Summary
Objectives
Parvovirus B19 (B19V) is a transfusion-transmissible virus. To obtain data about the prevalence, incidence, the course of B19V infection in blood donors and whether B19V might impair their blood counts, samples from blood donors with B19V infection were investigated.
Methods
Blood donations were screened for B19V DNA using the Cobas TaqScreen DPX Test® in mini-pools. B19V DNA concentration, anti-B19V IgG antibody titer and blood counts were determined in positive donors.
Results
157/23,889 (0.66%) donors provided 347 B19V DNA-positive samples. Prevalence of B19V infection was 0.45%, incidence 0.20%. B19V DNA concentrations were predominantly low; only in 8 samples were viral loads of ≥105 IU B19V DNA/ml plasma detectable. Besides a slight decrease in hemoglobin, hematocrit, mean corpuscular volume, mean cellular hemoglobin and mean hemoglobin concentration, no major differences in blood counts occurred in B19V DNA-positive samples. In samples with a low B19V DNA concentration, anti-B19V IgG titers were rather high. 98 donors provided at least 1 B19V DNA-positive follow-up sample, indicating a prolonged viremia.
Conclusions
B19V infection induced no major impairment in the blood counts. In donors with low-level viremia, infectivity through their donations is probably reduced by high antibody titers. Low-level viremia is prolonged, probably exceeding 1 year in many cases.
Key Words: Parvovirus B19, B19V infection, Blood donor, B19V DNA
Introduction
Parvovirus B19 (B19V) is a single-stranded, non-enveloped DNA virus and 1 of the smallest viruses (19-25 nm diameter) overall. Infections with B19V are widespread in human populations and usually occur by droplet transmission via the upper respiratory tract. However, B19V is also a common transfusion-transmissible agent, in particular through pooled plasma derivatives [1,2] but also by cellular blood components [3,4,5].
In the majority of the affected individuals, B19V infections cause rather harmless clinical pictures, e.g. erythema infectiosum (‘fifth disease’) in children or an arthropathy with favorable outcome in adults. In particular in adults, B19V infections often go unnoticed by the affected individuals due to the sub-clinical, asymptomatic course.
Nevertheless, there are 2 patients groups at risk for a more severe and more threatening clinical picture of the B19V infection: patients with increased red blood cell destruction resulting in high erythrocyte turnover, and pregnant women due to transplacental infection of the fetus. In the former patient group, B19V infection may result in a transient aplastic crisis and in the latter group in severe fetal anemia with consecutive hydrops fetalis and fetal death [6,7].
Etiological for both clinical manifestations is the tropism of B19V, which uses the P-antigen as the cellular receptor [8] to enter the host cells by endocytosis, resulting in their infection and apoptosis [9]. The P-antigen is mainly expressed on red blood cells and their precursor cells, but also on megakaryocytes, on placental cells and on fetal myocardium [7], explaining the distinct clinical picture of B19V infection.
The impact of B19V infection, either by transfusion or by droplet transmission, in the at-risk patients mentioned above has been well characterized. However, whether B19V infection impairs the blood count in asymptomatic blood donors is not known. An effect of the B19V infection on the blood counts of healthy individuals was described many years ago in a few experimentally infected volunteers [10,11], and B19V-associated anemia has been reported in otherwise healthy subjects [12,13,14].
Besides obtaining more epidemiological data and data on B19V DNA concentrations, antibody titer and the course of B19V infection, the aim of our study was to determine whether B19V infection in healthy blood donors has an effect on their blood counts.
Patients and Methods
Screening of Blood Donors for B19V
In 2011, all blood donations at the Institute of Transfusion Medicine of the University Hospital of Schleswig-Holstein in Northern Germany were screened for B19V DNA using the Cobas TaqScreen DPX Test® (Roche diagnostics GmbH, Mannheim, Germany; 95% limit of detection (LOD) provided by the manufacturer: 11.5 IU/ml, linear range 75-3.0 × 108 IU/ml) in minipools of up to 96 samples 6 weeks after donation. The Cobas TaqScreen DPX Test allows the simultaneous detection of B19V DNA and hepatitis A virus RNA, and was performed according to the manufacturer's recommendations on a Cobas AmpliPrep/Cobas TaqMan 96 System (Roche).
Plasma supernatant of each donor sample was separated from the cellular components within 18 h after sample drawing using a liquid handling workstation (Tecan Genesis RMP 150, Tecan, Crailsheim, Germany) and pooled. The minipools were stored at <-30 °C until B19V nucleic acid testing (NAT). Furthermore, for each donation, additional archive samples were obtained and stored at <-30 °C for resolution of minipools. Each minipool that tested positive for B19V DNA was resolved, irrespective of the DNA concentration. The DNA concentration of each B19V DNA-reactive sample was then assessed by individual-donor NAT. If the DNA concentration in a sample exceeded the linear range of the assay, the sample was investigated again after dilution.
Although cellular blood components already had been released by the time that B19V DNA testing was performed, fresh frozen plasmas (FFPs) were not released before B19V DNA testing. FFPs obtained from donations that presented B19V DNA concentrations of >104 IU/ml were discarded. If feasible, samples from donors who had already tested positive for B19V DNA at their previous donation were not pooled, but were directly investigated using individual-donor NAT 6 weeks after donation. From some of the donors testing positive for B19V DNA, additional blood samples were obtained between 2 donations for further B19V DNA testing, i.e. without taking a donation.
