Summary
Hepatitis E virus (HEV) has been recognized since 2004 as a transfusion-transmissible infectious agent, and recent epidemiological data suggest that it may pose a safety threat to the blood supply. It has recently become obvious that hepatitis E is endemic in industrialized countries, and that more infections are autochthonous than travel-associated. Epidemiological and phylogenetic analysis suggests that HEV infection has to be considered as a zoonosis and that viral transmission from animals (pigs, wild animals) occurs through food or direct contact. The seroprevalence and incidence of HEV in the general population and blood donors in European countries indicate an underestimated risk for transfusion transmissions. Recently reported cases of transfusion transmission of HEV infection, and detection of viremic, asymptomatic blood donors in nucleic acid amplification technique screening programs give an indication of the importance of this virus. Diagnostic assays for detection of anti-HEV antibodies, HEV antigens and RNA are discussed. Recent studies support the idea that active immunization can prevent hepatitis E, highlighting the need for vaccination programs. Here we review current knowledge of HEV and its epidemiology, blood transmission and prevention of this disease with emphasis on blood supply.
Key Words: Hepatitis E virus, Transfusion-transmitted HEV infection, Seroprevalence, Incidence, Transfusion transmission, HEV blood donor screening, HEV RNA
Introduction
Hepatitis E virus (HEV) is a non-enveloped single-stranded RNA virus approximately 27-34 nm in diameter belonging to the genus Hepevirus in the Hepeviridae family. This family contains mammalian HEV and the more distant avian HEV [1], as well as cut-throat trout virus [2]. The 2 latter groups represent a potential separate genus without association to human beings. Phylogenetic analysis of various mammalian HEV isolates circulating among human beings and animals has led to the recognition of 4 major genotypes (genotypes 1-4) and several subgenotypes [3,4,5]. All HEV genotypes represent a single serotype. Genotypes 1 and 2 are circulating in Africa and Asia, genotype 3 shows a broad distribution worldwide, and genotype 4 is restricted to Asia [6]. Genotypes 3 and 4 are generally less pathogenic, and are enzootic in a variety of wild and domestic animals, in particular wild boar and pigs [4,7,8]. Lately, HEV has been detected in bats and rodents [9,10,11], indicating that these mammals may be a reservoir for HEV and an additional source for transmission to humans. The classification of HEV variants is currently in transition without agreed definitions for genotypes and subtypes or for deeper taxonomic groupings into species and genera. Smith and coworkers [9] recommend a genetic classification of HEV into 4 species as follows: group A, HEV isolates that infect humans or are closely related to such isolates (genotypes 1-4, the 2 wild boar isolates, and the rabbit isolates); group B, avian HEV; group C, bat HEV; and group D, rat HEV and ferret HEV. The more divergent HEV-like virus from fish (cut-throat trout virus) would represent a plausible candidate member of a second genus within the Hepeviridae.
Zoonotic transmission of HEV occurs either by consumption of contaminated meat and meat products, or by contact with infected animals [12]. The virus is ubiquitous in the domestic pig and wild boar population in several European countries. In Germany, a high wild boar percentage was proven to be infected with HEV [13,14,15].
In developing countries, HEV is a major cause of acute hepatitis, transmitted by the fecal-oral route and associated with contamination of drinking water by HEV genotypes 1 and 2. In industrialized countries, HEV infection is being reported more frequently and, while some cases are imported after travel to endemic areas, autochthonous cases caused by genotype 3 are also increasing, and infection with HEV appears more prevalent than originally believed [6,16,17,18].
An alternative route of transmission is by transfusion of blood components, which has been reported in several countries [19,20,21,22,23,24,25]. Acute HEV infections have been identified in blood donors and confirmed by the detection of HEV RNA [26,27,28,29,30]. Recently, it has been demonstrated that HEV infection is underreported. Therefore, HEV infection should be considered in cases where other causes of acute hepatitis have been excluded [31].
The incidence of HEV in the blood donor population is unclear and likely underestimated due to the lack of well-validated assays, asymptomatic infections, lack of testing and the fact that HEV is not a reportable disease in all countries. In Germany, information on the incidence of HEV infections is available on the basis of a requirement to notify the authorities regarding hepatitis infections in compliance with the IfSG (Infektionsschutzgesetz; Infection Protection Act). Estimates of seroprevalence are also variable and thought to depend largely on the different performance characteristics of the assays used in the various studies. Limited studies in blood donors have suggested a significant seroprevalence, but the incidence in donors is not well characterized.
Here we present an update on HEV and its potential role in transfusion medicine, with particular focus on the German situation.
Clinical Picture of HEV Infection
After an incubation period of several weeks, HEV infection manifests itself by symptoms comparable to that of acute hepatitis induced by other hepatitic viruses: abdominal pain, nausea, vomiting and anorexia can occur, as well as pyrexia, jaundice, and hepatomegaly [32,33]. However, the clinical picture of HEV infection varies broadly and many HEV infections are self-limited without any symptoms [34].
Asymptomatic infection are probably very common for HEV genotype 3 infections. In 2008, acute hepatitis E infection was confirmed in passengers returning to the UK after a world cruise. An epidemiological investigation showed 33 cases of an acute HEV infection, but 67% were asymptomatic [35]. Epidemiological data from Southwest England showed that hepatitis E was anicteric in 25% of cases, and usually caused a self-limiting hepatitis predominantly in elderly Caucasian males [36].
However, HEV infection can also take a more severe or even fatal course, resulting in liver failure [33,37]. In particular, a mortality of up to 75% in patients with pre-existing chronic liver disease was described [38]. In developing countries, pregnant women are considered susceptible to such a fatal outcome of HEV infection [39,40]. In developed countries, patients are somewhat older (>45 years of age), are predominantly male with a higher frequency of underlying liver disease or alcohol abuse and a higher frequency of nonspecific symptoms; pregnant women do not show severe disease [6]. The mortality rate is somewhat higher in these areas, probably because of older age and coexistent illnesses.
Moreover, although HEV has been believed for many years to cause acute infections, but without progression to chronic infections, evidence is now emerging that HEV infections are not resolved in every case, and that in immunocompromised patients chronic infections, some with fatal outcome, also occur [41,42,43,44,45,46].
Mortality has been reported by the World Health Organization (WHO) to be 0.5-4.0% of the overall patient population, and to be up to 20% in pregnant women [34]. However, it must be mentioned that the origin of these older data has not been clearly specified, and it should be assumed that they derived from developing countries and thus from countries with insufficient health care and in which the more pathogenic HEV genotypes 1 and 2 are common.
Epidemiology
The spread of HEV among a population can be assessed by determination of antibodies of the IgG class against HEV. Using this method, epidemiological data have been obtained from several European countries: in Sweden, the seroprevalence of HEV has been reported to be 9.3% in the rural population, and to be somewhat, but not significantly, higher in pig farmers (13.0%) [47]. Using samples derived from a serological surveillance program of the Health Protection Agency in the UK, a seroprevalence of 13.0% was found in England in 1991, and of 13.5% in 2004 [48]. Subsequently, although the investigated study population of gynecological and orthopedic hospital admissions was smaller (n = 100) than in the aforementioned report, and probably less representative for the general population, a comparable seroprevalence of 14% was reported from Flanders (Belgium) [49]. This is a higher seroprevalence than that found in another serosurvey undertaken among homeless people in Marseille, which yielded a seroprevalence rate of 11.6% [50]. This value is rather high compared to other regions in Southern Europe: the seroprevalence has been estimated to be 1.5% in San Marino [51], 2.9% [52] in the Latium region in Italy and 1.08% in the community of Madrid [53]. However, before assuming a north-south divide in Europe concerning the seroprevalence of HEV, it should be noted that the compatibility of all these seroprevalence data is limited. In addition to the different antibody assays (with different performance characteristics) used in the reports mentioned, there were also differences in the study populations concerning both magnitude and consistence, and also whether ELISA reactive results were confirmed to be positive by Western blot [51,53].
Epidemiological data from Germany that have been obtained from individuals with occupational exposure to pigs (slaughterers, meat inspectors, veterinarians and pig farmers) [54] yielded a higher seroprevalence rate (28.3%) than the age- and gender-matched control group of non-exposed probands (15.5%). These results are in good accordance to the seroprevalence of 16.8% in the general population in Germany [55]. Thus, the former data reflect the zoonotic transmission of HEV genotype 3 rather than the common epidemiologic situation in Germany. The annual incidence of HEV in the general population in Germany was estimated to be 3.9 per 1,000 persons [55].
