Abstract
A series of macrocyclic biphenyl tetraoxazoles was synthesized. The latter stages of the synthetic approach allowed for the addition of varied N-protected α-amino acids, which were subsequently deprotected and condensed to provide the desired macrocycles. Improved yields could be realized in the macrocyclization step of their synthesis relative to other macrocyclic G-quadruplex stabilizers. These 24-membered macrocycles were evaluated for their ability to stabilize G-quadruplex DNA and for their relative cytotoxicity against human tumor cells. These biphenyl tetraoxazoles were not strong ligands for G-quadruplex DNA relative to other macrocyclic polyoxazoles. This reduced stabilizing potential did correlate with their comparatively lower cytotoxic activity as observed in the human tumor cell lines, RPMI 8402 and KB3-1. These studies provide useful insights into the conformational requirements for the development of selective and more potent G-quadruplex ligands.
Keywords: G-quadruplex, Biphenyl, Macrocycle, Oxazoles, Cytotoxicity, G-quadruplex ligand
1. Introduction
Sequences of DNA and RNA rich in guanosine bases are known to fold into G-quadruplexes.1,2 G-Tetrads (square-planar arrays of four guanines held together by hydrogen bonds) can be stacked upon each other to form G-quadruplexes. G-quadruplexes are stabilized by π–stacking interactions between the purines as well as by monovalent metal cations (usually K+ or Na+) sandwiched between the G-tetrads.3 G-quadruplexes have been associated with telomeres, the promoter regions of several oncogenes, and mRNA.4-11 As G-quad-ruplex stabilizers would be expected to effectively interfere with processes involving these nucleic acids, they have often been viewed as a promising new class of anticancer agents.12-15
Mechanistic insight into how G-quadruplex stabilizers principally function as anticancer agents requires compounds that are highly selective in stabilizing G-quadruplexes without interacting with the more common duplex structures of DNA or RNA.16 While a diverse array of compounds have been reported to stabilize G-quadruplex DNA17 with some degree of selectivity, most also have the ability to stabilize the more abundant duplex conformation of DNA or RNA.
Telomestatin (Fig. 1) is a natural product that was identified as a G-quadruplex ligand with improved selectivity relative to stabilization of duplex DNA. Using telomestatin as a template, the synthetic macrocyclic hexaoxazole HXDV (Fig. 1) was synthesized and identified as a highly selective G-quadruplex stabilizer exhibiting no affinity for stabilizing single-stranded, duplex, or triplex DNA.18,19 HXDV induces apoptosis in both telomerase positive and negative cells, induces M-phase cell cycle arrest, reduces the expression of the M-phase checkpoint regulator Aurora A, and is moderately cytotoxic towards several tumor cell lines with an average IC50 value of 0.5 μM.18,20 METV (Fig. 1) is another 24-membered macrocycle, which was synthesized using a ring-closing metathesis cyclization. METV also proved to be a highly selective G-quadruplex stabilizer and exhibited significantly greater cytotoxicity than HXDV against several human tumor cell lines with IC50 values typically ranging between 20 and 50 nM.21
Figure 1.
Structures of telomestatin, HXDV and METV.
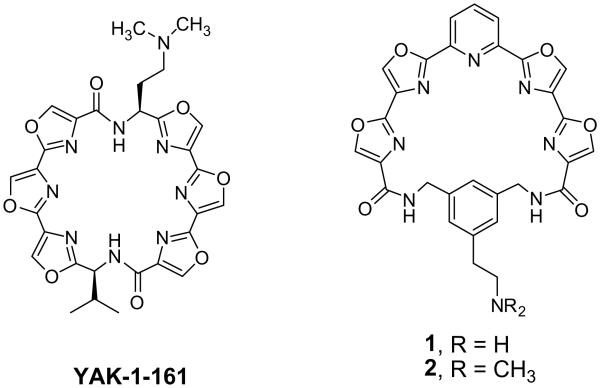
HXDV and METV have poor aqueous solubility making it difficult to formulate and assess their antitumor activity in vivo. Among a variety of analogs related to HXDV that were synthesized and evaluated for cytotoxicity, a 2-(N,N-dimethylamino)ethyl derivative, YAK-1-161 (Fig. 2), was shown to exhibit both improved cytotoxicity and solubility. When evaluated in vivo, YAK-1-161 had a T/C value of 21.6% and was well tolerated at doses levels ≤45 mg/kg.22
Figure 2.
Structures of YAK-1-161 and pyridyl polyoxazole macrocycles 1 and 2.
Efforts to develop additional highly selective G-quaduplex stabilizers that could be readily synthesized and explored with regard to their structure-activity led to the design of a series of 24-membered macrocyclic pyridyl polyoxazoles.23 Within this series, compounds having a 1,3-bis(aminomethyl)phenyl group linking the ends of a pyridyl tetraoxazole dicarboxylate intermediate were observed to be among the more cytotoxic. The presence of a 5-(2-aminoethyl) substituent attached to the phenyl moiety as in compound 1 (Fig. 2) or a 5-[2-(N,N-dimethylamino)ethyl] moiety as in compound 2 allowed not only for retention of notable cytotoxic activity, but also significantly enhanced their ability to be formulated for evaluation in vivo. These analogs had IC50 values of 30–40 nM when assayed against KB3-1 cells and 90–180 nM against RPMI 8402 cells and strongly stabilize G-quadruplex DNA with no observable stabilization of duplex DNA.23 Compound 2 was selected for in vivo evaluation against a human breast cancer xenograft (MDA-MB-435) in athymic nude mice. Results from this assay indicated that mice treated with the pyridyl polyoxazole macrocycle had a %T/C value of 27.7% which clearly demonstrated in vivo efficacy against this breast cancer xenograft.23
These data prompted further studies into the development of other macrocyclic G-quadruplex stabilizers. In the present study, biphenyl tetraoxazoles were explored for their potential as a new series of selective G-quadruplex DNA ligands. Modeling results suggested that these macrocycles would adapt a similar conformation to YAK-1-161, although not quite as planar, Figure 3.
Figure 3.
Overlaid structures of YAK-1-161 (in solid green) and biphenyl tetraoxazole macrocyle 21. ChemDraw3D Pro 12.0 was used for these illustrations and the structures were minimized using MM2. The minimized structures were comparable to the minimized structures obtained using SPARTAN 06 (equilibrium conformation/MMFF).
The synthetic strategy for this series of 24-membered macrocycles was designed to allow for versatility in terms of substituents that could be appended to the alkyl linkage within this macrocycle. In addition, it was hoped that improved yields might be realized in the synthetic step associated with the formation of the core macrocyclic structure.
2. Chemistry
Two key intermediates were selected as the principal subunits for the preparation of these biphenyl tetraoxazoles. These were 4, methyl 2-(3-bromophenyl)bis[4,2′]bioxazole-4′-carboxylate and 5, 2-(3-bromophenyl)-4′-(N-Boc-aminomethyl)[4,2′]-bioxazole, Scheme 1. The triisopropylsilyl protected methyl 2-(1-amino-2-hydroxyethyl)oxazole-4-carboxylate, 3, was prepared as previously described.18
Scheme 1.
Formation of the 2-phenyl[4,2′]bioxazoles 4 and 5. Reagents and conditions: (a) (COCl)2, CH2Cl2, rt (quant.); (b) Et3N, 53%; (c) HF/pyridine, 94%; (d) DAST, K2CO3, 79%; (e) DBU, BrCCl3; 88%; (f) LiBH4, THF/EtOH; 89%; (g) DPPA, DBU, THF, 71%; (h) PPh3, THF:H2O (10:1), 96%; (i) Boc2O, Et3N, 82%.
Treatment of 3 with the acid chloride prepared from 3-bromo-benzoic acid and oxalyl chloride provided the benzamide intermediate. Removal of the TIPS protecting group, followed by cyclization with DAST provided the 2,3-dihydro-2-(3-bromophenyl)[2′,4]bioxazole, which was aromatized using DBU and bromotrichloromethane to provide 4. The second key intermediate 5 was prepared from 4 by reduction of the methyl ester to the hydroxymethyl derivative, which was converted with DPPA to an azide. Reduction of this azide with triphenylphosphine and treatment with di-tert-butyl dicarbonate, provided intermediate 5.
Several biphenyl tetraoxazoles were synthesized as outlined in Scheme 2. Formation of the pinacolatoboranyl derivative of 4 was accomplished by coupling with bis(pinacolato)diborane. Suzuki-coupling of this intermediate with 5 provided the 3,3′-bis-[4,2′]bioxazole]biphenyl derivative, 7. Removal of the Boc-protecting group of 7 provided the aminomethyl derivative, which could be condensed with various N-Boc or Fmoc protected amino acids to form the intermediates 8–11. Hydrolysis of the ester and removal of the protecting group on the α-amino substituent provided the requisite intermediates 12–15 for the formation of the 24-membered biphenyltetraoxazole macrocycles. The macocyclization step proceeded in yields ranging from 31% to 65% for 16–19. The highest yield obtained was in the formation of 19, the intermediate required for the preparation of those analogs with improved drug-like physicochemical properties, 20 and 21. The N-Boc-(2-amino)ethyl) derivative 19 was smoothly converted to the primary amine 20, which could be subsequently converted to the N,N-dimethyl derivative 21.
Scheme 2.