The study was approved by the local ethics committee.
B19V Antibody Testing
The presence of IgG antibodies against B19V was determined by enzyme-linked immunosorbent assay (ELISA; anti-parvovirus B19-ELISA, EUROIMMUN, Lübeck, Germany). Assays were performed as recommended by the manufacturer.
Blood Count
No predonation hemoglobin (Hb) measurement, e.g. by finger-prick sampling, preceded the blood donation in the Institute of Transfusion Medicine. Instead, a complete blood count of each donor was performed using a UniCel®DxH 800 analyzer (BeckmanCoulter, Krefeld, Germany) using an EDTA blood sample drawn from the sampling pouch for diversion. The red blood cell unit of a donation was released if the Hb level of the donor was at least 11.6 g/dl, screening for B19V DNA was performed in any case. For donors presenting an Hb level of <12.5 g/dl (female) or <13.5 g/dl (male), at the next occasion Hb levels were determined before the blood donation and Hb levels of 12.5 g/dl and 13.5 g/dl, respectively, were mandatory for donation. Blood counts of 100 donors testing negative for B19V DNA served as controls.
Statistical Analysis
Comparisons of mean blood count values and anti-B19V IgG titers were performed by t-test using SPSS version 19 (IBM GmbH, Ehningen, Germany). A p value < 0.05 was considered statistically significant.
Results
Demographic Data, Prevalence and Incidence of B19V DNA
In 2011, 23,889 donors (6,658 first-time and 17,231 repeat blood donors) were screened for B19V DNA. 157/23,889 (0.66%) donors tested positive for B19V DNA. 79 were female, 78 male, with an overall median age of 36.0 years. From these donors, 53,789 (whole blood, platelet and plasma) donations were obtained, of which 326 (0.61%) tested positive for B19V DNA. 21 additional B19V DNA-positive samples were obtained from the 157 donors without taking a donation. Of the donors tested positive for B19V DNA, 46 (29.3%) were first-time donors and 111 (70.7%) repeat blood donors. 77/111 (69.4%) of the repeat blood donors were already B19V DNA-positive when tested the first time during the study period, and 34/111 (30.6%) provided at least 1 B19V DNA-negative sample and became infected within the study period. The resulting prevalence from these data was 0.45% (77/17,231 repeat blood donors already infected at the beginning of the study period) and the annual incidence was 0.20% (34/17,231 repeat blood donors infected during the study period).
Plasma B19V DNA Concentration in Infected Donors
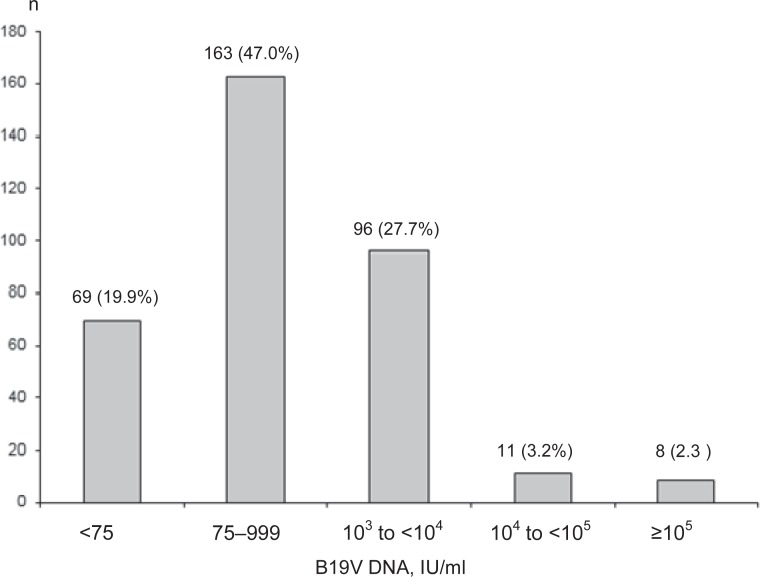
The majority of samples had a rather low B19V DNA concentration. While in 69/347 samples (19.9%) a DNA concentration of < 75 IU/ml was detectable, in 8 (2.3%) samples a high DNA concentration of >105 IU/ml was found, including 3 samples with DNA concentrations exceeding 1010 IU/ml. The distribution of the B19V DNA concentrations in the 347 samples is given in figure 1.
Fig. 1.
Distribution of B19V DNA concentration in 347 samples tested positive for B19V DNA.
Blood Count in B19V-Infected Blood Donors
Compared to the 100 B19V DNA-negative samples, there were no differences in the quantity of leukocytes, red cells and platelets in 345 B19V DNA-positive samples (table 1; in 2 samples, the blood count was not investigated). However, the mean Hb value, hematocrit (Hct), mean corpuscular volume (MCV), mean cellular Hb (MCH) value and mean Hb concentration (MCHC) of the 345 B19V DNA-positive samples were slightly, but statistically significantly lower (table 1) than in controls.
Table 1.