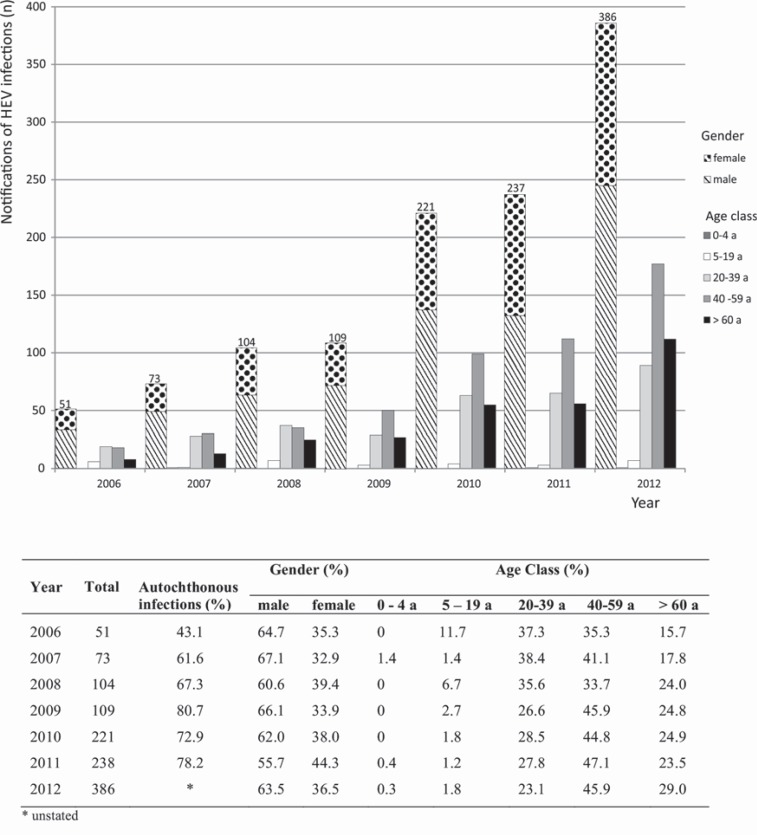
In Germany, information on the incidence of HEV infections (according to the Infection Protection Act) shows that the majority of notified German hepatitis E cases were acquired in Germany, and that there was no connection with travel to endemic areas (fig. 1). The few HEV cases that have so far been phylogenetically analyzed in Germany confirm the close relationship to pig isolates found in various other European countries [56]. The number of HEV infections reported in compliance with the IfSG has increased steadily over the past few years [57], while the proportion of HEV infections in men has remained constant, at about two thirds (fig. 1). In 2012, approximately half of the reported HEV infections in Germany were persons aged 40-59 years. Most blood donors are recruited from this collective. The same trend was seen in other European countries with autochthonous HEV infections, e.g. the UK [58,59] and the Netherlands [60].
Fig. 1.
Notifications of HEV infections in Germany in compliance with the IfSG (Infection Protection Act) (Source: Robert Koch-Institut: SurvStat, www3.rki.de/SurvStat). The overall notification of HEV infections, the proportion of autochthonous acquired HEV infections, and the age and gender distribution is given.
A clear correlation between the seroprevalence of HEV and age is obvious in the majority of the aforementioned reports. An increase in the seroprevalence has been noted in the older population, consistent with lifelong exposure to the virus. The reason for this observation is likely to be related to the mode of transmission of HEV genotype 3: meat or offal consumption is a major route of HEV transmission in Germany and other developed countries [57]. HEV has been detected in retail pork meat products [61,62,63]. The resistance to cooking in temperatures up to 60 °C [64], as well as the seasonal variation of autochthonous hepatitis E with peak in the summer [36], provides evidence that a higher consumption of meat, especially medium rare pork in the summer season (e.g. barbecues) represents a risk for HEV infections.
It might be expected that seroprevalence data obtained from blood donors in different industrialized countries should be more homogeneous due to a more homogeneous study population, but data from several European countries and Japan differ widely: they cover the considerable range of 0.4-52.2%. These data are provided in more detail in table 1, along with the number of blood donors included in the different studies and the methods for antibody screening and confirmation, if confirmation was performed at all. As already noted above, differences are attributed not only to the number of donors investigated, but also to the antibody tests used for the antibody screening, as well as supplemental, confirmatory assays performed. Several test strategies were applied: (1) ELISA was considered positive if a single reactive result was obtained; (2) a repeatedly reactive result was required for a positive result; (3) ELISA-reactive results were confirmed by a second ELISA; (4) confirmation was performed by Western blot analysis; (5) no confirmation of ELISA-reactive results was performed; and (6) Western blot analysis was primary used for screening. Beside differences in the tests, eating habits in the different countries may also be an explanation for the different seroprevalence values. The samples for a French study that determined a seroprevalence of 52.2% had all been collected in the Midi-Pyrénées region. A local delicacy in this region is a cured pig-liver sausage, which is usually eaten uncooked. HEV RNA was detectable in a high proportion of these sausages [65]. However, diet cannot explain the different seroprevalence found by another serosurvey that has been carried out among blood donors in the identical region but with another test [66]. Thus, there is convincing evidence that different sensitivity, as well as different specificity, of the tests used may contribute to the wide-ranging seroprevalence data. Data about the incidence of HEV in blood donors are available only for Germany (0.35% per year [67]).
Table 1.
Seroprevalence of HEV in blood donors
| Country | n | Seroprevalence, % | Method | Year [reference] |
|---|---|---|---|---|
| Japan | 12,600 | 3.4 | ELISA (in-house*, Cosmic corporation**) | 2010 [127] |
| Denmark | 461 | 20.6 | ELISA* (in-house) | 2008 [128] |
| Switzerland | 550 | 4.9 | ELISA* (MP Biomedicals) | 2011 [129] |
| The Netherlands | 1,275 | 0.4 | ELISA* (Abbott, Diagnostic Biotechnology), Westernblot** | 1993 [130] |
| Germany | 336 | 5.94 | Western blot (Mikrogen*), ELISA (MP Biomedicals**) | 2012 [28] |
| Germany | 1,019 | 6.8 | ELISA* (Mikrogen), Western blot** (Mikrogen) | 2013 [67] |
| Germany | 116 | 15.5 | Western blot* (Mikrogen) | 2011 [54] |
| England/Wales | 262 | 10.0 | ELISA* (Wantai), NAT*** (in-house) | 2011 [131] |
| Scotland | 1,559 | 4.7 | ELISA* (Wantai) | 2013 [59] |
| France | 1,998 | 3.2 | ELISA* (Genelabs Diagnostic) | 2007 [132] |
| France | 529 | 16.6 | ELISA* (Genelabs Diagnostics) | 2008 [66] |
| France | 512 | 52.2 | ELISA* (Wantai) | 2011 [65] |
Screening assay.
Supplemental assay for confirmation.
ELISA-positive samples were investigated by NAT.
Blood Transmission
Even if large differences in the seroprevalence in several countries are existent and seroprevalence cannot be estimated exactly, there is clear evidence that a considerable proportion of blood donors were infected with HEV. Furthermore, the common finding of an age-dependent increase in seroprevalence suggests that many infections occur in middle age, and thus during the period of blood donation activity (fig. 1).
HEV RNA was detected in 13 out of 16,125 (0.08%) blood donors [28]. In another study, out of 23,500 donors, 35 (0.14%) were found per year to have detectable HEV RNA [67]. The rate of HEV RNA-positive donations was reported to be 1:7,986 in Sweden and 1:4,525 in Germany [29]. The blood-borne transmission of HEV was demonstrated by the experimental infection of a Rhesus monkey with a plasma sample derived from a donor suffering from an acute HEV infection [68].
However, although presence of HEV RNA in blood donors is thus not a rare event, and HEV is obviously a blood-borne pathogen, only a few cases of transfusion-transmitted HEV infections have been documented from industrialized countries. The first was in 2004, when a case of a clinically manifested HEV infection after transfusion of 23 blood products in Japan was described. A nucleic acid amplification technique (NAT) investigation of archive samples and sequence analysis of the NAT products revealed that the HEV infection could be linked to a fresh frozen plasma: the HEV RNA detected in the donor showed complete identity for 2 distinct regions of HEV genome compared to those detected in the recipient. Although it could not be ruled out that the occurrence in both the patient and the donor was just an accidental coincidence of a HEV strain widespread in Japan, and even though the red blood unit obtained from the same donation as the plasma did not lead to HEV transmission, the report gave further evidence about the transfusion transmissibility of HEV [21]. 2 years later, a transfusion-transmitted HEV infection through a red blood cell unit was reported from the UK. While in the transfusion recipient the infection was asymptomatic apart from a mild jaundice and an elevation of liver enzymes, the donor became ill from an acute HEV infection, and the illness of the donor and diagnosis of HEV infection led to the investigation of the recipient [19]. In the following year, a further case of a child who suffered from transfusion-transmitted HEV infection after administration of a red blood cell unit was reported in France [20]. In both cases, from the UK and France, sequence homology in donor and recipient suggested a correlation between the transfusion and the HEV infection by genotype 3 of the recipients.