Synthesis of 3,3′-bis(2,4-bioxazol]-2′yl)-1,1′biphenylderivatives. Reagents and conditions: (a) PdCl2-(dppf)-CH2Cl2, bis(pinacolato)B2, KOAc, toluene, 93%; (b) PdCl2-(dppf)-CH2Cl2, Cs2CO3, dioxane:H2O (10:1), 59%; (c) TFA, CH2Cl2, 79%; (d) Boc-amino acid, EDC, HOBt, 2,6-lutidine for 8–10, Fmoc-Dab(Boc)-OH, EDC, HOBt, DIPEA for 11; (e) LiOH, THF:H2O (3:1) for 8–10, 1% piperidine/acetonitrile for 11; (f) TFA, CH2Cl2 for 12, 13, 14, LiOH, THF:H2O (3:1) for 15; (g) EDC, HOBt, 2,6-lutidine; (h) formaldehyde, NaBH (OAc)3, CH3OH:CH2Cl2 (1:4).
3. Pharmacology
The relative cytotoxic activities of each of the various biphenyl-tetraoxazole macrocycles 16–21 are summarized in Table 1. Among the tumor lines employed were the human lymphoblastoma RPMI 8402, a human epidermoid carcinoma KB3-1 as well as its variants, KBV-1, that overexpresses the efflux transporter MDR1, and KBH5.0 that overexpresses BCRP. None of these biphenyl macrocylic tetraoxazoles exhibited cytotoxic activity toward RPMI 8402 or KB3-1 comparable to other 24-membered macrocycles such as HXDV, YAK-1-161, METV, or 2, which have a much greater effect on stabilization of G-quadruplex DNA. Against RPMI 8402 cells, only 16 and 19 had IC50 values that were ≤3.5 μM. Against KB3-1 cells, 16, 17, 20, and 21 exhibited modest cytotoxicity with IC50 values that were ≥5.0 μM. Consistent with a greater potential to stabilize G-quadruplex DNA, HXDV, YAK-1-161, METV, and 2 had IC50 values against KB3-1 cells that were one or two orders of magnitude lower. Despite their relative weak cytotoxic activity to the parent cell line KB3-1, there is evidence to suggest for 16, 20, and 21 that these macrocyclic biphenyl tetraoxazoles are substrates for MDR1 and BCRP efflux transporters.
Table 1.
Relative cytotoxic activities (IC50; μM)a
| Compd | RPMI 8402 | KB3-1 wt | KBV-1 +MDR1 | KBH5.0 +BCRP |
|---|---|---|---|---|
| 16 | 2.5 | 2.3 | >10 | >10 |
| 17 | 10 | 5.0 | 6.5 | 10 |
| 18 | >10 | >10 | >10 | >10 |
| 19 | 3.5 | >10 | >10 | 6.0 |
| 20 | 7.0 | 4.0 | >10 | >10 |
| 21 | 6.5 | 3.5 | >10 | >10 |
| HXDV | 0.41 | 0.30 | >10 | 0.5 |
| YAK-1-161 | 0.07 | 0.03 | 5.0 | 0.03 |
| METV | 0.03 | 0.04 | 3.3 | 0.04 |
| 2 | 0.18 | 0.04 | >10 | >10 |
a Values are mean of at least two experiments.
Each of the macrocycles 16–21 was evaluated for its ability to selectively stabilize G-quadruplex versus duplex DNA. As listed in Table 2, the relative ability of these compounds to stabilize G-quadruplex and duplex DNA was measured by examining their effect on the thermal transition temperature (Ttran) of a human telomeric G-quadruplex-forming DNA sequence, (TTAGGG)4, or salmon testes (ST) duplex DNA in the presence of either 50 or 150 mM K+. Among these macrocyclic biaryl tetraoxazoles neither 18, the benzyl-substituted derivative, nor 19, the N-Boc derivative of the 2-aminoethyl derivative, had a significant impact on the melting profile of the quadruplex DNA (ΔTtran = 1.1–2.8 °C). Within this series of macrocycles, 16 and the isopropyl derivative 17 did stabilize quadruplex DNA, but only to a limited extent (ΔTtran = 4.1–6.4 °C). Among these macrocyclic biaryl tetraoxazoles, the 2-aminoethyl derivative 20 and the 2-(N,N-dimethylamino)ethyl derivative 21 had a more significant G-quadruplex stabilizing effect (ΔTtran = 8.4–14.6 °C) than either 16, 17, 18 or 19 (Fig. 4, Table 2). However, the ability of these aminoalkyl substituted macrocyclic biphenyl tetraoxazoles to stabilize G-quadruplex DNA was considerably less relative to several of the other 24-membered oxazole-containing macrocycles investigated in our laboratory, such as 2, YAK-1-161, and METV (Fig. 4, Table 2). None of the biaryl tetraoxazoles stabilized ST duplex DNA to a significant degree, with the observed ΔTtran values being close to or within the experimental uncertainty. Thus, the DNA stabilizing activities of 20 and 21 appear to be quite selective for the quadruplex relative to the duplex form.
Table 2.
Relative impacts on the thermal stabilities of d(TTAGGG)4 quadruplex and salmon testes (ST) duplex DNA
| Compound | d(TTAGGG)4 quadruplex DNA |
ST duplex DNA |
||||||
|---|---|---|---|---|---|---|---|---|
| 50 mM K+a |
150 mM K+b |
50 mM K+a |
150 mM K+b |
|||||
| Ttran c (°C) | ΔTtran d (°C) | Ttran c (°C) | ΔTtran d (°C) | Ttran c (°C) | ΔTtran d (°C) | Ttran c (°C) | ΔTtran d (°C) | |
| None | 55.3 | – | 63.1 | – | 78.2 | – | 86.1 | – |
| 16 | 60.1 | 4.8 | 67.3 | 4.2 | 78.2 | 0 | 86.7 | 0.6 |
| 17 | 61.7 | 6.4 | 67.2 | 4.1 | 78.2 | 0 | 86.2 | 0.1 |
| 18 | 58.1 | 2.8 | 64.7 | 1.6 | 77.2 | −1.0 | 85.7 | −0.4 |
| 19 | 57.7 | 2.4 | 64.2 | 1.1 | 78.3 | 0.1 | 86.5 | 0.4 |
| 20 | 68.2 | 12.9 | 77.7 | 14.6 | 81.1 | 2.9 | 87.1 | 1.0 |
| 21 | 63.7 | 8.4 | 73.7 | 10.6 | 79.1 | 0.9 | 86.7 | 0.6 |
| YAK-1-161 | 92.7 | 37.4 | 92.2 | 29.1 | 78.2 | 0 | 86.7 | 0.6 |
| METV | 92.2 | 36.9 | 93.2 | 30.1 | 78.2 | 0 | 86.2 | 0.1 |
| 2 | 91.2 | 35.9 | 91.2 | 28.1 | 78.7 | 0.5 | 86.8 | 0.7 |
Solution conditions were 10 mM potassium phosphate (pH 7.5) and sufficient KCl (32 mM) to bring the total K+ concentration to 50 mM.
Solution conditions were 10 mM potassium phosphate (pH 7.5) and sufficient KCl (132 mM) to bring the total K+ concentration to 150 mM.
Ttran reflects the thermal transition temperature, with values of Ttran being determined from the minima (for quadruplex DNA) or maxima (for duplex DNA) of first-derivative UV melting profiles exemplified by those in Figure 4. The uncertainty in the Ttran values is ±0.5 °C.
ΔTtran reflects the change in the Ttran of the quadruplex or duplex DNA induced by the presence of the compound. The uncertainty in the ΔTtran values is ±1.0 °C.
Figure 4.
First derivatives of UV melting profiles of a 24mer human telomeric quadruplex DNA sequence, d(TTAGGG)4, and salmon testes (ST) duplex DNA in the absence (filled circles) and presence of either 2 (filled triangles), 20 (open circles), or 21 (filled diamonds). The UV melting profiles of d(TTAGGG)4 were acquired at 295 nm, while those of ST DNA were acquired at 260 nm. The concentration of d(TTAGGG)4 was 5 μM in strand, while that of ST DNA was 30 μM in base pair. When present, all compounds were used at a concentration of 20 μM in the d(TTAGGG)4 experiments and 15 μM in the ST DNA experiments. The solution conditions were 10 mM potassium phosphate (pH 7.5) and sufficient KCl (132 mM) to bring the total K+ concentration to 150 mM.
The methodology employed in the syntheses of these macrocyclic biaryl tetraoxazoles provides for significant versatility in terms of the varied substitutents that could be attached to this macrocycle by the selection of the appropriately substituted a-amino acid. In addition, it appears that a considerable improvement in yield during the macrocyclization of these biaryl tetraoxazoles could be achieved relative to that observed for several other 24-membered macrocycles evaluated as G-quadruplex stabilizers. However, these macrocyclic biphenyl tetraoxazoles lack the potent stabilizing effect on G-quadruplex DNA observed with 2, HXDV, YAK-1-161, or METV. While the biphenyl macrocyle 21 structurally coincides with are large portion of YAK-1-161, it is clear from Figure 3 that it does not have the same planar character. The biphenyl macrocycles are also significantly less cytotoxic against the human tumor cells. It is known that replacement of a phenyl with a pyridyl moiety within macrocycles designed as G-quaduplex stabilizers can have a pronounced effect. Studies are in progress, therefore, to assess the promise of similarly structured macrocyclic 2-phenylpyridyl tetraoxazoles and 2,2′-bipyridyl tetraoxazoles.