Hematological data of 345 samples tested positive for B19V DNA compared to 100 B19V DNA-negative samples
| All donors tested positive, mean (min.–max.) | Control group (B19V DNA-negative), mean (min.–max.) | p value | |
|---|---|---|---|
| Number of donors, n | 345 | 100 | |
| Leukocyte count, μl | 6.7 (2.8–14.7) | 6.7 (3.2–11.2) | 0.963 |
| Erythrocyte count, × 106/μl | 4.6 (3.7–6.0) | 4.6 (3.9–5.4) | 0.405 |
| Hb, g/dl | 14.0 (10.8–16.8) | 14.4 (11.5–17.2) | <0.0001 |
| Hct, % | 39.9 (33.0–49.4) | 41.0 (33.7–48.1) | 0.002 |
| MCV, fl | 87.2 (70.3–96.6) | 88.7 (76.6–99.5) | 0.002 |
| MCH, pg | 30.5 (23.0–35.0) | 31.3 (27.0–36.0) | 0.001 |
| MCHC, g/dl | 35.0 (31.3–37.0) | 35.5 (33.0–46.0) | 0.001 |
| Platelet count, × 103/μl | 221 (89–454) | 219 (113–363) | 0.756 |
The blood counts were not influenced by the magnitude of the DNA concentration: the 8 B19V-positive samples with a DNA concentration of ≥105 IU/ml revealed no major differences compared to the control group, with the exception of the leukocyte count. Moreover, comparison of these 8 samples yielded no statistically significant differences in Hb, Hct, MCV, MCH and MCHC compared to the 100 control samples (table 2).
Table 2.
Hematological data of the 8 samples presenting B19V DNA concentrations >105 compared to 100 B19V DNA-negative samples
| DNA concentration > 105 IU/ml, mean (min.–max.) | Control group (B19V DNA negative), mean (min.–max.) | p value | |
|---|---|---|---|
| Number of donors, n | 8 | 100 | |
| Leukocyte count, μl | 5.4 (2.8–7.2) | 6.7 (3.2–11.2) | 0.036 |
| Erythrocyte count, × 106/µl | 4.6 (4.2–5.0) | 4.6 (3.9–5.4) | 0.877 |
| Hb, g/dl | 14.0 (11.5–15.0) | 14.4 (11.5–17.2) | 0.315 |
| Hct, % | 39.6 (34.3–42.6) | 41.0 (33.7–48.1) | 0.242 |
| MCV, fl | 86.2 (75.0–91.2) | 88.7 (76.6–99.5) | 0.120 |
| MCH, pg | 30.4 (25.0–33.0) | 31.3 (27.0–36.0) | 0.179 |
| MCHC, g/dl | 35.3 (33.0–37.0) | 35.5 (33.0–46.0) | 0.705 |
| Platelet count, × 103/μl | 226 (151–425) | 219 (113–363) | 0.831 |
Furthermore, all hematological data for the different subgroups displayed in figure 1 were compared with each other and to those of the B19V DNA-negative samples (table 3). Leukocyte counts of the samples with DNA concentration of ≥105 IU/ml were statistically significantly lower compared to samples with DNA concentrations of 103-104 IU/ml (p = 0.03), 999-75 IU/ml (p = 0.033), <75 IU/ml (p = 0.042) and to the negative controls (p = 0.036).
Table 3.
Hematological data in relation to the DNA concentration: p values of significant differences are specified in the result section
| DNA concentration > 105 IU/ml, mean (min.–max.) | DNA concentration 104–105 IU/ml, mean (min.-max.) | DNA concentration 103–104 IU/ml, mean (min.–max.) | DNA concentration 75–999 IU/ml, mean (min.–max.) | DNA concentration < 75 IU/ml, mean (min.–max.) | Control group (B19V DNA-negative), mean (min.–max.) | |
|---|---|---|---|---|---|---|
| Number of donors | 8 | 11 | 95 | 162 | 69 | 100 |
| Leukocyte count, μl | 5.4 (2.8–7.2) | 6.3 (4.4–8.1) | 6.7 (3.4–10.5) | 6.8 (3.6–14.7) | 6.7 (3.7–10.5) | 6.7 (3.2–11.2) |
| Erythrocyte count, × 106/μl | 4.6 (4.2–5.0) | 4.7 (3.1–6.0) | 4.6 (3.8–5.7) | 4.6 (3.8–6.0) | 4.6 (3.8–5.9) | 4.6 (3.9–5.4) |
| Hb, g/dl | 14.0 (11.5–15.0) | 13.8 (11.4–16.5) | 13.9 (10.8–16.7) | 13.9 (11.7–16.8) | 14.2 (11.7–16.5) | 14.4 (11.5–17.2) |
| Hct, % | 39.6 (34.3–42.6) | 40.3 (33.7–46.8) | 39.9 (33.0–49.4) | 39.9 (34.0–48.5) | 39.9 (34.0–46.5) | 41.0 (33.7–48.1) |
| MCV, fl | 86.2 (75.0–91.2) | 85.5 (74.5–93.8) | 87.4 (74.0–94.1) | 87.4 (70.3–96.6) | 86.7 (80.4–95.2) | 88.7 (76.6–99.5) |
| MCH, pg | 30.4 (25.0–33.0) | 29.5 (25.0–33.0) | 30.5 (24.0–34.0) | 30.6 (23.0–35.0) | 30.7 (28.0–33.0) | 31.3 (27.0–36.0) |
| MCHC, g/dl | 35.3 (33.0–37.0) | 34.4 (33.0–36.0) | 35.0 (32.0–37.0) | 34.9 (31.3–37.0) | 35.5 (33.0–37.0) | 35.5 (33.0–46.0) |
| Platelet count, × 103/μl | 226 (151–425) | 257 (157–440) | 222 (89–369) | 214 (109–374) | 229 (138–437) | 219 (113–363) |
Some statistically significant differences were found when samples with DNA concentrations of 104-105 IU/ml were compared to those with 999-75 IU/ml (platelet count: p = 0.008), <75 IU/ml (MCHC: p < 0.0001) and negative controls (MCV: p = 0.026, MCH: p = 0.031, MCHC: p = 0.045, and platelet count: p = 0.015). For samples containing DNA concentrations of 103-104 IU/ml, statistically significantly lower values were found compared to those with DNA concentrations of <75 IU/ml (MCHC: p = 0.001) and compared with the negative controls (Hb: p = 0.002, Hct: p = 0.013, MCV: p = 0.032, MCH: p = 0.003 and MCHC: p = 0.01).