Another case of transfusion-transmitted HEV infection was reported from Japan. A retrospective investigation revealed that the donor of a platelet concentrate became infected through consumption of grilled pork 23 days before donation. Subsequently, the infection had been transmitted to the recipient by transfusion [22].
Why transfusion-transmitted HEV infections are so rarely reported remains unclear: it is possible that a frequently subclinical or asymptomatic course occurs in the affected recipients, or that treating physicians may fail to recognize transfusion-transmitted HEV in many cases. An impressive example of the latter scenario was provided by the latest case report of a transfusion-transmitted HEV infection: the symptoms of the acute HEV infection in the recipient were initially misinterpreted as drug-induced liver toxicity and thereafter as an autoimmune disorder, before HEV infection was considered [69]. Moreover, it can also be assumed that the quantity of infectious virus remaining in blood components, and particularly in red blood cell concentrates, is insufficient to infect the recipients in many cases. In one report, no infection of a recipient occurred until 41 days after transfusion of a red blood cell unit taken from a donor with a low-level viremia, presumably below 125 IU/ml plasma [67].
So far there have been no reports about a transmission of HEV through plasma derivatives, although a recent report referred to a contamination of approximately 10% of plasma pools with HEV RNA. However, HEV RNA concentrations were rather low (≤1,000 copies/ml) [70] in all of these contaminated plasma pools. In contrast, no HEV RNA was present in the ready-for-use coagulation factor concentrates derived from 8 different manufacturers in another investigation [71].
As it possesses no envelope, HEV should not be affected by the solvent detergent process, and heat sensitivity of HEV varies depending on the plasma stabilizers and heating conditions. Nanofiltration seems to offer an appropriate measure to remove HEV, but due to the size of HEV, filters with a pore size of approximately 20 nm are required for this purpose [72].
Laboratory Diagnosis
In addition to clinical symptomatic diagnosis, hepatitis E infection is characterized by a number of typical biochemical markers, including bilirubinuria, elevated serum levels of bilirubin and alanine and aspartate aminotransferases (ALT, AST), and in some cases an increase in serum levels of alkaline phosphatase (ALP). However, these biomarkers are not specific to hepatitis E, and also occur in other forms of liver injury. In particular, the formerly used viral surrogate marker ALT is not always elevated in acute HEV infections [28]. Therefore, more specific and sensitive approaches are needed to diagnose HEV infection.
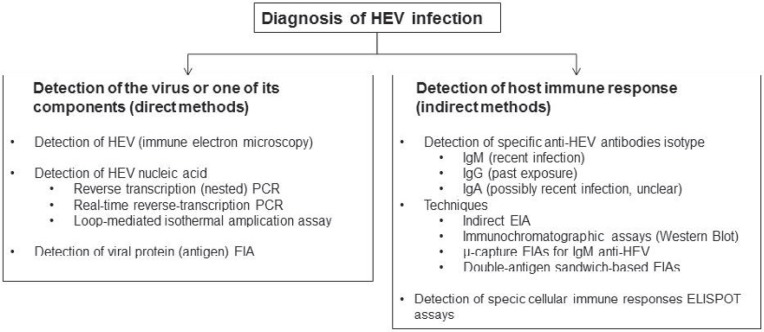
The laboratory diagnosis of HEV infection includes visualization of the pathogen by microscopic assays, detection of 1 of its components, e.g. protein (antigen assay) or nucleic acid (NAT), or indirect determination by detection of antibody against the virus [73]. A survey of direct and indirect approaches is summarized in figure 2.
Fig. 2.
Laboratory diagnosis of HEV infection (adapted from [73]).
Currently, direct cultivation of HEV in cell lines is not a routine method. Early studies reported the propagation of HEV in either primary hepatocytes or several established cell lines, but replication was inefficient. Recently, efficient cell culture systems for HEV in PLC/PRF/5, A549, PICM-19, and HepaRG cells have been established [74,75]. Time will show if these models represent tools for diagnosis, or for studying the viral biology of HEV.
Novel techniques, such as an HEV-specific interferon-gamma (IFN-γ) ELISPOT that measure HEV-specific cell-mediated immune responses are not yet suitable for routine use [76]. This assay might provide a better measure of prior HEV exposure than seroprevalence studies. However, further studies are needed and its application is only for selected cases.
The aims of testing for HEV infection are to differentiate between acute and recent infection. An acute infection status is relevant to blood safety because of the high potential of transfusion-transmitted infection, whereas the later is important for seroepidemiological studies to assess the risk of HEV infection in a population. So far, the most frequently used strategy is the testing for the presence of HEV IgG and IgM in combination with reverse transcriptase (RT) PCR on nucleic acids extracted from stool and serum/plasma in order to detect/exclude acute HEV infection.
Detection of Anti-HEV Antibodies
Diagnosis of hepatitis E is usually determined serologically by detection of the presence of IgM antibodies or rising anti-HEV IgG titers. The 4 HEV genotypes cause very similar antibody responses, suggesting a single serotype [16,77]. In the past, serodiagnosis of hepatitis E demonstrated limitations, including viremia with a relatively small or without any antibody response in symptomatic, as well as symptom-free individuals [78,79], a diverting IgM positivity [80] and undetectable or disappearing anti-HEV IgG antibodies [78,81]. The HEV antigens used until now in enzyme-linked immunosorbent assays (EIAs) were produced synthetically or recombinantly via at least 2 expression systems (Escherichia coli and baculovirus, [82]), differing in the viral strain origin (Pakistani, Burmese, or Mexican) and the viral gene product (ORF2 or ORF3 [83]). Unfortunately, this resulted in a significant variation of assay sensitivities, specificities and performances [84,85,86]. Antigens of most HEV immunoassays were derived from genotype 1 viruses; therefore, their applicability to HEV genotype 3 infections is indeterminate [84]. Vollmer et al. [87] systematically characterized serological assays using seroconversion panels of virologically confirmed HEV genotype 3-infected individuals. The presence of anti-HEV antibodies was determined using various immunological assays: recomWell HEV IgM, recomWell HEV IgG (Mikro-gen), HEV-IgM-ELISA3.0, HEV-ELISA, HEV-ELISA4.0, AssureHEV-IgM Rapid Test (MP Biomedicals), and the Anti-HEV-ELISA (IgM, IgG, Euroimmun). Assay sensitivities were evaluated by testing a serially diluted WHO reference reagent for hepatitis E virus antibody and 1 patient sample. Comparison of anti-hepatitis E virus antibody seroconversion was performed in 10 blood donors. Anti-HEV assays differ in their sensitivities for detecting HEV infection, with anti-HEV IgM assays being more divergent than anti-HEV IgG assays. Furthermore, the detection period of IgM antibodies significantly varies between the different assays: anti-HEV IgM antibodies are detectable over a considerably longer time period using the HEV-IgM-ELISA3.0.
Previous studies have reported that the detection of anti-HEV IgA is a convenient complementary marker for the diagnosis of HEV infection [88,89,90], especially regarding the enhanced specificity of a combination of both anti-HEV IgM and IgA immunoglobulins. Like IgM, IgA anti-HEV antibodies appear during acute hepatitis E. However, Herremans and coworkers [90] reported infections with HEV genotype 3 without an increase of IgA antibodies. Detection of anti-HEV IgA can be a useful supplement for diagnosis of acute HEV infection, especially in patients negative for anti-HEV IgM. In conclusion, little is known about the time course of the IgA response and the diagnostic importance in HEV infection.
Detection of HEV Antigens
As is the case for other transfusion-transmitted viruses (antigens), e.g. hepatitis B virus (HBsAg), HIV-1 (p24 antigen) or hepatitis C virus (core antigen), a suitable HEV antigen assay would be a reasonable addition to the test portfolio, and could allow the easy direct detection of the pathogen in samples such as serum or stool. Single testing of blood donors would then be easier in practice than molecular genetic screening.
The antigenic epitopes used in HEV antibody assays are primarily the capsid (ORF2) protein, and occasionally the ORF3 protein [87]. Despite the genetic variability of HEV genotypes, antibody response targets the capsid epitopes, corresponding to neutralization of the pathogen. Therefore, indirect sandwich EIA detection uses monoclonal antibodies against HEV capsid protein for HEV antigen capture and detection with another biotin-labeled anti-HEV-ORF2 antibody [91,92]. Approximately 44.6% of sera positive for anti-HEV IgM alone, 28.6% positive for both anti-HEV IgM and IgG, and 0% positive for anti-HEV IgG alone were also positive for HEV antigen using this EIA. For 42 HEV antibody-positive sera tested for HEV RNA and antigen in parallel, the concordance was 81.0% (34/42). All PCR products were found to belong to HEV genotype 4. To evaluate the temporal relationship among HEV antigen positivity and HEV RNA, anti-HEV IgG and IgM, and ALT concentrations, macaques were infected with HEV genotypes 1 and 4 and serial samples were collected. The results showed that the antigen EIA can detect the capsid proteins of both genotypes. HEV antigen was detectable prior to ALT elevation and the appearance of anti-HEV antibodies in the infected monkeys, and lasted for several weeks in all cases. HEV antigen became detectable in the serum at almost the same time as HEV RNA in feces, but persisted for 4 weeks less than HEV RNA. This assay should be valuable for the diagnosis of acute hepatitis E, particularly in the window period prior to seroconversion to anti-HEV [91].