4. Experimental
4.1. Chemistry: general methods
All reactions, unless otherwise stated, were done under nitrogen atmosphere. Reaction monitoring and follow-up were done using aluminum backed Silica G TLC plates with UV254 (Sorbent Technologies), visualizing with ultraviolet light. Flash column chromatography was done on a Combi Flash Rf Teledyne ISCO using hexane, ethyl acetate, dichloromethane, and methanol. The 1H (400 MHz) and 13C (100 MHz) NMR spectra were done in CDCl3, methanol-d4, or DMSO-d6 and recorded on a Bruker Avance III (400 MHz) Multinuclear NMR Spectrometer. Data is expressed in parts per million relative to the residual non-deuterated solvent signals, spin multiplicities are given as s (singlet), d (doublet), dd (doublet of doublets), t (triplet), dt (doublet of triplets), q (quartet), m (multiplet), and bs (broad singlet), and coupling constants (J) are reported in Hertz. Melting points were determined using Mel-temp II apparatus and are uncorrected. IR data was recorded on a Thermo Nicolet Avatar Model 360 FTIR. HRMS experiments were conducted by Washington University Resource for Biomedical and Bioorganic Mass Spectrometry Department of Chemistry.
4.2. Compound (4)
4.2.1. Methyl 2-(1-(3-bromobenzamido)-2-((triisopropylsilyl)oxy)ethyl)oxazole-4-carboxylate
3-Bromobenzoic acid (1.7 g, 8.4 mmol) was dissolved in dry dichloromethane (5 mL) and oxalyl chloride (1.5 mL, 16.8 mmol) was added. A catalytic amount of DMF (two drops) was added and the reaction stirred at room temperature for 2 h. The solvent was concentrated under reduced pressure and the residue was placed under the vacuum to dry. The residue was redissolved in dichloromethane (5 mL) and methyl 2-(1-amino-2-((triisopropylsilyl)oxy)ethyl)oxazole-4-carboxylate (241 mg, 7 mmol) was added followed by triethylamine (2.9 mL, 21 mmol). The reaction mixture was allowed to stir overnight at room temperature under N2. The mixture was then washed with saturated NaHCO3 and organic layer concentrated and purified by flash chromatography using a gradient of 0–40% ethyl acetate/hexane to give a colorless oil (1.83 g, 53% yield); 1H NMR (400 MHz) (CDCl3) δ 8.21 (s, 1H), 7.96 (s, 1H), 7.73–7.71 (m, 1H), 7.64–7.62 (m, 1H), 7.28–7.32 (m, 1H), 7.22 (d, 1H, J = 8), 5.51 (m, 1H), 4.29 (m, 1H), 4.14 (m, 1H), 3.89 (s, 3H), 0.87–1.05 (m, 21H); 13C NMR (CDCl3) δ 165.5, 163.3, 161.3, 144.1, 135.6, 134.8, 133.4, 130.5, 130.1, 125.6, 122.7, 64.6, 52.1, 50.2, 17.7, 11.7; IR 3324, 1740, 1668 cm−1.
4.2.2. Methyl 2-(1-(3-bromobenzamido)-2-hydroxyethyl) oxazole-4-carboxylate
Methyl 2-[(1-(3-bromobenzamido)-2-[(triisopropylsilyl)oxy] ethyl]oxazole-4-carboxylate (1.83 g, 3.5 mmol) was dissolved in THF:pyridine (5:1, 6 mL) and cooled to 0 °C under nitrogen. This was treated dropwise with HF-pyridine (1 mL) and the reaction was stirred at room temperature for 18 h. On completion of the reaction, the reaction mixture was poured slowly into saturated solution of NaHCO3. This mixture was extracted with chloroform and the chloroform layer dried with sodium sulfate. The solvent was concentrated under reduced pressure and the residue azeotroped with toluene to give a white solid (1.2 g, 94% yield); mp = 168–170 °C; 1H NMR (CD3OD) δ 8.75–8.76 (m, 1H), 8.52 (s, 1H), 8.07–8.08 (m, 1H), 7.84–7.87 (m, 1H), 7.70–7.73 (m, 1H), 7.38–7.42 (m, 1H), 5.38–5.41 (m, 1H), 4.04–4.07 (m, 2H), 3.88 (s, 3H); 13C NMR (CD3OD) δ 168.3, 164.5, 162.7, 146.1, 136.6, 135.6, 133.3, 131.5, 131.1, 127.2, 123.2, 62.9, 52.3, 51.7.
4.2.3. Methyl 2′-(3-bromophenyl)-4′,5′-dihydro-[2,4′-bioxazole]-4-carboxylate)
Methyl 2-(1-(3-bromobenzamido)-2-hydroxyethyl)oxazole-4-carboxylate (1.2 g, 3.2 mmol) was suspended in dry dichloromethane (10 mL) and placed under nitrogen. After cooling to −78 °C, DAST (787 mg, 4.88 mmol) was added and the reaction stirred at −78 °C for 3 h. This was followed by addition of potassium carbonate (675 mg, 4.88 mmol) and the mixture was allowed to warm to room temperature. Upon completion of the reaction, the reaction mixture was poured carefully into saturated solution of sodium bicarbonate and extracted with dichloromethane to give a pale yellow solid (894 mg, 79%); mp = 82–84 °C; 1H NMR (CDCl3) δ 8.17 (s, 1H), 7.97 (s, 1H), 7.74 (dd, 1H, J = 0.96, 7.76), 7.46–7.48 (m, 1H), 7.13–7.18 (m, 1H), 5.45 (t, 1H, J = 8.44), 4.76 (t, 1H, J = 8.44), 4.67 (t, 1H, J = 8.44), 3.76 (s, 3H); 13C NMR (CDCl3) δ 165.0, 163.2, 161.1, 144.8, 134.7, 133.2, 131.3, 129.9, 128.6, 127.0, 122.2, 70.6, 63.7, 52.0; IR 1741 cm−1.
4.2.4. Methyl 2′-(3-bromophenyl)-[2,4′-bioxazole]-4-carboxylate (4)
Methyl 2′-(3-bromophenyl)-4′,5′-dihydro-[2,4′-bioxazole]-4-carboxylate (100 mg, 0.28 mmol) was dissolved in dry dichloromethane (5 mL), placed under nitrogen and cooled to 0 °C. DBU (0.09 mL, 0.6 mmol) was added dropwise and the solution became brown. BrCCl3 (0.07 mL, 0.72 mmol) was added and the reaction was stirred at room temperature overnight. On completion of the reaction, reaction mixture was poured into saturated solution of ammonium chloride. The aqueous layers were extracted with dichloromethane and the combined organic layers dried over sodium sulfate, concentrated and purified by flash chromatography using a gradient of 0–100% ethyl acetate/hexane to give a white solid (87 mg, 88%); mp = 175–177 °C, 1H NMR (CDCl3) δ 8.41 (s, 1H), 8.32 (s, 1H), 8.26–8.27 (m, 1H), 8.01–8.04 (m, 1H), 7.59–7.62 (m, 1H), 7.32–7.37 (m, 1H), 3.94 (s, 3H); 13C NMR (CDCl3) δ 161.3, 161.2, 155.6, 143.8, 139.7, 134.4, 134.2, 134.1, 130.4, 129.7, 128.1, 125.2, 123.0, 52.2; IR 1745 cm−1 HRMS calculated for C14H9BrN2O4 (M+H)+, 348.9818; found, 348.9820.
4.3. Compound (5)
4.3.1. (2′-(3-Bromophenyl)-[2,4′-bioxazol]-4-yl)methanol
Methyl 2′-(3-bromophenyl)-[2,4′-bioxazole]-4-carboxylate, 4, (310 mg, 0.89 mmol) was dissolved in 11 mL of THF:EtOH (10:1) and cooled to 0 °C under nitrogen. Lithium borohydride (61.7 mg, 2.83 mmol) was added and the reaction was warmed to room temperature overnight. The resulting mixture was poured into water and extracted with ethyl acetate. The combined organic layers were washed with brine and dried over sodium sulfate. The organic layer was concentrated and purified by flash chromatography using a gradient of 0–100% ethyl acetate/hexane to give a white solid (253 mg, 89%); mp = 142–144 °C; 1H NMR (400 MHz, CDCl3) δ 8.35–8.36 (m, 1H), 8.31 (s, 1H), 8.08–8.11 (m, 1H), 7.70–7.71 (m, 1H), 7.63–7.66 (m, 1H), 7.36–7.40 (m, 1H), 4.72 (d, 2H, J = 0.84); 13C NMR (CDCl3) δ 161.3, 155.4, 141.7, 138.5, 135.1, 134.0, 131.8, 130.4, 129.8, 128.2, 125.3, 123.0, 57.0; IR 3054, 2986, 2305, 1552, 1421, 1265, 895, 735, 705, 447 cm−1.
4.3.2. 4-(Azidomethyl)-2′-(3-bromophenyl)-2,4′-bioxazole
(2′-(3-Bromophenyl)-[2,4′-bioxazol]-4-yl)methanol (246 mg, 0.77 mmol) and DPPA (0.25 mL, 1.16 mmol) was dissolved in 10 mL of THF. The reaction was cooled to 0 °C under nitrogen. Neat DBU (0.17 mL, 1.16 mmol) was added and the reaction stirred for 2 h at 0 °C and for 16 h at room temperature. The resulting mixture was washed with water, 5% HCl and extracted with ethyl acetate. The organic layer was washed with brine and dried over sodium sulfate. The organic layer was then concentrated under reduced pressure and purified by flash chromatography using a gradient of 0–50% ethyl acetate/hexane to give a white solid (189 mg, 71%); mp = 130–132 °C; 1H NMR (CDCl3) δ 8.21–8.25 (m, 1H), 8.21 (s, 1H), 7.97–7.99 (m, 1H), 7.65 (s, 1H), 7.56–7.53 (m, 1H), 7.26–7.30 (m, 1H), 4.32 (s, 2H); 13C NMR (CDCl3) δ 161.3, 155.6, 138.8, 137.2, 136.1, 134.0, 131.6, 130.4, 129.8, 128.2, 125.0, 123.0, 46.29; IR 3053, 2986, 2305, 2104, 1552, 1421, 1265, 1115, 909, 736, 650 cm−1.
