 152.Van der Hoek W, Schneeberger PM, Oomen T, Wegdam-Blans MC, Dijkstra F, Notermans DW, Bijlmer HA, Groeneveld K, Wijkmans CJ, Rietveld A, Kampschreur LM, van Duynhoven Y. Shifting priorities in the aftermath of a Q fever epidemic in 2007 to 2009 in the Netherlands: from acute to chronic infection. Euro Surveill. 2012;17:20059. [PubMed] [Google Scholar]