The concordances between HEV antigen and HEV RNA, and between HEV antigen and anti-HEV IgM, were 77.1% and 72.9%, respectively, with significant correlations, while that between HEV RNA and anti-HEV IgM was 61.4% with no significant correlation. 11 of 25 samples negative for anti-HEV IgM were positive for HEV antigen. The ALT, AST, ALP, total iron-binding capacity (TBA), gamma-glutamyl transferase (GGT), total bilirubin, and direct bilirubin levels did not differ significantly between the anti-HEV IgM-positive and -negative groups. However, the ALT, AST, ALP, TBA, and GGT levels were significantly higher in the HEV antigen-positive group than in the HEV antigen-negative group. All of the HEV isolates cloned belonged to genotype 4. HEV antigen was highly correlated with HEV RNA and elevated ALT, AST, ALP, TBA, and GGT levels. Testing for HEV antigen in combination with anti-HEV IgM is useful for the diagnosis of HEV infection [92].
In an Indian study, the use of hepatitis E virus antigen detection as an early diagnostic marker in an outbreak, in comparison to anti-HEV IgM and RT-PCR analyses, was determined [92]. The positivity for anti-HEV IgM, HEV antigen, and RT-PCR was 91.6%, 69.4%, and 47.2%, respectively. RT-PCR and HEV antigen detection gave the highest positive results (100%) in the first 3 days of illness. Positive HEV PCR declined to 54% by days 4-7, whereas HEV antigen and IgM detection were 88% and 100%, respectively. HEV antigen was found to be an early diagnostic marker of acute infection, and was detected in 3 additional cases in the early phase (1-3 days), without detectable anti-HEV IgM antibodies. These 3 samples were also positive for HEV RNA. After day 7, anti-HEV IgM was the main diagnostic indicator of infection [93].
Detection of HEV RNA
Today, HEV can be reliably detected using NATs in the active phase of infection in serum, plasma or fecal samples. HEV RNA screening is primarily performed in blood samples (plasma). Several in-house NAT methods have been described using nested RT-PCR [56,93,94,95], real-time RT-PCR [97,98,99,100,101,102] or loop-mediated isothermal amplification [103]. For the later technique, sensitivity higher than that for RT-PCR was shown but experience with this assay is limited to 1 study [103].
The main focus is on real-time detection methods using fluorescent probes. For diagnosis, the majority of HEV RT-PCR assays have used conserved HEV genomic regions as the target for amplification. Considering the wide genetic heterogeneity of HEV isolates, it is critical to design primer and probes that guarantee the development of highly sensitive and broadly reactive assays [17]. One of the most widely used of these real-time RT-PCRs is that developed by Jothikumar and colleagues [97]. Garson et al. [104] recommended an improvement using a minor-groove-binder (MGB)-modified probe. They demonstrated that the MGB-modified probe detected HEV RNA in plasma samples from 6 patients with serologically confirmed hepatitis E in whom the unmodified probe had failed to detect HEV RNA. The sequence analysis of the RT-PCR target ORF3 segment revealed an identical C→T single nucleotide mutation in the probe-binding region in each case.
Therefore, a defined panel of HEV genotypes and isolates is necessary to test the HEV NAT assays. In a collaborative study the performance of HEV NAT assays was evaluated using a panel of HEV-containing plasma samples of genotypes 3a, 3b, 3f, and 4c [105]. The results of the study demonstrated a 100- to 1,000-fold difference in sensitivity between the majority of assays, independent of the virus strain. The broad variability in assay sensitivity between different laboratories illustrated the need for a well-characterized reference standard for use in standardizing NAT assays to detect and quantify HEV RNA. This study was instrumental in establishing a WHO standard for HEV RNA for NAT-based assays. This was endorsed by the WHO Expert Committee on Biological Standardization (ECBS) in 2009 (WHO/BS/09,2126) and, following the initial study, 2 virus strains were selected for further development as a candidate international standard for the WHO and a candidate Japanese National Standard in collaboration with the National Institute of Infectious Diseases (NIID) in Japan. The viral strains being developed as standards are genotype 3a and 3b HEV strains, which were equally well detected in the initial study and belong to the widely distributed genotype 3. Results from the collaborative study to evaluate candidate standards for HEV RNA for use in NAT-based assays were published in October 2011 [106].
In 2012, the first WHO international standard for HEV RNA was established as a genotype 3a strain with a unitage of 250,000 IU/ml [106]. First studies using this standard have provided data to compare HEV NAT assays [28,29].
Currently, there are several commercially available HEV RT-PCR assays that are used for HEV screening studies. Vollmer et al. [28] have demonstrated high sensitivity of real-time PCR assay and applicability for routine blood donor mini-pool screening. Compared to published in-house HEV RT-PCRs, the RealStar HEV RT-PCR Kit (Altona Diagnostics, Hamburg, Germany) showed a 10-fold higher analytical sensitivity. Using a nucleic acid extraction from high-volume plasma (4.8 ml, chemagic viral DNA/RNA reagent kit, PerkinElmer Chemagen Technologie GmbH, Baesweiler, Germany), this real-time assay revealed a 95% lower limit of detection (LOD) of 4.66 IU/ml. Novel assays, e.g. HepatitisE@ceeram Tools™. health kit (Ceeram, La Chapelle-Sur-Erdre, France), ampliCube HEV RT-PCR (Mikrogen, Neuried, Germany) show a lower analytical sensitivity (data not shown [107]).
To estimate the risk of HEV transmission through transfusion, the incidence of HEV infections in the blood donor population has to be analyzed. The NAT screening study recently observed a widespread distribution of HEV in plasma fractionation pools and plasma donations from Sweden and Germany, whereas plasma fractionation pools from the Middle East, as well as 51,075 individual donations from the USA, showed no contamination with HEV RNA [29,70]. Approximately 10% of plasma pools were positive for HEV RNA (sources: North America, Europe, Southeast Asia, [70]) and the rate of individual HEV RNA-positive donors varies from 1:7,986 (0.012%, Sweden), 1:7,040 (0.014%, UK) [109], and 1:4,525 (0.022%, Germany) [29]. Other studies from Asia revealed hepatitis E viremia among blood donors of at least 0.3% (Japan, [110], donors with elevated ALT: 1.1% [27]). However, in Germany, a considerably higher rate of 1:1,240 HEV RNA-positive donors (0.08%) has also been observed [28]. Possible explanations are, on the one hand, effects of the particular donor population, especially within the context of the zoonotic potential and transmission of HEV. On the other hand, this might be due to the fact that this screening method showed a 95% LOD of 4.66 IU/ml, which is increased by factor 50 compared to the assay used by Baylis and coworkers (250 IU/ml [29]). Observed viral loads in this study varied from 18.6 to 2.6 × 104 IU/ml, showing values in similar ranges recently reported for German or Swedish donations (1.6 × 103 to 4.8 ×104 IU/ml [29]) or Japanese blood donors (79 to 3.1 × 107 copies/ml [27].
HEV Prevention and Control of Infection
To prevent HEV infection, the provision of safe drinking water, proper disposal of human feces, and personal hygiene are required. During outbreaks, chlorination or boiling of water is useful. In areas with zoonotic transmission of HEV, proper cooking of meat, especially from pigs, is recommended to prevent food-borne transmission.
As already implemented for the hepatitis A virus, the development of active immunization should be the goal. No HEV vaccine is currently licensed for marketing. The development of a vaccine has so far been hampered because HEV has been difficult to replicate in cell culture [111]. Recent successes in cell culture of HEV have been recorded [112], possibly leading to vaccine development and a better understanding of the virus biology. At present, no vaccines on the basis of inactivated viruses or nonpathogenic isolates are available [112]. However, other approaches, such as DNA-based vaccines, or recombinant proteins, able to induce both cellular and antibody response are under evaluation [111,114]. Several HEV immunization studies are based mainly on recombinant proteins. The ORF2 protein (capsid) has been considered the best candidate for HEV vaccine because it contains a neutralization epitope [115] and is cross-reactive with all mammalian HEV [116].
Although HEV vaccine trials, including trials conducted in populations in southern Asia, have shown candidate vaccines to be effective and well-tolerated, these vaccines have not yet been produced or made available to susceptible populations [117,118]. However, prospects for control of HEV infection are encouraged by recent efforts in vaccine development [119,120]. A new recombinant HEV vaccine has been developed by Chinese scientists that protects recipients from both infection (> 70% efficacy) and disease (>90% efficacy) for up to 3 years [120]. This vaccine will be the first that is commercially available and has been licensed for production and sale by the State Food and Drug Administration of China [121]. However, no comparative data on the safety and immunogenicity of these vaccines are available and the focus of studies has been on clinical disease and not HEV infection rates. It is thus unclear whether these vaccines can reduce HEV transmission.