If samples with DNA concentrations between 999 and 75 IU/ml were compared to those with DNA concentrations of <75 IU/ml, differences that were statistically significant in 2 parameters were calculated (MCHC: p < 0.0001, platelet count: p = 0.047). Comparisons of the samples with rather low DNA concentrations (999-75 IU/ml and <75 IU/ml) with the negative controls yielded several statistically significant differences: 999-75 IU/ml to negative controls: Hb: p = 0.001, Hct: p = 0.007, MCV: p = 0.029, MCH: p = 0.004 and MCHC: p = 0.001; <75 IU/ml to negative controls: Hct: p = 0.036, MCV: p = 0.001, MCH: p = 0.029. All other differences were not statistically significant.
To exclude a significant myelosuppression in the context of acute B19V infection, the blood counts of 20 donors presenting an acute B19V infection during the study period were compared to the values measured in the preceding, B19V DNA-negative donation as well as to the values measured by the consecutive donation, which was already available. However, again no differences were obvious (table 4).
Table 4.
Hematological data of 20 donors with newly acquired B19V infection compared to the values provided by the last B19V DNA-negative donation and to their first follow-up donation
| Index donation, mean (min.–max.) | Previous, negative donation, mean (min.–max.) | p value | Consecutive donation, mean (min.–max.) | p value | |
|---|---|---|---|---|---|
| No. of donors, n | 20 | 20 | 20 | ||
| Leukocyte count, μl | 6.0 (2.8–9.0) | 6.7 (3.9–9.9) | 0.192 | 6.9 (3.6–14.7) | 0.195 |
| Erythrocyte count, × 106/µl | 4.6 (4.1–5.0) | 4.6 (4.0–5.2) | 0.433 | 4.7 (4.0–5.3) | 0.191 |
| Hb, g/dl | 14.0 (11.4–15.4) | 14.3 (12.9–16.5) | 0.249 | 14.3 (12.1–16.2) | 0.352 |
| Hct, % | 39.6 (33.7–44.8) | 40.7 (35.2–46.4) | 0.234 | 40.7 (36.1–45.5) | 0.246 |
| MCV, fl | 86.8 (75.0–95.2) | 87.7 (78.8–95.0) | 0.508 | 86.8 (78.3–95.2) | 0.994 |
| MCH, pg | 30.6 (25.0–33.0) | 31.0 (27.0–33.0) | 0.522 | 30.4 (26.0–33.0) | 0.827 |
| MCHC, g/dl | 35.3 (33.0–37.0) | 35.5 (34.0–37.0) | 0.439 | 35.2 (33.0–37.0) | 0.777 |
| Platelet count, × 103/μl | 218 (118–425) | 217 (119–344) | 0.967 | 210 (97–334) | 0.703 |
Anti-B19V IgG Titer
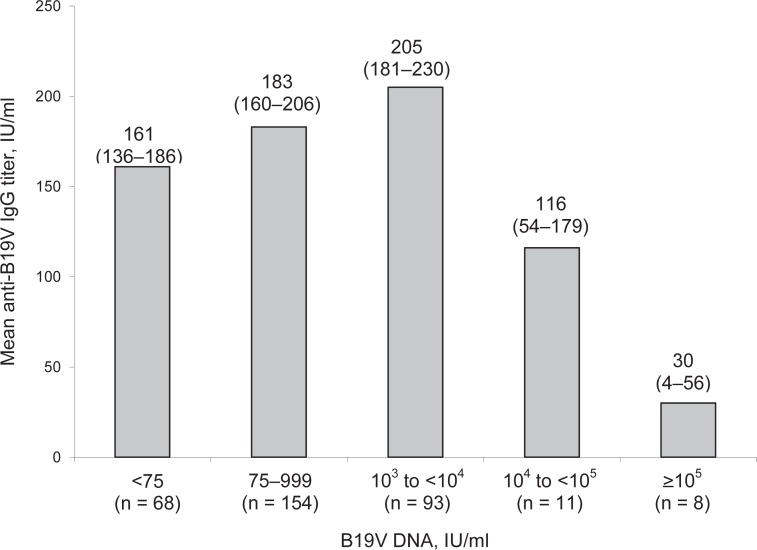
The anti-B19V IgG titers according to B19V DNA concentration are given in figure 2. In 2 of 3 samples with a DNA concentration of >1010, no anti-B19V IgG was detectable; 1 of these samples showed a result close to the cut-off of the assay.