4.3.3. (2′-(3-Bromophenyl)-[2,4′-bioxazol]-4-yl)methanamine
4-(Azidomethyl)-2′-(3-bromophenyl)-2,4′-bioxazole (181 mg, 0.52 mmol) was dissolved in 11 mL of THF:water (10:1) and polymer-bound triphenylphosphine (262 mg, 0.79 mmol) was added and the reaction mixture stirred at room temperature overnight. The reaction mixture was then filtered, concentrated under reduced pressure, and the residue azeotroped with toluene to give a white solid (161.4 mg, 96%); mp = 125–127 °C; 1H NMR (CDCl3) δ 8.20 (s, 1H), 8.14 (s, 1H), 7.93–7.95 (m, 1H), 7.49–7.50 (m, 2H), 7.21–7.25 (m, 1H), 3.76 (s, 2H), 2.69 (br s, 2H); 13C NMR (CDCl3) δ 161.1, 155.1, 142.9, 134.4, 133.9, 131.9, 130.3, 129.73, 125.4, 122.9, 120.2, 119.98, 119.93, 37.75; IR 3053, 2985, 2305, 2253, 1714, 1645, 1591, 1552, 1490, 1463, 1430, 1474, 1311, 1265, 1163, 1113, 1054, 1025, 908, 793, 734, 650 cm−1.
4.3.4. tert-Butyl [[2′-(3-Bromophenyl)-[2,4′-bioxazol-4-yl]methyl]carbamate (5)
(2′-(3-Bromophenyl)-[2,4′-bioxazol]-4-yl)methanamine (160 mg, 0.5 mmol) was dissolved in dry dichloromethane (5 mL) and triethylamine (50 mg, 0.5 mmol) was maintained under nitrogen. The solution was cooled to 0 °C and a solution of Boc2O (152 mg, 0.7 mmol) in dichloromethane (2 mL) was added and stirred at room temperature overnight. The solvent was removed under reduced pressure and the residue purified by flash chromatography (0–50% ethyl acetate/hexane) to give a white solid (122.8 mg, 59%); mp = 145–147 °C; 1H NMR (CDCl3) δ 8.30 (s, 1H), 8.23 (s, 1H), 8.02–8.04 (m, 1H), 7.58–7.63 (m, 2H), 7.30–7.34 (m, 1H), 5.19 (br s, 1H), 4.29 (d, 2H, J = 8), 1.44 (s, 9H); 13C NMR (CDCl3) δ 161.2, 155.6, 155.1, 139.6, 138.4, 135.2, 133.9, 131.8, 130.3, 129.7, 128.2, 125.2, 122.9, 79.6, 36.5, 28.3; IR 1679, 1263, 748, 421 cm−1; HRMS calculated for C18H19BrN3O4 (M+H)+, 420.0553; found, 420.0551.
4.4. Compound (6)
4.4.1. Methyl 2′-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-[2,4′-bioxazole]-4-carboxylate (6)
Methyl 2′-(3-bromophenyl)-[2,4′-bioxazole]-4-carboxylate (4.2.4) (500 mg, 1.43 mmol) in toluene (5 mL) was treated with bis(pinacolato)diboron (436 mg, 1.72 mmol) and then potassium acetate (544 mg, 4.29 mmol) was added. The solution was purged with nitrogen and PdCl2-(dppf)-CH2Cl2 (35 mg, 0.049) added and the reaction mixture heated at 95 °C overnight. The reaction was cooled to room temperature and filtered. The filtrate was concentrated under reduced pressure and purified by flash chromatography (0–50% ethyl acetate/hexane) to give a white solid (526 mg, 93%) mp = 160–162 °C; 1H NMR (CDCl3) δ 8.59 (s, 1H), 8.43 (s, 1H), 8.35 (s, 1H), 8.20–8.23 (m, 1H), 7.93–7.99 (m, 1H), 7.48– 7.52 (m, 1H), 3.96 (s, 3H), 1.37 (s, 12H); 13C NMR (CDCl3) δ 162.9, 161.3, 155.9, 143.7, 139.3, 137.4, 134.3, 133.2, 130.9, 129.4, 128.2, 125.7, 84.1, 52.5, 24.8; IR 3052, 2983, 2253, 1742, 1638, 1608, 1439, 1408, 1370, 1326, 1238, 1265, 1144, 1104, 1003, 907, 970, 861, 733, 650 cm−1.
4.5. Compound (7)
4.5.1. Methyl 2′-(3′-(4-(((t-butoxycarbonyl)amino)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (7)
A mixture of t-butyl ((2′-(3-bromophenyl)-[2,4′-bioxazol]-4-yl) methyl)carbamate (4.3.4) (100 mg, 0.25 mmol), methyl 2′-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)[2,4′-bioxazole]-4-carboxylate (3.4.1) (113 mg, 0.29 mmol), cesium carbonate (233 mg, 0.72 mmol) was dissolved in 11 mL of dioxane:water (10:1) and the solution purged under nitrogen. PdCl2-(dppf)-CH2Cl2 (24.5 mg, 0.03 mmol) was added and the mixture heated at 90 °C for 4 h. The reaction mixture was then cooled to room temperature and filtered. The filtered solution was concentrated and purified by flash chromatography using a gradient of 0–100% ethyl acetate/ hexane to give a white solid (85.2 mg, 59%); mp = 115–117 °C; 1H NMR (CDCl3) δ 8.46 (s, 2H), 8.42–8.43 (m, 1H), 8.34 (s, 1H), 8.28 (s, 1H), 8.14–8.18 (m, 2H), 7.78–7.81 (m, 2H), 7.64 (s, 1H), 7.57– 7.64 (m, 2H), 5.12 (br s, 1H), 4.31 (d, 2H, J = 5.84), 3.96 (s, 3H), 1.45 (s, 9H); 13C NMR (CDCl3) δ 162.8, 161.9, 156.0, 155.8, 143.9, 141.1, 141.0, 140.9, 139.7, 138.4, 135.1, 134.5, 131.9, 131.2, 130.2, 130.0, 129.7, 129.6, 127.3, 127.2, 127.1, 126.4, 126.35, 126.31, 125.8, 125.7. 79.8, 52.4, 36.7, 28.53; IR 3452, 3166, 2981, 2253, 1711, 1638, 1554, 1503, 1438, 1368, 1323, 1249, 1157, 1115, 1044, 1002, 971, 906, 798, 730, 650, 445; HRMS calculated for C32H28·N5O8 (M+H)+, 610.1932; found, 610.1927.
4.5.2. Removal of Boc protecting group
Methyl 2′-(3′-(4-(((t-butoxycarbonyl)amino)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (3.5.1) (159.9 mg, 0.26 mmol) was dissolved in anhydrous dichloromethane (2 mL) and cooled to 0 °C. Trifluoroacetic acid (2 mL) was added and stirred at that temperature for 3 h. The solvents were removed under reduced pressure and dissolved in dichloromethane. This solution was poured into saturated sodium bicarbonate solution and the organic layer separated. The organic layer was dried over sodium sulfate and concentrated to give a colorless oil; (105 mg, 79%); 1H NMR (CDCl3) δ 8.41 (s, 1H), 8.40 (s, 1H), 8.37 (s, 1H), 8.28 (s, 1H), 8.23 (s, 1H), 8.08–8.12 (m, 2H), 7.72–7.76 (m, 2H), 7.52–7.56 (m, 3H), 3.90 (s, 3H), 3.80 (s, 2H); 13C NMR (CDCl3) 162.7, 162.5, 161.3, 155.8, 155.4, 154.0, 143.9, 143.8, 140.9, 140.8, 139.5, 138.2, 134.4, 134.0, 131.9, 131.0, 130.0, 129.8, 129.6, 129.5, 127.2, 127.0, 126.2, 126.1, 125.6, 52.3, 38.1.
4.6. Compound (16)
4.6.1. Methyl 2′-(3′-(4-((2-((t-butoxycarbonyl)amino) acetamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (8)
Boc-protected glycine (31 mg, 0.18 mmol), EDC (37 mg, 0.20 mmol) and HOBt (26 mg, 0.20 mmol) were dissolved in dry DMF (5 mL) and stirred for 5 min under nitrogen. A solution of methyl 2′-(3′-(4-(aminomethyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (4.5.2) (54 mg, 0.09 mmol), 2,6-lutidine (0.04 mL, 0.36 mmol) in dry DMF (5 mL) was added and reaction stirred at room temperature overnight. On completion of the reaction, the DMF was removed and the mixture purified with flash chromatography (0–5% methanol/dichloromethane) to obtain a white solid (41.2 mg, 70%), mp = 132–134 °C; 1H NMR (CDCl3) δ 8.46 (s, 1H), 8.42 (s, 1H), 8.41 (s, 1H), 8.40 (s, 1H), 8.35 (s, 1H), 8.12–8.14 (m, 2H), 7.77–7.79 (m, 2H), 7.68 (s, 1H), 7.56–7.60 (m, 2H), 6.97 (t, 1H, J = 5.40), 5.36 (br s, 1H), 4.47 (d, 2H, J = 5.92), 3.96 (s, 3H), 3.37 (d, 2H, J = 5.68), 1.43 (s, 9H); 13C NMR (CDCl3) δ 169.6, 162.6, 162.5, 161.3, 155.8, 155.4, 143.8, 140.8, 140.7, 139.5, 138.6, 138.4, 136.2, 135.6, 134.3, 131.6, 131.0, 129.9, 129.8, 129.6, 129.5, 127.1, 126.9, 126.1, 126.1, 125.5, 124.9, 80.3, 52.2, 44.4, 35.2, 28.2; IR 3625, 3054, 3429, 2986, 2305, 1721, 1678, 1421, 1368, 1265, 1156, 1114, 1018, 970, 896, 737, 705, 439 cm−1; HRMS calculated for C34H31O6N9 (M+H)+, 667.2147; found, 667.2143.