Additionally, whether HEV vaccines should be used for the general population in high endemic areas or only for high-risk groups (such as patients with chronic liver disease, immunosuppressed persons, pregnant women or children) has to be discussed.
Treatment for acute or chronic HEV infection is generally supportive. Most patients need no specific treatment because the illness is self-limiting. Some patients with severe acute HEV infection have responded to ribavirin therapy, but this treatment is contraindicated in pregnant women. IFN-α-2a/-2b and ribavirin therapy, separately or in combination, has been used for patients with chronic infection [122,123,124,125].
Conclusion
The risk of transfusion-transmitted HEV infection is still under discussion. Risk assessment is difficult to determine due to the large proportion of asymptomatic and undiagnosed HEV infections, the unknown efficiency of pathogen reduction techniques, and the lack of data from long-term systematic routine NAT screening. Severe or fatal HEV infections with a high morbidity and mortality have been observed in pregnant women, immunosuppressed individuals, and patients with pre-existing liver disease. Chronic disease progression, particularly observed in solid organ transplant recipients, raise concerns because treatment is still evolving and vaccines are not yet available. Basically information on HEV virology and epidemiology is required combined with the development of seroconversion and/or genotype-specific panels and standards to allow validation of NAT and serological assays. In the short-term view, studies in blood donors using validated, standardized NAT assays are feasible to evaluate the potential exposure risk to transfusion recipients. For this purpose, the first WHO International Standard for HEV RNA [105,107] and a panel of virologically confirmed clinical samples [87] would help to characterize NAT assays. The NAT-based screening of blood donors in large-scale studies will provide data on HEV incidence, and will increase the safety of blood components by exclusion of viremic (NAT-positive) donors. Recipients of blood transfusion should be analyzed using serological and NAT assays to assess whether transfusion-transmitted HEV can be confirmed in available repository samples or in prospective studies.
The risk of HEV transmission by plasma products is currently estimated to be low since steps have been introduced for most of the products (except for solvent/detergent-treated plasma) that are considered to be at least partly effective in deactivating or removing HEV [30]. The data available on non-enveloped model viruses, such as feline calicivirus, cannot clearly be transferred to HEV. In addition, the effectiveness of the deactivation methods developed for plasma and cellular blood products is currently unknown. Therefore, it has to be considered whether HEV NAT screening should be adopted in the routine testing procedure for blood donations. Currently, HEV NAT screening is the only precautionary measure to prevent transfusion-transmitted HEV infection, as neither antibody detection nor surrogate markers for HEV infection, especially ALT measurement, correlate with the acute HEV infection and detection of HEV RNA in plasma, respectively. Only HEV antigen tests may possibly offer a feasible screening method in the future. This is very important for cellular blood components that cannot be treated with pathogen inactivation, or retesting after quarantine storage.
Actually, the proposal is to amend the European pharmacopoeia monograph 1646 – human plasma (pooled and treated for virus inactivation) [126]. The amendment would see the introduction of HEV NAT with a possible implementation in January 2015. The authors conclude that recent studies provide clear evidence for a transfusion-associated risk of HEV. Therefore, HEV NAT screening for blood products is a meaningful consideration for the near future.
Recent successful clinical testing of HEV vaccines bodes well for the future, perhaps establishing a vaccination program for children and/or blood donors combining hepatitis A, B and E virus.
Disclosure Statement
The authors declare that they have no conflict of interest.
| Gender (%) | Age Class (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Total | Autochthonous infections (%) | |||||||
| male | female | 0 - 4 a | 5 – 19 a | 20-39 a | 40-59 a | > 60 a | |||
| 2006 | 51 | 43.1 | 64.7 | 35.3 | 0 | 11.7 | 37.3 | 35.3 | 15.7 |
| 2007 | 73 | 61.6 | 67.1 | 32.9 | 1.4 | 1.4 | 38.4 | 41.1 | 17.8 |
| 2008 | 104 | 67.3 | 60.6 | 39.4 | 0 | 6.7 | 35.6 | 33.7 | 24.0 |
| 2009 | 109 | 80.7 | 66.1 | 33.9 | 0 | 2.7 | 26.6 | 45.9 | 24.8 |
| 2010 | 221 | 72.9 | 62.0 | 38.0 | 0 | 1.8 | 28.5 | 44.8 | 24.9 |
| 2011 | 238 | 78.2 | 55.7 | 44.3 | 0.4 | 1.2 | 27.8 | 47.1 | 23.5 |
| 2012 | 386 | * | 63.5 | 36.5 | 0.3 | 1.8 | 23.1 | 45.9 | 29.0 |
unstated
Acknowledgements
The authors thank Tanja Vollmer and Holger Hennig for critical reading of this manuscript, as well as Sarah Kirkby for her linguistic advice.
References
- 1.Payne CJ, Ellis TM, Plant SL, et al. Sequence data suggests big liver and spleen disease virus (blsv) is genetically related to hepatitis E virus. Vet Microbiol. 1999;68:119–125. doi: 10.1016/s0378-1135(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 2.Batts W, Yun S, Hedrick R, Winton J. A novel member of the family hepeviridae from cutthroat trout (oncorhynchus clarkii) Virus Res. 2011;158:116–123. doi: 10.1016/j.virusres.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: Genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5–36. doi: 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- 4.Meng XJ. Hepatitis E virus: Animal reservoirs and zoonotic risk. Vet Microbiol. 2010;140:256–265. doi: 10.1016/j.vetmic.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purdy MA, Khudyakov YE. The molecular epidemiology of hepatitis E virus infection. Virus Res. 2011;161:31–39. doi: 10.1016/j.virusres.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal R, Jameel S. Hepatitis E. Hepatology. 2011;54:2218–2226. doi: 10.1002/hep.24674. [DOI] [PubMed] [Google Scholar]
- 7.Casas M, Martin M. Hepatitis E virus and pigs: A zoonotic risk in Europe? Vet J. 2010;186:135–136. doi: 10.1016/j.tvjl.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Bachlein C, Grummer B. [Hepatitis E – a new zoonotic disease in Germany?] Berl Munch Tierarztl Wochenschr. 2010;123:198–204. [PubMed] [Google Scholar]
- 9.Smith DB, Purdy MA, Simmonds P. Genetic variability and the classification of hepatitis E virus. J Virol. 2013;87:4161–4169. doi: 10.1128/JVI.02762-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drexler JF, Seelen A, Corman VM, et al. Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family hepeviridae. J Virol. 2012;86:9134–9147. doi: 10.1128/JVI.00800-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johne R, Heckel G, Plenge-Bonig A, et al. Novel hepatitis E virus genotype in norway rats, Germany. Emerg Infect Dis. 2010;16:1452–1455. doi: 10.3201/eid1609.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purcell RH, Emerson SU. Hidden danger: The raw facts about hepatitis E virus. J Infect Dis. 2010;202:819–821. doi: 10.1086/655900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schielke A, Sachs K, Lierz M, et al. Detection of hepatitis E virus in wild boars of rural and urban regions in Germany and whole genome characterization of an endemic strain. Virol J. 2009;6:58. doi: 10.1186/1743-422X-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaci S, Nockler K, Johne R. Detection of hepatitis E virus in archived German wild boar serum samples. Vet Microbiol. 2008;128:380–385. doi: 10.1016/j.vetmic.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Baechlein C, Schielke A, Johne R, et al. Prevalence of hepatitis E virus-specific antibodies in sera of German domestic pigs estimated by using different assays. Vet Microbiol. 2010;144:187–191. doi: 10.1016/j.vetmic.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 17.Pelosi E, Clarke I. Hepatitis E: A complex and global disease. Emerg Health Threats J. 2008;1:e8. doi: 10.3134/ehtj.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis HC, Wichmann O, Duizer E. Transmission routes and risk factors for autochthonous hepatitis E virus infection in europe: A systematic review. Epidemiol Infect. 2010;138:145–166. doi: 10.1017/S0950268809990847. [DOI] [PubMed] [Google Scholar]