Fig. 2.
Mean anti-B19V IgG titer in relation to the B19V DNA concentration of 334 samples. In 13 samples, antibody titer was not determined. The numbers in parentheses below the DNA concentration give the number of investigated samples in each group. The numbers at the top of the columns give the mean anti-B19V IgG titer, and (in parenthesis) the 95% confidence intervals.
The mean IgG titer of the samples with ≥105 IU/ml B19V DNA was significantly lower than that for all other groups (p = 0.033 vs. 104-105, and p < 0.001 vs. 103-104, 75-999 and <75 IU/ml). Moreover, there were differences in antibody titer of the donors with a DNA concentration 104-105 versus 103-104 (p = 0.023) and 103-104 versus <75 (p = 0.016). None of the other differences were significant.
Duration of B19 Viremia
Of the 157 donors, 98 (62.4%) provided at least 1 further donation or sample during the study period: mostly, these consecutive samples, drawn after a mean interval of 92 days, also tested positive in individual NAT for B19V DNA. In 9/98 (9.2%) donors, the first follow-up sample tested negative for B19V DNA, and in 1/98 (1.0%) donor, the second follow-up sample tested negative for B19V DNA. However, 3/10 donors who in between tested negative became positive again for B19V DNA in 1 of the further follow-up samples.
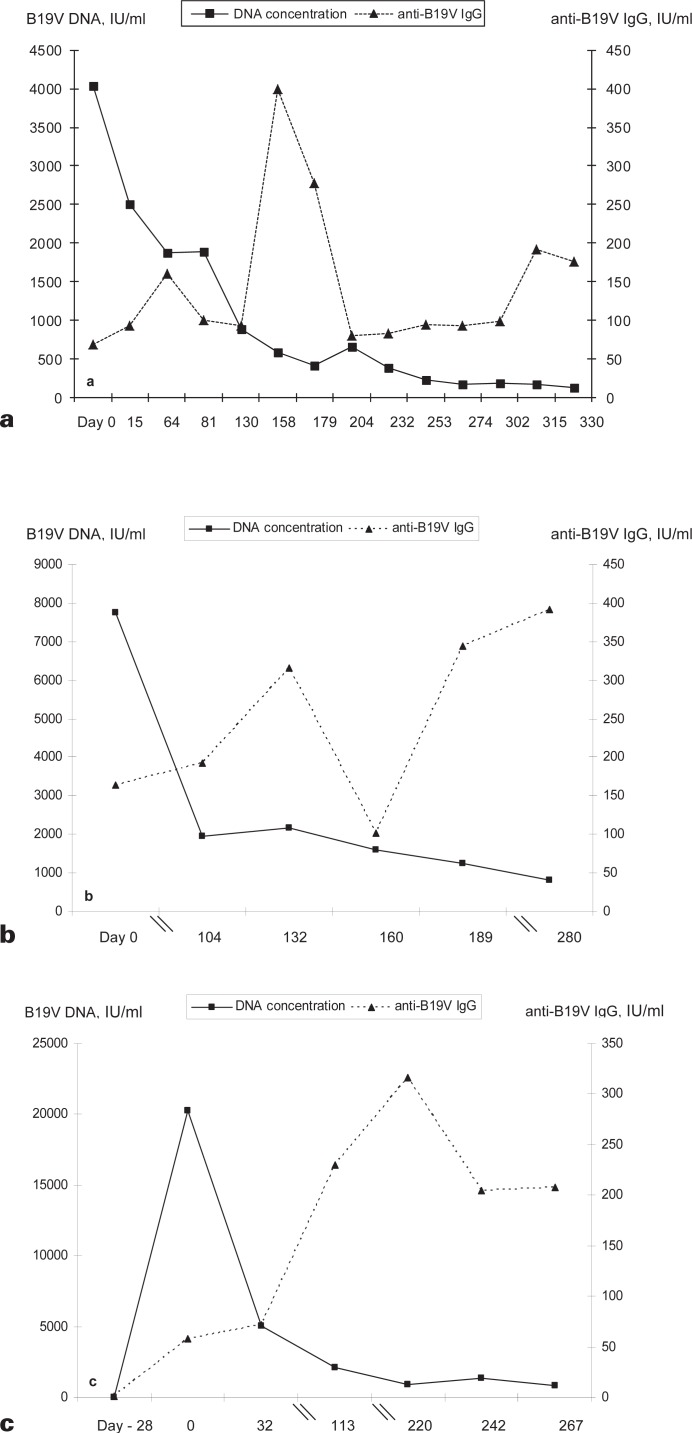
Figures 3a and b display the time course of viremia and the anti-B19V IgG titer in 2 donors for whom several samples were obtained over a longer period. The donor presented in figure 3c became infected sometime between day −28 (date of the last B19V DNA-negative donation in 2011) and day 0, the first positive donation. The DNA concentration had already decreased to 20,252 IU/ml, indicating a rather short period of high level viremia at some time within the last 4 weeks.
Fig. 3.