4.6.2. Lithium 2′-(3′-(4-((2-((tert-butoxycarbonyl)amino)acetamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate
Methyl 2′-(3′-(4-((2-((tert-butoxycarbonyl)amino)acetamido)methyl)[2,4′-bioxazol]-2′-yl)[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (40 mg, 0.06 mmol) was dissolved in THF/H2O (3:1, 2 mL) and treated with lithium hydroxide (3.0 mg, 0.07 mmol) at 0 °C. This was stirred at room temperature for 4 h. The solvents were concentrated under reduced pressure and dried over vacuum to yield a white solid (39 mg, 100%) mp = 239–240 °C (decomposed); 1H NMR (DMSO) δ 8.89 (s, 1H), 8.83 (s, 1H), 8.29–8.30 (m, 3H), 7.95–8.03 (m, 3H), 7.91–7.95 (m, 3H), 7.63–7.67 (m, 2H), 6.92 (t, 1H, J = 5.68), 4.17 (d, 2H, J = 5.28), 3.50 (d, 2H, J = 5.72), 1.30 (s, 9H); 13C NMR (DMSO) δ 169.4, 162.9, 161.3, 161.2, 155.9, 155.4, 154.3, 152.8, 143.0, 140.2, 140.1, 139.9, 139.8, 139.7, 139.5, 138.6, 136.2, 134.3, 131.4, 131.0, 130.2, 129.6, 129.5, 127.0, 126.8, 125.8, 124.3, 78.0, 43.2, 34.6, 28.1.
4.6.3. 2′-(3′-(4-((2-Aminoacetamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylic acid (12) as its trifluoroacetic acid salt
Lithium 2′-(3′-(4-((2-((tert-butoxycarbonyl)amino)acetamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (39 mg, 0.06 mmol) was suspended in dry DCM (1 mL) and cooled to 0 °C. Trifluoroacetic acid (1 mL) was added and the reaction stirred at that temperature for 2 h. The solvent was concentrated under reduced pressure and azeotroped with toluene to give product (38 mg, 100%); mp = 153–155 °C; 1H NMR (DMSO) δ 9.10 (s, 1H), 8.98 (s, 1H), 8.83–8.86 (m, 2H), 8.39 (d, 2H), 8.11–8.15 (m, 3H), 8.02–8.04 (m, 2H), 7.74–77.79 (m, 2H), 4.35 (d, 2H, J = 5.28), 3.61 (s, 2H); 13C (DMSO) δ 166.0, 162.9, 161.3, 161.2, 157.7, 154.3, 152.8, 144.9, 141.1, 140.3, 140.0, 139.8, 139.5, 138.6, 136.5, 134.3, 131.4, 130.9, 130.4, 130.3, 129.7, 127.0, 126.8, 125.9, 124.3, 115.9, 54.8, 34.6.
4.6.4. (16)
2′-(3′-(4-((2-Aminoacetamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylic acid (12) as its trifluoroacetic acid salt (38 mg, 0.06 mmol) was dissolved in dry DMF (30 mL), EDC (23 mg, 0.12 mmol) and HOBt (16 mg, 0.12 mmol) was added. 2,6-Lutidine (0.04 mL, 0.3 mmol) was added and reaction stirred at room temperature overnight. The DMF was removed on Kugelrohr and the residue purified by flash chromatography (0–10% MeOH/DCM) to give a white solid (6.4 mg, 24%); mp = 350–352 °C (decomposed); 1H NMR (DMSO) δ 9.08 (s, 1H), 8.95 (s, 1H), 8.76–8.77 (m, 2H), 8.65 (s, 1H), 8.61 (s, 1H), 8.46 (m, 1H), 8.12–8.16 (m, 3H), 7.97–8.01 (m, 2H), 7.68– 7.73 (m, 2H), 4.19 (d, 2H, J = 5.48), 3.84 (d, 2H, J = 6.36); 13C NMR (DMSO) δ 168.5, 161.7, 159.8, 154.8, 142.1, 138.5, 138.2, 136.1, 130.8, 130.4, 128.6, 126.9, 126.8, 125.4, 123.5, 45.7; HRMS calculated for C28H19N6O6 (M+H)+, 535.1361; found, 535.1347.
4.7. Compound (17)
4.7.1. Methyl 2′-(3′-(4-((2-((t-butoxycarbonyl)amino)-3-methylbutanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (9)
Boc-protected L-valine (52 mg, 0.24 mmol), EDC (50.5 mg, 0.26 mmol) and HOBt (36 mg, 0.26 mmol) were dissolved in dry DMF (5 mL) and stirred for 5 min under nitrogen. A solution of methyl 2′-(3′-(4-(aminomethyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (4.5.2) (72.8 mg, 0.12 mmol), 2,6-lutidine (0.06 mL, 0.48 mmol) in dry DMF (5 mL) was added and reaction stirred at room temperature overnight. On completion of the reaction, the DMF was removed and the mixture purified with flash chromatography (0–100% ethyl acetate/ hexane) to obtain a white solid (66.2 mg, 78%); mp = 135–138 °C; 1H NMR (CDCl3) δ 8.40 (s, 1H), 8.39 (s, 1H), 8.37 (s, 1H), 8.28 (s, 1H), 8.22 (s, 1H), 8.08–8.12 (m, 2H), 7.73–7.75 (m, 2H), 7.62 (s, 1H), 7.52–7.56 (m, 2H), 6.49 (br s, 1H), 4.95 (br s, 1H), 4.39 (d, J = 5.48), 3.90 (s, 3H), 3.84–3.86 (m, 1H), 2.08–2.15 (m, 1H), 1.26 (s, 9H), 0.88 (d, 3H), 0.83 (d, 3H); 13C (CDCl3) δ 171.9, 162.6, 161.3, 155.9, 155.4, 143.8, 140.8, 140.7, 139.5, 138.7, 138.4, 135.7, 134.3, 131.5, 130.9, 129.9, 129.8, 129.5, 129.4, 127.0, 126.9, 126.2, 126.1, 125.5, 125.0, 118.1, 110.6, 79.9, 60.1, 52.2, 35.2, 30.8, 28.2, 19.3; IR 3054, 2986, 2305, 1724, 1421, 1265, 1156, 895, 737, 705 cm−1; HRMS calculated for C37H37O9N6 (M+H)+, 709.2617; found, 709.2607.
4.7.2. Lithium 2′-(3′-(4-((2-((t-butoxycarbonyl)amino)-3-methylbutanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate
Methyl 2′-(3′-(4-((2-((tert-butoxycarbonyl)amino)-3-methylb-utanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (31 mg, 0.05 mmol) was dissolved in THF/H20 (3:1, 2 mL) and treated with lithium hydroxide (2.5 mg, 0.06 mmol) at 0 °C. This was stirred at room temperature for 4 h. The solvents were concentrated under reduced pressure and dried over vacuum to yield a white solid (35 mg, 100%); mp = 209–210 °C (decomposed); 1H NMR (CD3OD) δ 8.35 (s, 2H), 8.22 (s, 1H), 8.03–8.06 (m, 2H), 7.81–7.84 (m, 3H), 7.58–7.67 (m, 4H), 7.21–7.24 (m, 2H), 4.37 (s, 2H), 3.87 (d, 1H), 2.00–2.07 (m, 1H), 1.41 (s, 9H), 0.94 (d, 6H, J = 7.16); 13C (CD3OD) δ 174.7, 169.8, 168.1, 166.6, 163.8, 156.7, 155.9, 145.9, 144.6, 144.0, 142.5, 142.0, 141.4, 140.7, 137.6, 132.4, 130.9, 130.8, 128.9, 128.4, 128.3, 127.1, 126.1, 125.9, 124.8, 118.6, 112.5, 80.6, 63.3, 35.6, 30.93, 28.7, 18.5.
4.7.3. (S)-2′-(3′-(4-((2-Amino-3-methylbutanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylic acid (13) as its trifluoroacetic acid salt
Lithium 2′-(3′-(4-((2-((tert-butoxycarbonyl)amino)-3-methylbutanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (35 mg, 0.05 mmol) was suspended in dry DCM (2 mL) and cooled to 0 °C. Trifluoroacetic acid (2 mL) was added and the reaction stirred at that temperature for 2 h. The solvent was concentrated under reduced pressure and azeotroped with toluene to give of the desired product (34 mg, 100%); mp = 105–107 °C; 1H NMR (CD3OD) δ 8.58 (s, 1H), 8.50 (s, 1H), 8.48 (s, 1H), 8.29 (s, 2H), 7.98–8.00 (m, 2H), 7.83 (s, 1H), 7.76–7.80 (m, 2H), 7.66 (d, 1H, J = 8.24), 7.52–7.56 (m, 2H), 4.30–4.41 (m, 2H), 3.63 (d, 1H, J = 6), 2.11–2.16 (m, 1H), 0.99 (d, 3H, J = 2.44), 0.97 (d, 3H, J = 2.44); 13C (CD3OD) 169.7, 164.1, 164.0, 161.1, 158.7, 145.9, 142.0, 141.7, 140.8, 139.8, 137.8, 132.4, 131.9, 131.0, 128.3, 128.2, 127.3, 127.1, 126.2, 118.7, 118.5, 117.4, 115.8, 114.6, 113.0, 111.5, 60.0, 36.0, 31.4, 18.8, 18.0.