- 19.Boxall E, Herborn A, Kochethu G, et al. Transfusion-transmitted hepatitis E in a ‘nonhyperendemic’ country. Transfus Med. 2006;16:79–83. doi: 10.1111/j.1365-3148.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 20.Colson P, Coze C, Gallian P, et al. Transfusion-associated hepatitis E, france. Emerg Infect Dis. 2007;13:648–649. doi: 10.3201/eid1304.061387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsubayashi K, Nagaoka Y, Sakata H, et al. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion. 2004;44:934–940. doi: 10.1111/j.1537-2995.2004.03300.x. [DOI] [PubMed] [Google Scholar]
- 22.Matsubayashi K, Kang JH, Sakata H, et al. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion. 2008;48:1368–1375. doi: 10.1111/j.1537-2995.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 23.Tamura A, Shimizu YK, Tanaka T, et al. Persistent infection of hepatitis E virus transmitted by blood transfusion in a patient with T-cell lymphoma. Hepatol Res. 2007;37:113–120. doi: 10.1111/j.1872-034X.2007.00024.x. [DOI] [PubMed] [Google Scholar]
- 24.Mitsui T, Tsukamoto Y, Yamazaki C, et al. Prevalence of hepatitis E virus infection among hemodialysis patients in Japan: Evidence for infection with a genotype 3 HEV by blood transfusion. J Med Virol. 2004;74:563–572. doi: 10.1002/jmv.20215. [DOI] [PubMed] [Google Scholar]
- 25.Khuroo MS, Kamili S, Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol. 2004;19:778–784. doi: 10.1111/j.1440-1746.2004.03437.x. [DOI] [PubMed] [Google Scholar]
- 26.Guo QS, Yan Q, Xiong JH, et al. Prevalence of hepatitis E virus in chinese blood donors. J Clin Microbiol. 2010;48:317–318. doi: 10.1128/JCM.01466-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakata H, Matsubayashi K, Takeda H, et al. A nationwide survey for hepatitis E virus prevalence in japanese blood donors with elevated alanine aminotransferase. Transfusion. 2008;48:2568–2576. doi: 10.1111/j.1537-2995.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- 28.Vollmer T, Diekmann J, Johne R, et al. Novel approach for detection of hepatitis E virus infection in German blood donors. J Clin Microbiol. 2012;50:2708–2713. doi: 10.1128/JCM.01119-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baylis SA, Gartner T, Nick S, et al. Occurrence of hepatitis E virus RNA in plasma donations from sweden, Germany and the United States. Vox Sang. 2012;103:89–90. doi: 10.1111/j.1423-0410.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- 30.Arbeitskreis Blut UBBK. Hepatitis E virus. Transfus Med Hemother. 2009;36:40–47. doi: 10.1159/000197321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waar K, Herremans MM, Vennema H, et al. Hepatitis E is a cause of unexplained hepatitis in the netherlands. J Clin Virol. 2005;33:145–149. doi: 10.1016/j.jcv.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Balayan MS, Andjaparidze AG, Savinskaya SS, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecaloral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 33.Sainokami S, Abe K, Kumagai I, et al. Epidemiological and clinical study of sporadic acute hepatitis E caused by indigenous strains of hepatitis E virus in Japan compared with acute hepatitis A. J Gastroenterol. 2004;39:640–648. doi: 10.1007/s00535-003-1359-5. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization DoCDSaR Hepatitis E. 2001
- 35.Said B, Ijaz S, Kafatos G, et al. Hepatitis E outbreak on cruise ship. Emerg Infect Dis. 2009;15:1738–1744. doi: 10.3201/eid1511.091094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalton HR, Stableforth W, Thurairajah P, et al. Autochthonous hepatitis E in southwest England: Natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784–790. doi: 10.1097/MEG.0b013e3282f5195a. [DOI] [PubMed] [Google Scholar]
- 37.Pfefferle S, Frickmann H, Gabriel M, et al. Fatal course of an autochthonous hepatitis E virus infection in a patient with leukemia in Germany. Infection. 2012;40:451–454. doi: 10.1007/s15010-011-0220-7. [DOI] [PubMed] [Google Scholar]
- 38.Dalton HR, Hazeldine S, Banks M, et al. Locally acquired hepatitis E in chronic liver disease. Lancet. 2007;369:1260. doi: 10.1016/S0140-6736(07)60595-9. [DOI] [PubMed] [Google Scholar]
- 39.Jilani N, Das BC, Husain SA, et al. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J Gastroenterol Hepatol. 2007;22:676–682. doi: 10.1111/j.1440-1746.2007.04913.x. [DOI] [PubMed] [Google Scholar]
- 40.Patra S, Kumar A, Trivedi SS, et al. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med. 2007;147:28–33. doi: 10.7326/0003-4819-147-1-200707030-00005. [DOI] [PubMed] [Google Scholar]
- 41.Gerolami R, Moal V, Picard C, Colson P. Hepatitis E virus as an emerging cause of chronic liver disease in organ transplant recipients. J Hepatol. 2009;50:622–624. doi: 10.1016/j.jhep.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Gerolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med. 2008;358:859–860. doi: 10.1056/NEJMc0708687. [DOI] [PubMed] [Google Scholar]
- 43.Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 44.Halac U, Beland K, Lapierre P, et al. Cirrhosis due to chronic hepatitis E infection in a child post-bone marrow transplant. J Pediatr. 2012;160:871–874. doi: 10.1016/j.jpeds.2012.01.028. e871. [DOI] [PubMed] [Google Scholar]
- 45.de Niet A, Zaaijer HL, ten Berge I, et al. Chronic hepatitis E after solid organ transplantation. Neth J Med. 2012;70:261–266. [PubMed] [Google Scholar]
- 46.Pischke S, Stiefel P, Franz B, et al. Chronic hepatitis E in heart transplant recipients. Am J Transplant. 2012;12:3128–3133. doi: 10.1111/j.1600-6143.2012.04200.x. [DOI] [PubMed] [Google Scholar]
- 47.Olsen B, Axelsson-Olsson D, Thelin A, Weiland O. Unexpected high prevalence of IgG-antibodies to hepatitis E virus in Swedish pig farmers and controls. Scand J Infect Dis. 2006;38:55–58. doi: 10.1080/00365540500321470. [DOI] [PubMed] [Google Scholar]
- 48.Ijaz S, Vyse AJ, Morgan D, et al. Indigenous hepatitis E virus infection in England: More common than it seems. J Clin Virol. 2009;44:272–276. doi: 10.1016/j.jcv.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Van Hoecke F, Van Maerken T, De Boulle M, et al. Hepatitis E seroprevalence in East and West Flanders, Belgium. Acta Gastroenterol Belg. 2012;75:322–324. [PubMed] [Google Scholar]
- 50.Kaba M, Brouqui P, Richet H, et al. Hepatitis E virus infection in sheltered homeless persons, France. Emerg Infect Dis. 2010;16:1761–1763. doi: 10.3201/eid1611.091890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rapicetta M, Kondili LA, Pretolani S, et al. Seroprevalence and anti-HEV persistence in the general population of the republic of San Marino. J Med Virol. 1999;58:49–53. doi: 10.1002/(sici)1096-9071(199905)58:1<49::aid-jmv7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 52.Vulcano A, Angelucci M, Candelori E, et al. HEV prevalence in the general population and among workers at zoonotic risk in Latium region. Ann Ig. 2007;19:181–186. [PubMed] [Google Scholar]
- 53.Fogeda M, Avellon A, Echevarria JM. Prevalence of specific antibody to hepatitis E virus in the general population of the community of Madrid, Spain. J Med Virol. 2012;84:71–74. doi: 10.1002/jmv.22270. [DOI] [PubMed] [Google Scholar]
- 54.Krumbholz A, Mohn U, Lange J, et al. Prevalence of hepatitis E virus-specific antibodies in humans with occupational exposure to pigs. Med Microbiol Immunol. 2012;201:239–244. doi: 10.1007/s00430-011-0210-5. [DOI] [PubMed] [Google Scholar]
- 55.Faber MS, Wenzel JJ, Jilg W, et al. Hepatitis E virus seroprevalence among adults, Germany. Emerg Infect Dis. 2012;18:1654–1657. doi: 10.3201/eid1810.111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Preiss JC, Plentz A, Engelmann E, et al. Autochthonous hepatitis E virus infection in Germany with sequence similarities to other European isolates. Infection. 2006;34:173–175. doi: 10.1007/s15010-006-4132-x. [DOI] [PubMed] [Google Scholar]
- 57.Wichmann O, Schimanski S, Koch J, et al. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis. 2008;198:1732–1741. doi: 10.1086/593211. [DOI] [PubMed] [Google Scholar]
- 58.Lewis HC, Boisson S, Ijaz S, et al. Hepatitis E in England and Wales. Emerg Infect Dis. 2008;14:165–167. doi: 10.3201/eid1401.070307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cleland A, Smith L, Crossan C, et al. Hepatitis E virus in Scottish blood donors. Vox Sang. 2013;105:283–289. doi: 10.1111/vox.12056. [DOI] [PubMed] [Google Scholar]