Course of viremia and anti-B19V IgG titer in 3 different donors (a-c). The DNA concentration is given by the squares and a continuous line, and the anti-B19V IgG titer by triangles and a broken line. Time point 0 is that of the index donation leading to the inclusion in the study.
Discussion
Using the Cobas TaqScreen DPX Test® for the screening our blood donors for B19V DNA, we determined the prevalence of ongoing B19V infection to be 0.45% with an annual incidence of 0.20%. The analytical sensitivity of test (95% LOD: 11.5 IU/ml as given by the manufacturer) was somewhat higher in an investigation by Koppelman et al. [15]; they determined the analytical sensitivity to be 20.3 IU/ml; however, Müller et al. [16] did confirm the LOD given by the manufacturer in their evaluation of the test.
We found a higher prevalence of B19V DNA-positive donations than Schmidt et al. [17] in another B19V DNA survey in Germany and Austria. They calculated an average rate of B19V infection of 12.7 per 100,000 donations (with DNA concentration of >105 IU/ml) and 261.5 per 100,000 donations (DNA concentration of <105 IU/ml), respectively, after a longer observation period of 4 years. Based on our prevalence data (326/53,789 donations B19V DNA positive), we calculated an overall rate of 606.1 B19V DNA-positive donations per 100,000 (or 1 in 165 donations), including 14.9 of 100,000 (or 1 in 6,724) donations with high DNA concentrations of ≥105 IU/ml within 1 year. Although the analytical sensitivity of the NAT tests used in our survey and that of Schmidt et al. are comparable, there are differences in the procedure for preparing pooled samples for nucleic acid extraction. Furthermore, not all B19V DNA-reactive minipools were resolved by Schmidt et al., but only those containing > 105 IU/ml B19V DNA. Therefore, comparisons between the B19V DNA surveys are difficult. However, the incidence of B19V infection is dependent not only on the season (with a higher incidence in the spring [18]) but also – with intervals of several years – recurrent small BV19 epidemics occur [7,17,18]. It is possible that our study happened to be performed during such a B19V epidemic. Moreover, it can be assumed that numerous donations with low B19V DNA concentration below 1,000 IU/ml were missed due to B19V DNA screening in plasma pools containing up to 96 samples, and therefore, prevalence of B19V infection was yet higher in the study period.
Although we did not perform any specific inquiry among our donors concerning symptoms of B19V infection, we can assume that the overwhelming majority did not suffer from any symptoms. If B19V infection in adults is accompanied by any clinical manifestations, these are usually unspecific, rather flu-like symptoms, and blood donors are asked about such unspecific symptoms in our questionnaire. However, a hematological impairment, even if only laboratory confirmed, has to be assumed (due to the distinct tropism of B19V [7,10,11,19,20]), not only in at-risk patient groups, but also in otherwise healthy individuals. For that reason, we investigated the blood counts of the B19V DNA-positive samples and compared them to the values measured in 100 B19V DNA-negative samples. While the differences in the samples with higher DNA concentrations (>104 IU/ml), especially the leukocyte count in samples with DNA concentrations ≥105 IU/ml, are most probably attributed to the low number of samples, we could not detect any major hematological changes with regards to the counts of red cells or platelets, in any B19V DNA-positive sample, or in those presenting high B19V DNA concentrations (tables 1 and 2). Even in the context of newly acquired, acute B19V infection, there were no numeric differences in the red cells or platelets (table 4). The incidental finding of a slight but significant decrease in Hb, Hct, MCV, MCH and MCHC in 345 B19V DNA-positive samples remains unexplained; we can only speculate on whether and how an ongoing B19V infection might influence the iron metabolism and/or iron recovery in erythroid precursor cells. It is possible that these changes reflect a slight iron deficiency, which is frequently found in ongoing infections. This is in line with the assumption that these changes in hematological parameters were mainly detectable in the samples with lower DNA concentrations (<104 IU/ml, table 3), obtained from donors with a probably more long-lasting B19V infection. Moreover, to detect these changes, a large number of B19V DNA-positive samples had to be investigated. The changes were not detectable in lower numbers of samples with high DNA concentration (table 2) or in the context of newly acquired B19V infection (table 4). Moreover, the decrease in these parameters did not fall below their normal values, and, therefore, ongoing B19V infections and the associated, slight hematological changes are probably without any clinical relevance for otherwise healthy individuals.
However, it is now certain that blood donors with B19V infection may transmit their infection to the recipients of their donated blood products. There have been numerous reports describing the transmission of B19V infection through plasma derivatives (for overview see [21]), resulting in the recommendation of a general B19V NAT testing of plasma pools, which should be discarded if the B19V DNA concentration exceed 104 IU/ml [1].