4.7.4. (17)
(S)-2′-(3′-(4-((2-Amino-3-methylbutanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylic acid (13) as its trifluoroacetic acid salt (34 mg, 0.05 mmol) was dissolved in dry DMF (25 mL), EDC (19 mg, 0.1 mmol) and HOBt (13 mg, 0.1 mmol) was added. 2,6-Lutidine (0.03 mL, 0.25 mmol) was added and reaction stirred at room temperature overnight. The DMF was removed on Kugelrohr and residue purified by flash chromatography using a gradient of 0–10% MeOH/DCM) to give a white solid (8.7 mg, 31%); mp = 290–292 (decomposed) °C; 1H NMR (400 MHz) (CDCl3) δ 8.98 (s, 1H), 8.94 (s, 1H), 8.24 (s, 2H), 8.15 (s, 1H), 7.84–7.91 (m, 5H), 7.59 (s, 1H), 7.50–7.55 (m, 3H), 4.94 (dd, 1H, J = 8, 16), 4.6 (dd, 1H, J = 4, 8), 3.79 (dd, 1H, J = 4, 8), 2.40–2.46 (m, 1H), 0.85 (d, 3H, J = 8), 0.71 (d, 3H, J = 8); 13C NMR (100 MHz) (CDCl3)d 170.9, 162.9, 162.6, 160.6, 155.9, 155.2, 141.7, 139.0, 138.9, 138.7, 138.5, 138.0, 137.0, 134.9, 131.4, 130.9, 129.5, 129.4, 128.2, 128.0, 127.2, 126.9, 126.1, 126.0, 125.2, 125.1, 57.7, 34.1, 30.79, 19.3, 16.8; IR 3053, 2986, 2305, 2253, 1421, 1265, 909, 734, 705, 650 cm−1; HRMS calculated for C31H25·N6O6 (M+H)+, 577.1830; found, 577.1821.
4.8. Compound (18)
4.8.1. (S)-Methyl 2′-(3′-(4-((2-((tert-butoxycarbonyl)amino)-3-phenylpropanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazol]-4-carboxylate (10)
Boc-protected L-phenylalanine (53 mg, 0.2 mmol), EDC (76 mg, 0.4 mmol) and HOBt (53 mg, 0.4 mmol) were dissolved in dry DMF (5 mL) and stirred for 5 min under nitrogen. A solution of methyl 2′-(3′-(4-(aminomethyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (4.5.2) (30 mg, 0.05 mmol), 2,6-lutidine (0.07 mL, 0.64 mmol) in dry DMF (5 mL) was added and reaction stirred at room temperature overnight. On completion of the reaction, the DMF was removed and the mixture purified with flash chromatography (0–100% ethyl acetate/hexane) to obtain a white solid (19.7 mg, 52%); mp = 153–156 °C; 1H NMR (CDCl3) δ 8.39 (s, 1H), 8.37–8.38 (m, 1H), 8.35–8.36 (m, 1H), 8.27 (s, 1H), 8.20 (s, 1H), 8.06–8.10 (m, 2H), 7.94 (s, 1H), 7.71–7.74 (m, 2H), 7.50–7.55 (m, 2H), 7.47 (s, 1H), 7.07–7.17 (m, 5H), 6.41 (t, 1H, J = 5.64), 4.98 (br s, 1H), 4.30 (d, 2H, J = 5.72), 3.89 (s, 3H), 2.99 (d, 2H, J = 6.78), 1.32 (s, 9H); 13C NMR (CDCl3) δ 171.2, 162.6, 162.5, 161.3, 161.2, 155.8, 155.3, 154.3, 143.8, 140.9, 140.8, 139.5, 138.3, 136.5, 135.5, 134.4, 131.7, 131.0, 130.0, 129.9, 129.8, 129.5, 129.2, 128.6, 127.1, 127.0, 126.9, 126.2, 126.1, 125.5, 79.0, 60.3, 55.9, 52.3, 38.5, 35.2, 31.4, 28.2; IR 3432, 2253, 1672, 1493, 1385, 1157, 1115, 906, 730, 650, 416 cm−1; HRMS calculated for C41H37·N6O9 (M+H)+, 757.2617; found, 757.2615.
4.8.2. Lithium 2′-(3′-(4-((2-((tert-butoxycarbonyl)amino)-3-phenylpropanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate
Methyl 2′-(3′-(4-((2-((t-butoxycarbonyl)amino)-3-phenylprop-anamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (19.7 mg, 0.026 mmol) was dissolved in THF:H2O (3:1, 1.2 mL) and treated with lithium hydroxide (1.3 mg, 0.03 mmol) at 0 °C. This was stirred at room temperature for 4 h. The solvents were concentrated under reduced pressure and the residue dried under vacuum to yield a white solid (16 mg, 100%); mp = 235–236 °C; 1H NMR (400 MHz) (DMSO) δ 8.89 (s, 1H), 8.84 (s, 1H), 8.28 (d, 2H, J = 1.64), 8.07 (s, 1H), 8.02 (d, 2H, J = 7.76), 7.91 (d, 2H, J = 7.56), 7.80 (s, 1H), 7.63–7.67 (m, 2H), 7.08–7.14 (m, 7H), 4.17 (s, 2H), 4.13 (m, 1H), 2.88 (d, 1H, J = 4.52), 2.71 (d, 1H, J = 4.52), 1.22 (s, 9H); 13C NMR (100 MHz) (DMSO) δ δ171.75, 163.1, 162.5, 161.3, 161.2, 155.8, 154.3, 152.9, 143.8, 140.9, 140.8, 140.2, 139.9, 138.1, 136.0, 135.5, 134.4, 131.3, 130.2, 129.8, 129.6, 129.2, 123.6, 127.9, 126.9, 126.8, 126.1, 125.8, 124.3, 77.9, 55.8, 34.9, 37.4, 28.1.
4.8.3. (S)-2′-(3′-(4-((2-Amino-3-phenylpropanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazol]-4-carboxylic acid (14) as its trifluoroacetate salt
Lithium 2′-(3′-(4-((2-((tert-butoxycarbonyl)amino)-3-phenylpropanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (16 mg, 0.02 mmol) was suspended in dry dichloromethane (1 mL) and cooled to 0 °C. Trifluoroacetic acid (1 mL) was added and the reaction stirred at that temperature for 2 h. The solvent was concentrated under reduced pressure and azeotroped with toluene to give the desired product as a pale yellow solid (16 mg, 100%) mp = 135–137 °C; 1H NMR (400 MHz) (DMSO) δ 9.16 (s, 1H), 9.03 (s, 1H), 8.99 (m, 1H), 8.94 (s, 1H), 8.44 (s, 1H), 8.43 (s, 1H), 8.16–8.20 (m, 2H), 8.07–8.09 (m, 2H), 7.97 (s, 1H), 7.79–7.84 (m, 2H), 7.28–7.38 (m, 5H), 4.33–4.35 (m, 2H), 4.05–4.08 (m, 1H), 3.12 (d, 1H, J = 6.25), 3.07 (d, 1H, J = 7.32); 13C NMR (100 MHz) (CDCl3) δ171.7, 163.1, 162.5, 161.3, 157.4, 154.3, 152.9, 143.8, 140.9, 140.8, 140.2, 139.9, 138.1, 136.0, 135.5, 134.4, 131.3, 130.2, 129.4, 129.6, 129.2, 123.6, 127.9, 126.9, 126.8, 126.1, 125.8, 124.3, 55.8, 48.5, 37.4.
4.8.4. (18)
(S)-2′-(3′-(4-((2-Amino-3-phenylpropanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylic acid, 14, as its trifluoroacetate salt (16 mg, 0.02 mmol) was dissolved in dry DMF (11 mL), EDC (8 mg, 0.04 mmol) and HOBt (6 mg, 0.04 mmol) was added. 2,6-Lutidine (0.02 mL, 0.11 mmol) was added and reaction stirred at room temperature overnight. The DMF was removed on Kugelrohr and residue purified by flash chromatography using a gradient of 0–10% MeOH/DCM to give a white solid (3.7 mg, 27%); mp = 240–243 °C (decomposed); 1H NMR (400 MHz) (CDCl3) 9.00 (s, 1H), 8.95 (s, 1H), 8.21 (s, 1H), 8.19 (s, 1H), 8.10 (s, 1H), 7.86–7.93 (m, 4H),7.68 (d, 1H, J = 10.2), 7.62 (s, 1H), 7.51–7.55 (m, 2H), 7.36–7.39 (m, 1H), 6.95–7.09 (m, 5H), 5.16–5.22 (m, 1H), 4.85–4.91 (m, 1H), 3.81 (dd, 1H, J = 3.32, 18.44), 3.43 (dd, 1H, J = 3.76, 14.64), 2.81–2.88 (m, 1H); 13C NMR (100 MHz) (CDCl3) δ 170.6, 162.9, 162.6, 160.4, 155.8, 155.1, 141.4, 139.0, 138.8, 138.7, 138.4, 138.0, 136.8, 136.6, 135.1, 131.6, 130.8, 129.6, 129.5, 128.9, 128.3, 128.1, 128.0, 127.3, 126.9, 126.5, 126.0, 125.9, 125.2, 53.5, 38.3, 34.5; IR 3054, 2986, 2305, 1421 cm−1; HRMS calculated for C35H24·N6O6 (M+H)+, 625.1830; found, 625.1828.