- 60.Borgen K, Herremans T, Duizer E, et al. Non-travel related hepatitis E virus genotype 3 infections in the Netherlands; a case series 2004-2006. BMC Infect Dis. 2008;8:61. doi: 10.1186/1471-2334-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yazaki Y, Mizuo H, Takahashi M, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003;84:2351–2357. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- 62.Rutjes SA, Lodder WJ, Lodder-Verschoor F, et al. Sources of hepatitis E virus genotype 3 in the Netherlands. Emerg Infect Dis. 2009;15:381–387. doi: 10.3201/eid1503.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colson P, Borentain P, Queyriaux B, et al. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis. 2010;202:825–834. doi: 10.1086/655898. [DOI] [PubMed] [Google Scholar]
- 64.Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J Infect Dis. 2005;192:930–933. doi: 10.1086/432488. [DOI] [PubMed] [Google Scholar]
- 65.Mansuy JM, Bendall R, Legrand-Abravanel F, et al. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis. 2011;17:2309–2312. doi: 10.3201/eid1712.110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mansuy JM, Legrand-Abravanel F, Calot JP, et al. High prevalence of anti-hepatitis E virus antibodies in blood donors from south west France. J Med Virol. 2008;80:289–293. doi: 10.1002/jmv.21056. [DOI] [PubMed] [Google Scholar]
- 67.Juhl D, Baylis SA, Blumel J, et al. Seroprevalence and incidence of hepatitis E virus infection in German blood donors. Transfusion 2013; 10.1111/trf.12121. [DOI] [PubMed]
- 68.Xia NS, Zhang J, Zheng YJ, et al. Transfusion of plasma from a blood donor induced hepatitis E in rhesus monkey. Vox Sang. 2004;86:45–47. doi: 10.1111/j.0042-9007.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- 69.Haim-Boukobza S, Ferey MP, Vetillard AL, et al. Transfusion-transmitted hepatitis E in a misleading context of autoimmunity and drug-induced toxicity. J Hepatol. 2012;57:1374–1378. doi: 10.1016/j.jhep.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Baylis SA, Koc O, Nick S, Blumel J. Widespread distribution of hepatitis E virus in plasma fractionation pools. Vox Sang. 2012;102:182–183. doi: 10.1111/j.1423-0410.2011.01527.x. [DOI] [PubMed] [Google Scholar]
- 71.Modrow S, Wenzel JJ, Schimanski S, et al. Prevalence of nucleic acid sequences specific for human parvoviruses, hepatitis A and hepatitis E viruses in coagulation factor concentrates. Vox Sang. 2011;100:351–358. doi: 10.1111/j.1423-0410.2010.01445.x. [DOI] [PubMed] [Google Scholar]
- 72.Yunoki M, Yamamoto S, Tanaka H, et al. Extent of hepatitis E virus elimination is affected by stabilizers present in plasma products and pore size of nanofilters. Vox Sang. 2008;95:94–100. doi: 10.1111/j.1423-0410.2008.01078.x. [DOI] [PubMed] [Google Scholar]
- 73.Aggarwal R. Diagnosis of hepatitis E. Nat Rev Gastroenterol Hepatol. 2012;10:24–33. doi: 10.1038/nrgastro.2012.187. [DOI] [PubMed] [Google Scholar]
- 74.Okamoto H. Hepatitis E virus cell culture models. Virus Res. 2011;161:65–77. doi: 10.1016/j.virusres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 75.Rogee S, Talbot N, Caperna T, et al. New models of hepatitis E virus replication in human and porcine hepatocyte cell lines. J Gen Virol. 2013;94:549–558. doi: 10.1099/vir.0.049858-0. [DOI] [PubMed] [Google Scholar]
- 76.Shata MT, Barrett A, Shire NJ, et al. Characterization of hepatitis E-specific cell-mediated immune response using IFN-gamma ELISPOT assay. J Immunol Methods. 2007;328:152–161. doi: 10.1016/j.jim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engle RE, Yu C, Emerson SU, et al. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J Clin Microbiol. 2002;40:4576–4580. doi: 10.1128/JCM.40.12.4576-4580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mansuy JM, Peron JM, Bureau C, et al. Immunologically silent autochthonous acute hepatitis E virus infection in France. J Clin Microbiol. 2004;42:912–913. doi: 10.1128/JCM.42.2.912-913.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nicand E, Grandadam M, Teyssou R, et al. Viraemia and faecal shedding of HEV in symptom-free carriers. Lancet. 2001;357:68–69. doi: 10.1016/S0140-6736(05)71568-3. [DOI] [PubMed] [Google Scholar]
- 80.Zhang JZ, Im SW, Lau SH, et al. Occurrence of hepatitis E virus IgM, low avidity IgG serum antibodies, and viremia in sporadic cases of non-A, -B, and -C acute hepatitis. J Med Virol. 2002;66:40–48. doi: 10.1002/jmv.2109. [DOI] [PubMed] [Google Scholar]
- 81.Bendall R, Ellis V, Ijaz S, et al. Serological response to hepatitis E virus genotype 3 infection: IgG quantitation, avidity, and IgM response. J Med Virol. 2008;80:95–101. doi: 10.1002/jmv.21033. [DOI] [PubMed] [Google Scholar]
- 82.Ferguson M, Walker D, Mast E, Fields H. Report of a collaborative study to assess the suitability of a reference reagent for antibodies to hepatitis E virus. Biologicals. 2002;30:43–48. doi: 10.1006/biol.2001.0315. [DOI] [PubMed] [Google Scholar]
- 83.Yu C, Engle RE, Bryan JP, et al. Detection of immunoglobulin M antibodies to hepatitis E virus by class capture enzyme immunoassay. Clin Diagn Lab Immunol. 2003;10:579–586. doi: 10.1128/CDLI.10.4.579-586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bendall R, Ellis V, Ijaz S, et al. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 85.Drobeniuc J, Meng J, Reuter G, et al. Serologic assays specific to immunoglobulin M antibodies against hepatitis E virus: pangenotypic evaluation of performances. Clin Infect Dis. 2010;51:e24–27. doi: 10.1086/654801. [DOI] [PubMed] [Google Scholar]
- 86.Myint KS, Endy TP, Gibbons RV, et al. Evaluation of diagnostic assays for hepatitis E virus in outbreak settings. J Clin Microbiol. 2006;44:1581–1583. doi: 10.1128/JCM.44.4.1581-1583.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vollmer T, Diekmann J, Eberhardt M, Knabbe C, Dreier J.Anti-hepatitis E virus antibody seroconversion in ten blood donors with hepatitis E virus infection: Systematic evaluation of test characteristic of seven anti-HEV assays. submitted for publication 2013
- 88.Takahashi M, Kusakai S, Mizuo H, et al. Simultaneous detection of immunoglobulin A (IgA) and IgM antibodies against hepatitis E virus (HEV) is highly specific for diagnosis of acute HEV infection. J Clin Microbiol. 2005;43:49–56. doi: 10.1128/JCM.43.1.49-56.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang S, Tian D, Zhang Z, et al. Clinical significance of anti-HEV IgA in diagnosis of acute genotype 4 hepatitis E virus infection negative for anti-HEV IgM. Dig Dis Sci. 2009;54:2512–2518. doi: 10.1007/s10620-008-0657-4. [DOI] [PubMed] [Google Scholar]
- 90.Herremans M, Duizer E, Jusic E, Koopmans MP. Detection of hepatitis E virus-specific immunoglobulin A in patients infected with hepatitis E virus genotype 1 or 3. Clin Vaccine Immunol. 2007;14:276–280. doi: 10.1128/CVI.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang F, Li X, Li Z, et al. Detection of HEV antigen as a novel marker for the diagnosis of hepatitis E. J Med Virol. 2006;78:1441–1448. doi: 10.1002/jmv.20717. [DOI] [PubMed] [Google Scholar]
- 92.Zhao C, Li L, Harrison TJ, et al. Relationships among viral diagnostic markers and markers of liver function in acute hepatitis E. J Gastroenterol. 2009;44:139–145. doi: 10.1007/s00535-008-2281-7. [DOI] [PubMed] [Google Scholar]
- 93.Majumdar M, Singh MP, Pujhari SK, et al. Hepatitis E virus antigen detection as an early diagnostic marker: report from India. J Med Virol. 2013;85:823–827. doi: 10.1002/jmv.23529. [DOI] [PubMed] [Google Scholar]
- 94.Inoue J, Takahashi M, Yazaki Y, et al. Development and validation of an improved RT-PCR assay with nested universal primers for detection of hepatitis E virus strains with significant sequence divergence. J Virol Methods. 2006;137:325–333. doi: 10.1016/j.jviromet.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 95.Zaki Mel S, Foud MF, Mohamed AF. Value of hepatitis E virus detection by cell culture compared with nested PCR and serological studies by IgM and IgG. FEMS Immunol Med Microbiol. 2009;56:73–79. doi: 10.1111/j.1574-695X.2009.00552.x. [DOI] [PubMed] [Google Scholar]
- 96.Choi C, Ha SK, Chae C. Development of nested RT-PCR for the detection of swine hepatitis E virus in formalin-fixed, paraffin-embedded tissues and comparison with in situ hybridization. J Virol Methods. 2004;115:67–71. doi: 10.1016/j.jviromet.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Jothikumar N, Cromeans TL, Robertson BH, et al. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65–71. doi: 10.1016/j.jviromet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 98.Ward P, Poitras E, Leblanc D, et al. Comparative analysis of different TaqMan real-time RT-PCR assays for the detection of swine hepatitis E virus and integration of feline calicivirus as internal control. J Appl Microbiol. 2009;106:1360–1369. doi: 10.1111/j.1365-2672.2008.04104.x. [DOI] [PubMed] [Google Scholar]