There is no such recommendation for single plasma units for therapeutic use and for cellular blood products, although single cases of transfusion-transmitted (TT) B19V infections have been reported. In a donor-recipient-linked study, Klein-man et al. [4] showed that TT-B19V infections through blood products from donors with lower DNA concentrations (< 106 IU/ml B19V-DNA) are uncommon: the amount of infectious B19V is either insufficient or high titers of protective, neutralizing antibodies are existent. Although we have not traced the recipients of our B19V DNA-positive blood products so far, our data endorse at least the last assumption: in samples with B19V DNA of <105 IU/ml, more anti-B19V IgG was present (fig. 2, 3). We did not perform a Western blot to distinguish between neutralizing and non-neutralizing antibodies. However, antibodies against VP1, which are considered to be neutralizing [7], are detectable in the advanced stage of B19V infection, when DNA concentrations already decline [17]. Therefore, it can be assumed that the antibodies in the samples with B19V DNA levels of <105 IU/ml are also directed against VP1 [17]. That could lead to a sufficient neutralization of infectious virions, while lower IgG titers in samples with B19V DNA of ≥105 IU/ml could indicate either antibody adsorption from the plasma onto the virus surface (and complete or partial neutralization), or (more probable) a recently acquired B19V infection without any previous antibody formation and the presence of a large quantity of infectious virus. However, in neither case can the presence of B19V IgG prevent TT-B19V infection through low-level viremic donations. A single case has been reported of TT-B19V infection through a red blood cell unit from a donor with a B19V DNA concentration of <104, despite the presence of anti-B19V IgG [3]. The cause might be the immaturity of these antibodies and their presumably low avidity, but no antibody titer was given in the report.
While persistent B19V infections initially seemed to be limited mainly to immunocompromised individuals [22,23], it has now become obvious that occurrence of long-lasting B19V infections (6 months or longer) is not only a phenomenon in immunocompetent patients [24,25] but it also occurs in healthy blood donors [17]. Considering our data, a long-standing viremia, even if at low levels, may occur in the overwhelming majority of B19V-infected blood donors. Many of our B19V DNA-positive donors joined our study through their first donation in 2011. Thus, for some of our donors the follow-up period comprised not a year. However, in the 3 exemplary donors displayed in figures 3a-c, a longer follow-up was possible. Their courses of infection suggested that viremia existed for more than 1 year: if a high-level viremia was present at all, it was rapidly resolved (fig. 3c) and B19V DNA then persisted for a longer period, approaching the detection limit of the assay without definite resolution of the low-level viremia, despite an increasing anti-B19V IgG titer (fig. 3a-c).
In conclusion, prevalence and incidence in our donor population was higher than in a comparable survey, presumably attributed to a B19V epidemic during the year 2011. No major impairment occurred in the blood count of the infected blood donors, with only a slight decrease in Hb, Hct, MCV, MCH and MCHC being observed, without any clinical relevance. Decrease in B19V DNA concentration was accompanied by an increase in anti-B19V IgG titer, which might be protective for transfusion recipients, but which cannot definitely resolve an infection within 1 year. To determine the time period of the prolonged B19 viremia is an issue for future research, as are the reasons for the incomplete elimination of B19V from the plasma.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors are indebted to Ursula Thiessen for their excellent technical assistance.
References
- 1.Food and Drug Administration Guidance for Industry: Nucleic acid testing (nat) to reduce the possible risk of parvovirus B19 transmission by plasma-derived products. www.fda.gov/biologics-bloodvaccines/guidancecomplianceregulatoryinformation/guidances/blood/ucm071592.htm.
- 2.Blümel J, Schmidt I, Effenberger W, Seitz H, Willkommen H, Brackmann HH, Löwer J, Eis-Hübinger AM. Parvovirus B19 transmission by heat-treated clotting factor concentrates. Transfusion. 2002;42:1473–1481. doi: 10.1046/j.1537-2995.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- 3.Satake M, Hoshi Y, Taira R, Momose SY, Hino S, Tadokoro K. Symptomatic parvovirus B19 infection caused by blood component transfusion. Transfusion. 2011;51:1887–1895. doi: 10.1111/j.1537-2995.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 4.Kleinman SH, Glynn SA, Lee TH, Tobler LH, Schlumpf KS, Todd DS, Qiao H, Yu MY, Busch MP, National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study-II (NHLBI REDS-II) A linked donor-recipient study to evaluate parvovirus B19 transmission by blood component transfusion. Blood. 2009;114:3677–3683. doi: 10.1182/blood-2009-06-225706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hourfar MK, Mayr-Wohlfart U, Themann A, Sireis W, Seifried E, Schrezenmeier H, Schmidt M. Recipients potentially infected with parvovirus B19 by red blood cell products. Transfusion. 2011;51:129–136. doi: 10.1111/j.1537-2995.2010.02780.x. [DOI] [PubMed] [Google Scholar]