4.9. Compound (19)
4.9.1. Methyl 2′-(3′-(4-((2-((((9H-Fluoren-9-yl)methoxy) carbonyl)amino)-4-((tert-butoxycarbonyl)amino)butanamido) methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazol]-4-carboxylate (11)
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-4-((t-butoxycarbonyl)amino)butanoic acid (39 mg, 0.09 mmol), EDC (17.8 mg, 0.09 mmol) and HOBt (17 mg, 0.10 mmol) were dissolved in dry DMF (2 mL) and stirred for 5 min under nitrogen. A solution of methyl 2′-(3′-(4-(aminomethyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (4.5.2) (44.8 mg, 0.074 mmol), diisopropylethylamine (0.02 mL, 0.08 mmol) in dry DMF (5 mL) was added and reaction stirred at room temperature overnight. On completion of the reaction, the DMF was removed and the mixture purified with flash chromatography (0–100% ethyl acetate/hexane) to obtain a white solid (54.4 mg, 78%); mp = 185–187 °C; 1H NMR (400 MHz) (CDCl3) δ 8.41 (s, 1H), 8.35 (s, 2H), 8.28 (s, 1H), 8.20 (s, 1H), 8.06–8.08 (m, 2H), 7.72–7.74 (m, 2H), 7.62–7.66 (m, 3H), 7.50–7.55 (m, 4H), 7.22–7.30 (m, 5H), 6.12 (br s, 1H), 5.23 (br s, 1H), 4.38 (s, 2H), 4.31 (d, 2H, J = 6.84), 4.11–4.18 (m, 2H), 3.89 (s, 3H), 3.28–3.34 (m, 1H), 2.92–2.95 (m, 1H), 1.85–1.90 (m, 1H), 1.58–1.61 (m, 1H), 1.35 (s, 9H); 13C (100 MHz) (CDCl3) δ 171.6, 162.6, 162.5, 161.4, 156.3, 155.8, 155.42, 143.87, 143.6, 141.2, 140.9, 140.7, 139.6, 138.6, 138.6, 138.4, 135.6, 134.3, 131.5, 130.9, 130.0, 129.8, 129.6, 129.5, 127.6, 127.1, 127.0, 127.0, 126.9, 126.1, 125.5, 125.0, 119.9, 79.7, 67.00, 52.3, 47.1, 35.6, 28.3, 28.2; IR 3054, 2986, 2685, 2305, 1684, 1637 cm−1; HRMS calculated for C51H46O11N7 (M+H)+, 932.3250; found, 932.3225.
4.9.2. Methyl 2′-(3′-(4-((2-amino-4-((t-butoxycarbonyl) amino)butanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazol]-4-carboxylate
Methyl 2′-(3′-(4-((2-((((9H-fluoren-9-yl)methoxy)carbonyl) amino)-4-((t-butoxycarbonyl)amino) butanamido) methyl)[2,4′-bioxazol]-2′-yl)[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (22 mg, 0.024 mmol) was stirred in a solution of 1% piperidine in acetonitrile (4 mL) for 3 h at room temperature. The solvent was removed under reduced pressure and purified by flash chromatography (0–10% CH3OH/CH2Cl2) to yield a white solid (15.3 mg, 91%); mp = 113–115 °C 1H NMR (400 MHz) (CDCl3) δ 8.40 (s, 1H), 8.37 (s, 1H), 8.35 (s, 1H), 8.28 (s, 1H), 8.23 (s, 1H), 8.07–8.08 (m, 2H). 7.98 (br s, 1H), 7.71–7.74 (m, 2H), 7.62 (s, 1H), 7.51–7.58 (m, 2H), 4.93 (br s, 1H), 4.38 (d, 2H, J = 4), 3.89 (s, 3H), 3.23–3.27 (m, 1H), 3.15–3.18 (m, 1H), 1.93–1.98 (m, 1H), 1.60–1.66 (m, 1H), 1.35 (s, 9H); 13C NMR (100 MHz) (CDCl3) δ 175.0, 162.6, 162.5, 161.3, 156.4, 155.8, 155.4, 143.8, 140.9, 140.8, 139.5, 138.9, 138.3, 135.6, 134.4, 131.7, 131.0, 130.0, 129.8, 129.6, 129.5, 127.1, 126.9, 126.2, 126.1, 125.5, 79.4, 52.8, 52.3, 37.1, 35.8, 35.1, 28.3.
4.9.3. (S)-2′-(3′-(4-((2-Amino-4-((tert-butoxycarbonyl)amino) butanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazol]-4-carboxylate (15)
Methyl 2′-(3′-(4-((2-amino-4-((tert-butoxycarbonyl)amino) butanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate (25 mg, 0.037 mmol) was dissolved in THF:H2O (3:1, 2 mL) and treated with lithium hydroxide (2.0 mg, 0.05 mmol) at 0 °C. This was stirred at room temperature for 4 h. The solvents were removed under reduced pressure and the residue dried under vacuum, 1 M HCl solution was added and a white solid precipitate formed. The solid was filtered and then azeotroped with toluene to give a white solid (25 mg, 100%); mp = 203–205 °C; 1H NMR (400 MHz) (DMSO) δ 9.09–9.12 (m, 2H), 8.97 (s, 1H), 8.85 (s, 1H), 8.35–8.36 (m, 2H), 8.21 (s, 1H), 8.08–8.12 (m, 2H), 7.99–8.01 (m, 2H), 7.71–7.76 (m, 2H), 4.49 (s, 2 h), 3.80 (m, 1H), 3.02–3.07 (m, 2H), 1.86–1.87 (m, 2H), 1.37 (s, 9H); 13C (400 MHz) (DMSO) δ 175.0, 162.6, 162.5, 161.3, 156.4, 155.8, 155.4, 145.2, 141.3, 140.6, 139.5, 138.9, 137.0, 135.6, 134.4, 131.7, 130.5, 130.0, 129.8, 129.6, 129.5, 127.1, 126.9, 126.3, 126.1, 124.5, 79.4, 50.7, 37.1, 36.4, 31.9, 28.5.
4.9.4. (19)
(S)-2′-(3′-(4-((2-Amino-4-((t-butoxycarbonyl)amino)butanamido)methyl)-[2,4′-bioxazol]-2′-yl)-[1,1′-biphenyl]-3-yl)-[2,4′-bioxazole]-4-carboxylate, (4.9.3), (25.3 mg, 0.036 mmol) was dissolved in dry DMF (18 mL), EDC (14 mg, 0.073 mmol) and HOBt (9.8 mg, 0.073 mmol) was added 2,6-lutidine (0.017 mL, 0.145 mmol) and the reaction stirred at room temperature overnight. The DMF was removed on Kugelrohr and residue purified by flash chromatography using a gradient of 0–10% MeOH/DCM)to give a white solid (16 mg, 65%); mp = 200–202 °C (decomposed); 1H NMR (400 MHz) (CDCl3) δ 8.99 (s, 1H0, 8.91 (s, 1H), 8.23 (s, 2H), 8.16 (s, 1H), 7.84–7.90 (m, 4H), 7.60 (s, 1H), 7.50–7.58 (m, 4H), 4.91–4.95 (m, 2H), 4.77–4.83 (m 1H), 3.83–3.87 (m, 1H), 3.27–3.30 (m, 1H), 2.83–2.89 (m, 1H), 2.28–2.31 (m, 1H), 1.60–1.66 (m, 1H), 1.31 (s, 9H); 13C (100 MHz) (CDCl3) δ 170.8, 162.9, 162.5, 160.9, 155.9, 155.7, 155.1, 141.7, 139.1, 138.6, 138.4, 138.2, 138.1, 136.4, 135.3, 131.3, 130.7, 129.5, 129.4, 128.2, 127.9, 127.2, 126.7, 125.0, 125.9, 125.3, 125.2, 79.0, 55.1, 50.3, 34.6, 28.3, 28.6; IR 1681 cm−1; HRMS calculated for C35H31·N7O8 (M+H)+, 678.2307; found, 678.2293.
4.10. Compound (20)
4.10.1. (20)
Compound 19 (4.9.4) (14 mg, 0.02 mmol) was suspended in dry DCM (1 mL) and cooled to 0 °C. Trifluoroacetic acid (1 mL) was added and the reaction stirred at that temperature for 2 h. The solvent was concentrated under reduced pressure and washed with saturated NaHCO3. The organic layer was dried over sodium sulfate and concentrated to give a white solid (12.4 mg, 100%); mp = 210– 212 °C; 1H NMR (CD3OD) δ 8.61 (s, 1H), 8.57 (s, 1H), 8.56 (s, 1H), 8.48 (s, 1H), 8.41 (s, 1H), 7.78–7.87 (m, 6H), 7.48–7.53 (m, 3H), 4.50–4.53 (m, 2H), 3.90–3.94 (m, 1H), 2.89–2.95 (m, 2H), 1.88–1.92 (m, 2H); 13C NMR (400 MHz) (CD3OD) δ 170.8, 162.9, 162.5, 160.9, 155.7, 155.1, 141.7, 139.1, 138.6, 138.4, 138.2, 138.1, 136.4, 135.3, 131.3, 130.7, 129.5, 129.4, 128.2, 127.9, 127.2, 126.7, 125.0, 125.9, 125.3, 125.2, 55.1, 50.3, 34.6, 28.5; HRMS calculated for C30H23O6N7 (M+H)+, 578.1783; found, 578.1783.