- 99.Gyarmati P, Mohammed N, Norder H, et al. Universal detection of hepatitis E virus by two real-time PCR assays: TaqMan and primer-probe energy transfer. J Virol Methods. 2007;146:226–235. doi: 10.1016/j.jviromet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 100.Adlhoch C, Kaiser M, Pauli G, et al. Indigenous hepatitis E virus infection of a plasma donor in Germany. Vox Sang. 2009;97:303–308. doi: 10.1111/j.1423-0410.2009.01211.x. [DOI] [PubMed] [Google Scholar]
- 101.Ahn JM, Rayamajhi N, Gyun Kang S, Sang Yoo H. Comparison of real-time reverse transcriptase-polymerase chain reaction and nested or commercial reverse transcriptase-polymerase chain reaction for the detection of hepatitis E virus particle in human serum. Diagn Microbiol Infect Dis. 2006;56:269–274. doi: 10.1016/j.diagmicrobio.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 102.Enouf V, Dos Reis G, Guthmann JP, et al. Validation of single real-time taqman PCR assay for the detection and quantitation of four major genotypes of hepatitis E virus in clinical specimens. J Med Virol. 2006;78:1076–1082. doi: 10.1002/jmv.20665. [DOI] [PubMed] [Google Scholar]
- 103.Lan X, Yang B, Li BY, et al. Reverse transcription-loop-mediated isothermal amplification assay for rapid detection of hepatitis E virus. J Clin Microbiol. 2009;47:2304–2306. doi: 10.1128/JCM.00498-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garson JA, Ferns RB, Grant PR, et al. Minor groove binder modification of widely used taqman probe for hepatitis E virus reduces risk of false negative real-time PCR results. J Virol Methods. 2012;186:157–160. doi: 10.1016/j.jviromet.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 105.Baylis SA, Hanschmann KM, Blumel J, Nubling CM. Standardization of hepatitis E virus (HEV) nucleic acid amplification technique-based assays: An initial study to evaluate a panel of HEV strains and investigate laboratory performance. J Clin Microbiol. 2011;49:1234–1239. doi: 10.1128/JCM.02578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baylis SA, Mizusawa S, Okada Y, Hanschmann KO. Collaborative study to establish a World Health Organization International Standard for hepatitis E virus RNA for nucleic acid amplification technology (NAT)-based assays. WHO Report 2011;WHO/BS/2011.2175.
- 107.Baylis SA, Blümel J, Mizusawa S, et al. World Health Organization International Standard to harmonize assays for detection of hepatitis E RNA. Emerg Infect Dis. 2013;19:729–735. doi: 10.3201/eid1905.121845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abravanel F, Chapuy-Regaud S, Lhomme S, et al. Performance of two commercial assays for detecting HEV RNA in acute or chronic infections. J Clin Microbiol. 2013;51:1913–1916. doi: 10.1128/JCM.00661-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ijaz S, Szypulska R, Tettmar KI, et al. Detection of hepatitis E virus RNA in plasma mini-pools from blood donors in England. Vox Sang. 2012;102:272. doi: 10.1111/j.1423-0410.2011.01554.x. [DOI] [PubMed] [Google Scholar]
- 110.Fukuda S, Ishikawa M, Ochiai N, et al. Unchanged high prevalence of antibodies to hepatitis E virus (HEV) and HEV RNA among blood donors with an elevated alanine aminotransferase level in Japan during 1991-2006. Arch Virol. 2007;152:1623–1635. doi: 10.1007/s00705-007-0996-z. [DOI] [PubMed] [Google Scholar]
- 111.Worm HC, Wirnsberger G. Hepatitis E vaccines: progress and prospects. Drugs. 2004;64:1517–1531. doi: 10.2165/00003495-200464140-00002. [DOI] [PubMed] [Google Scholar]
- 112.Tanaka T, Takahashi M, Takahashi H, et al. Development and characterization of a genotype 4 hepatitis E virus cell culture system using a HE-Jf5/15f strain recovered from a fulminant hepatitis patient. J Clin Microbiol. 2009;47:1906–1910. doi: 10.1128/JCM.00629-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Emerson SU, Purcell RH. Recombinant vaccines for hepatitis E. Trends Mol Med. 2001;7:462–466. doi: 10.1016/s1471-4914(01)02106-2. [DOI] [PubMed] [Google Scholar]
- 114.Kamili S, Spelbring J, Carson D, Krawczynski K. Protective efficacy of hepatitis E virus DNA vaccine administered by gene gun in the cynomolgus macaque model of infection. J Infect Dis. 2004;189:258–264. doi: 10.1086/380801. [DOI] [PubMed] [Google Scholar]
- 115.Zhou YH, Purcell RH, Emerson SU. A truncated orf2 protein contains the most immunogenic site on orf2: Antibody responses to non-vaccine sequences following challenge of vaccinated and non-vaccinated macaques with hepatitis E virus. Vaccine. 2005;23:3157–3165. doi: 10.1016/j.vaccine.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 116.Meng J, Dai X, Chang JC, et al. Identification and characterization of the neutralization epitope(s) of the hepatitis E virus. Virology. 2001;288:203–211. doi: 10.1006/viro.2001.1093. [DOI] [PubMed] [Google Scholar]
- 117.Purcell RH, Nguyen H, Shapiro M, et al. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine. 2003;21:2607–2615. doi: 10.1016/s0264-410x(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 118.Li SW, Zhang J, Li YM, et al. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine. 2005;23:2893–2901. doi: 10.1016/j.vaccine.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 119.Shrestha MP, Scott RM, Joshi DM, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356:895–903. doi: 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- 120.Zhu FC, Zhang J, Zhang XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, doubleblind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 121.Labrique AB, Sikder SS, Krain LJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18:1401–1404. doi: 10.3201/eid1809.120241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kamar N, Rostaing L, Abravanel F, et al. Ribavirin therapy inhibits viral replication on patients with chronic hepatitis E virus infection. Gastroenterology. 2010;139:1612–1618. doi: 10.1053/j.gastro.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 123.Kamar N, Abravanel F, Garrouste C, et al. Three-month pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol Dial Transplant. 2010;25:2792–2795. doi: 10.1093/ndt/gfq282. [DOI] [PubMed] [Google Scholar]
- 124.Kamar N, Rostaing L, Abravanel F, et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis. 2010;50:e30–33. doi: 10.1086/650488. [DOI] [PubMed] [Google Scholar]
- 125.Chaillon A, Sirinelli A, De Muret A, et al. Sustained virologic response with ribavirin in chronic hepatitis E virus infection in heart transplantation. J Heart Lung Transplant. 2011;30:841–843. doi: 10.1016/j.healun.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 126.Baylis SA. Hepatitis E virus issues for plasma products. Oral presentation, 20th IPFA/PEI Workshop, Helsinki Finland 2013.
- 127.Takeda H, Matsubayashi K, Sakata H, et al. A nationwide survey for prevalence of hepatitis E virus antibody in qualified blood donors in Japan. Vox Sang. 2010;99:307–313. doi: 10.1111/j.1423-0410.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- 128.Christensen PB, Engle RE, Hjort C, et al. Time trend of the prevalence of hepatitis E antibodies among farmers and blood donors: a potential zoonosis in Denmark. Clin Infect Dis. 2008;47:1026–1031. doi: 10.1086/591970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaufmann A, Kenfak-Foguena A, Andre C, et al. Hepatitis E virus seroprevalence among blood donors in southwest Switzerland. PloS One. 2011;6:e21150. doi: 10.1371/journal.pone.0021150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zaaijer HL, Kok M, Lelie PN, et al. Hepatitis E in the Netherlands: imported and endemic. Lancet. 1993;341:826. doi: 10.1016/0140-6736(93)90599-c. [DOI] [PubMed] [Google Scholar]
- 131.Beale MA, Tettmar K, Szypulska R, et al. Is there evidence of recent hepatitis E virus infection in English and North Welsh blood donors? Vox Sang. 2011;100:340–342. doi: 10.1111/j.1423-0410.2010.01412.x. [DOI] [PubMed] [Google Scholar]
- 132.Boutrouille A, Bakkali-Kassimi L, Cruciere C, Pavio N. Prevalence of anti-hepatitis E virus antibodies in French blood donors. J Clin Microbiol. 2007;45:2009–2010. doi: 10.1128/JCM.00235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]