- 6.Blümel J, Burger R, Drosten C, Gröner A, Gürtler L, Heiden M, Hildebrandt M, Jansen B, Montag-Lessing T, Offergeld R, Pauli G, Seitz R, Schlenkrich U, Schottstedt V, Strobel J, Willkommen H, von König CH. Parvovirus B19 – revised. Transfus Med Hemother. 2010;37:339–350. doi: 10.1159/000322190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350:586–597. doi: 10.1056/NEJMra030840. [DOI] [PubMed] [Google Scholar]
- 8.Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: Cellular receptor for B19 parvovirus. Science. 1993;262:114–117. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 9.Servant-Delmas A, Lefrère JJ, Morinet F, Pillet S. Advances in human B19 erythrovirus biology. J Virol. 2010;84:9658–9665. doi: 10.1128/JVI.00684-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potter CG, Potter AC, Hatton CS, Chapel HM, Anderson MJ, Pattison JR, Tyrrell DA, Higgins PG, Willman JS, Parry HF, Cotes PM. Variation of erythroid and myeloid precursors in the marrow and peripheral blood of volunteer subjects infected with human parvovirus (B19) J Clin Invest. 1987;79:1486–1492. doi: 10.1172/JCI112978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson MJ, Higgins PG, Davis LR, Willman JS, Jones SE, Kidd IM, Pattison JR, Tyrrell DA. Experimental parvoviral infection in humans. J Infect Dis. 1985;152:257–265. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- 12.Osaki M, Matsubara K, Iwasaki T, Kurata T, Nigami H, Harigaya H, Baba K. Severe aplastic anemia associated with human parvovirus B19 infection in a patient without underlying disease. Ann Hematol. 1999;78:83–86. doi: 10.1007/s002770050477. [DOI] [PubMed] [Google Scholar]
- 13.Qian XH, Zhang GC, Jiao XY, Zheng YJ, Cao YH, Xu DL, Chen CS. Aplastic anaemia associated with parvovirus B19 infection. Arch Dis Child. 2002;87:436–437. doi: 10.1136/adc.87.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ideguchi H, Ohno S, Ishigatsubo Y. A case of pure red cell aplasia and systemic lupus erythematosus caused by human parvovirus B19 infection. Rheumatol Int. 2007;27:411–414. doi: 10.1007/s00296-006-0227-z. [DOI] [PubMed] [Google Scholar]
- 15.Koppelman MH, Cuijpers HT, Wessberg S, Valkeajärvi A, Pichl L, Schottstedt V, Saldanha J. Multicenter evaluation of a commercial multiplex polymerase chain reaction test for screening plasma donations for parvovirus B19 DNA and hepatitis A virus RNA. Transfusion. 2012;52:1498–1508. doi: 10.1111/j.1537-2995.2012.03705.x. [DOI] [PubMed] [Google Scholar]
- 16.Müller MM, Fraile MI, Hourfar MK, Peris LB, Sireis W, Rubin MG, López EM, Rodriguez GT, Seifried E, Saldanha J, Schmidt M. Evaluation of two, commercial, multi-dye, nucleic acid amplification technology tests, for HBV/HCV/HIV-1/HIV-2 and B19V/HAV, for screening blood and plasma for further manufacture. Vox Sang. 2013;104:19–29. doi: 10.1111/j.1423-0410.2012.01635.x. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M, Themann A, Drexler C, Bayer M, Lanzer G, Menichetti E, Lechner S, Wessin D, Prokoph B, Allain JP, Seifried E, Hourfar MK. Blood donor screening for parvovirus B19 in Germany and Austria. Transfusion. 2007;47:1775–1782. doi: 10.1111/j.1537-2995.2007.01443.x. [DOI] [PubMed] [Google Scholar]
- 18.Kooistra K, Mesman HJ, de Waal M, Koppelman MH, Zaaijer HL. Epidemiology of high-level parvovirus B19 viraemia among Dutch blood donors, 2003-2009. Vox Sang. 2011;100:261–266. doi: 10.1111/j.1423-0410.2010.01423.x. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava A, Bruno E, Briddell R, Cooper R, Srivastava C, van Besien K, Hoffman R. Parvovirus B19-induced perturbation of human megakaryocytopoiesis in vitro. Blood. 1990;76:1997–2004. [PubMed] [Google Scholar]
- 20.Klein HG. Transfused B19V: B-nign, B-ware, B-gone? Blood. 2009;114:3509–3511. doi: 10.1182/blood-2009-09-239939. [DOI] [PubMed] [Google Scholar]
- 21.Parsyan A, Candotti D. Human erythrovirus B19 and blood transfusion – an update. Transfus Med. 2007;17:263–278. doi: 10.1111/j.1365-3148.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- 22.Frickhofen N, Abkowitz JL, Safford M, Berry JM, Antunez-de-Mayolo J, Astrow A, Cohen R, Halperin I, King L, Mintzer D, Cohen B, Young NS. Persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1 (HIV-1): A treatable cause of anemia in AIDS. Ann Intern Med. 1990;113:926–933. doi: 10.7326/0003-4819-113-12-926. [DOI] [PubMed] [Google Scholar]
- 23.Flunker G, Peters A, Wiersbitzky S, Modrow S, Seidel W. Persistent parvovirus B19 infections in immunocompromised children. Med Microbiol Immunol. 1998;186:189–194. doi: 10.1007/s004300050063. [DOI] [PubMed] [Google Scholar]
- 24.LaMonte AC, Paul ME, Read JS, Frederick MM, Erdman DD, Han LL, Anderson LJ, Women and Infants Transmission Study Persistent parvovirus B19 infection without the development of chronic anemia in HIV-infected and -uninfected children: The Women and Infants Transmission Study. J Infect Dis. 2004;189:847–851. doi: 10.1086/381899. [DOI] [PubMed] [Google Scholar]
- 25.Lefrère JJ, Servant-Delmas A, Candotti D, Mariotti M, Thomas I, Brossard Y, Lefrère F, Girot R, Allain JP, Laperche S. Persistent B19 infection in immunocompetent individuals: Implications for transfusion safety. Blood. 2005;106:2890–2895. doi: 10.1182/blood-2005-03-1053. [DOI] [PubMed] [Google Scholar]