4.11. Compound (21)
4.11.1. (21)
Compound 20 (4.10.1) (8.6 mg, 0.015 mmol) was dissolved in 20% MeOH in dichloromethane (3 mL) and a solution of 37% aqueous formaldehyde (1 mL) was added under nitrogen. The reaction mixture was allowed to stir at room temperature for 10 min and sodium triacetoxyborohydride (46.6 mg, 0.22 mmol) was then added. The reaction was stirred at room temperature overnight. The reaction mixture was then poured into saturated sodium bicarbonate solution and extracted with dichloromethane. The organic extracts were dried over sodium sulfate, concentrated and purified using a gradient of 1–10% MeOH/CH2Cl2 with 0.1% NH4OH to give a white solid (4.2 mg, 47%); mp = 218–220 °C (decomposed); 1H NMR (400 MHz) (CDCl3) 8.91 (s, 1H), 8.88 (s, 1H), 8.22 (s, 1H), 8.21 (s, 1H), 8.16 (s, 1H), 7.86–7.92 (m, 5H), 7.62 (s, 1H), 7.52–7.56 (m, 4H), 4.79–4.88 (m, 2H), 3.86–3.88 (m, 2H), 2.24–2.35 (m, 1H), 2.19 (s, 6H), 2.03–2.05 (m, 1H), 1.92–1.97 (m, 1H), 1.91–1.92 (m, 1H); 13C NMR (100 MHz) (CDCl3) δ 170.8, 162.6, 162.5, 160.9, 155.7, 155.1, 141.0, 139.1, 139.0, 138.6, 138.4, 138.1, 136.4, 135.3, 131.3, 131.0, 129.5, 129.4, 128.2, 127.3, 127.2, 126.7, 125.9, 125.7, 125.4, 125.1, 55.7, 44.6, 34.4, 28.2; HRMS calculated for C30H23·N7O6 (M+H)+, 578.1783; found, 578.1783.
4.12. Cytotoxicity assays
The cytotoxicity was determined using the MTT-microtiter plate tetrazolinium cytotoxicity assay (MTA). The KB3-1 cell line and its multidrug-resistant variant KBV-1 were obtained from K.V. Chin (The Cancer Institute of New Jersey, New Brunswick, NJ, USA).24 The KBH5.0 cell line as noted previously was derived from KB3-1 by stepwise selection against Hoechst 33342.25 The cytotoxicity assay was performed using 96-well microtiter plates. Cells were grown in suspension at 37 °C in 5% CO2 and maintained by regular passage in RPMI medium supplemented with 10% heat inactivated fetal bovine serum, L-glutamine (2 mM), penicillin (100 U/mL), and Streptomycin (0.1 mg/mL). For determination of IC50, cells were exposed continuously for FOUR days to varying concentrations of drug, and MTT assays were performed at the end of the fourth day. Each assay was performed with a control that did not contain any drug. All assays were performed at least twice in six replicate wells.
4.13. UV melting studies
UV melting experiments were conducted on an AVIV Model 14DS Spectrophotometer (Aviv Biomedical, Lakewood, NJ, USA) equipped with a thermoelectrically controlled cell holder. Quartz cells with a pathlength of 1.0 cm were used for all the temperature-dependent absorbance studies. UV melting profiles were acquired at either 260 nm (for duplex) or 295 nm (for quadruplex) with a 5 second averaging time. The temperature was raised in 0.5 °C increments, and the samples were allowed to equilibrate for 1 min at each temperature setting. In the quadruplex melting studies, the concentration of d(TTAGGG)4 was 5 μM in strand (120 μM in nucleotide). When present in these quadruplex studies, all test compounds were used at a concentration of 20 μM. In the duplex melting studies, the salmon testes (ST) DNA concentration was 15 μM in base pair (30 μM in nucleotide), and all test compounds, when present, were used at concentration of 15 μM. The solution conditions for all the UV melting studies were 10 mM potassium phosphate (pH 7.5) and sufficient KCl (132 mM) to bring the total K+ concentration to 150 mM. Prior to their use in the UV melting experiments, all d(TTAGGG)4 solutions were preheated at 90 °C for 5 min and slowly cooled to room temperature over a period of 4 h.
Acknowledgments
The Bruker Avance III 400 MHz NMR spectrometer used for this study was purchased with funds from NCRR Grant No. 1S10RR23698-1A1. Mass spectrometry was provided by the Washington University Mass Spectrometry Resource with support from the NIH National Center for Research Resources Grant No. P41RR0954.
References and notes
- 1.Monchaud D, Teulade-Fichou M-P. Org. Biomol. Chem. 2008;6:627. doi: 10.1039/b714772b. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen M, Ulven T. Curr. Med. Chem. 2010;17:3438. doi: 10.2174/092986710793176320. [DOI] [PubMed] [Google Scholar]
- 3.Keniry MA. Biopolymers. 2000/2001;56:123. doi: 10.1002/1097-0282(2000/2001)56:3<123::AID-BIP10010>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Oganesian L, Bryan TM. BioEssays. 2007;29:155. doi: 10.1002/bies.20523. [DOI] [PubMed] [Google Scholar]
- 5.Simonsson T, Pecinka P, Kubista M. Nucleic Acids Res. 1998;26:1167. doi: 10.1093/nar/26.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cogoi S, Xodo LE. Nucleic Acids Res. 2006;34:2536. doi: 10.1093/nar/gkl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai J, Dexheimer TS, Chen D, Carver M, Ambrus A, Jones RA, Yang D. J. Am. Chem. Soc. 2006;128:1096. doi: 10.1021/ja055636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rankin S, Reszka AP, Huppert J, Zloh M, Parkinson GN, Todd AK, Laname S, Balasubramanian S, Neidle S. J. Am. Chem. Soc. 2005;127:10584. doi: 10.1021/ja050823u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo K, Pourpak A, Beetz-Rogers K, Gokhale V, Sun D, Hurley LH. J. Am. Chem. Soc. 2007;129:10220. doi: 10.1021/ja072185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagihara M, Yoneda K, Yabuuchi H, Okuno Y, Nakatani K. Bioorg. Med. Chem. Lett. 2010;20:2350. doi: 10.1016/j.bmcl.2010.01.158. [DOI] [PubMed] [Google Scholar]
- 11.Joachimi A, Benz A, Hartig JS. Bioorg. Med. Chem. 2009;17:6811. doi: 10.1016/j.bmc.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 12.Danzhou Y, Keika O. Future Med. Chem. 2010;2:619. [Google Scholar]
- 13.Huang Z-S, Tan J-H, Ou T-M, Li D, Gu L-Q. In: Medicinal Chemistry Nucleic Acids. Zhang L-H, Xi Z, Chattopadhyaya J, editors. John Wiley & Sons Inc; New York: 2011. pp. 206–257. [Google Scholar]
- 14.Balasubramanian S, Hurley L, Neidle S. Nat. Rev. Drug Disc. 2011;10:261. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duchler MJ. Drug Target. 2012;20:389. doi: 10.3109/1061186X.2012.669384. [DOI] [PubMed] [Google Scholar]
- 16.Perry PJ, Jenkins TC. Mini-Rev. Med. Chem. 2001;1:31. doi: 10.2174/1389557013407304. [DOI] [PubMed] [Google Scholar]
- 17.Kim MY, Gleason-Guzman M, Izbicka E, Nishioka D, Hurley LH. Cancer Res. 2003;63:3247. [PubMed] [Google Scholar]
- 18.Minhas GS, Pilch DS, Kerrigan EJ, LaVoie EJ, Rice JE. Bioorg. Med. Chem. Lett. 2006;16:3891. doi: 10.1016/j.bmcl.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 19.Barbieri CM, Srinivasan AR, Rzuczek SG, Rice JE, LaVoie EJ, Pilch DS. Nucleic Acids Res. 2007;35:3272. doi: 10.1093/nar/gkm188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai YC, Qi H, Lin CP, Lin RK, Kerrigan JE, Rzuczek SG, LaVoie EJ, Rice JE, Pilch DS, Lyu YL, Liu LF. J. Biol. Chem. 2009;284:22535. doi: 10.1074/jbc.M109.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satyanarayana M, Rzuczek SG, LaVoie EJ, Pilch DS, Liu A, Liu LF, Rice JE. Bioorg. Med. Chem. Lett. 2008;18:3802. doi: 10.1016/j.bmcl.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satyanarayana M, Kim Y-A, Rzuczek SG, Pilch DS, Liu AA, Liu LF, Rice JE, LaVoie EJ. Bioorg. Med. Chem. Lett. 2010;20:3150. doi: 10.1016/j.bmcl.2010.03.086. [DOI] [PubMed] [Google Scholar]
- 23.Rzuczek SG, Pilch DS, Liu A, Liu L, LaVoie EJ, Rice JE. J. Med. Chem. 2010;53:3632. doi: 10.1021/jm1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gervasoni JE, Jr., Fields SZ, Krishna S, Baker MA, Rosado M, Thuraisamy K, Hindenburg AA, Taub RN. Cancer Res. 1991;51:4955. [PubMed] [Google Scholar]
- 25.Li T-K, Houghton PJ, Desai SD, Daroui P, Liu AA, Hars ES, Ruchelman AL, LaVoie EJ, Liu LF. Cancer Res. 2003;63:8400. [PubMed] [Google Scholar]