Summary
Autoantibodies to the thyrotrophin (TSH) receptor (anti-TSHR) are unique, in that they are involved directly in the pathophysiology of certain autoimmune thyroid diseases (AITD). Thyroid-stimulating antibodies (TSAb) act as agonists that activate the thyroid gland and cause Graves' disease. Other anti-TSHR antibodies block TSH and can cause hypothyroidism. Thyroid-blocking antibodies (TBAb) have not been studied as extensively as TSAb. We developed a TBAb bioassay based on a cell line that expresses a chimeric TSHR. The 50% inhibitory concentration of the chimeric Chinese hamster ovary (CHO)-Luc cells was more than five-fold lower compared with the wild-type CHO-Luc cells. We tested the performance of this bioassay using a thyroid-blocking monoclonal antibody K1-70, established an assay cut-off and detected TBAb in 15 of 50 (30%) patients with AITD. Interestingly, the assay detects both TSAb and TBAb and measures the net activity of a mixture of both types of antibodies. There was a high correlation (R2 0·9, P < 0·0001) between the results of the TSAb assay and the negative percentage inhibition of the TBAb assay. The TBAb bioassay was approximately 20-fold more sensitive than a commercially available TSHR binding assay (TRAb). In contrast to TRAb, sera with high levels of TBAb activity were able to be diluted several hundred-fold and still exhibit blocking activity above the cut-off level. Thus, this TBAb bioassay provides a useful tool for measuring the activity of anti-TSHR antibodies and may help clinicians to characterize the diverse clinical presentations of patients with AITD.
Keywords: autoimmune thyroid disease, bioassay, thyroid-blocking antibody, thyrotrophin receptor autoantibody
Introduction
Autoimmune thyroid disease (AITD) is associated with the presence of autoantibodies to a number of thyroid antigens, such as thyroid peroxidase (TPO), thyroglobulin (TG) and thyrotrophin or thyroid-stimulating hormone (TSH) receptor (TSHR). Anti-TPO and anti-TG antibodies, although detected frequently in patients with AITD, are not thought to be involved directly in any specific pathology or disease entity. Autoantibodies to the TSHR are, however, unique in that they are involved directly in the pathophysiology of certain types of AITD. Some anti-TSHR autoantibodies act as agonists which mimic the action of TSH and stimulate thyroid growth and thyroid hormone production, albeit in an unregulated manner. These thyroid-stimulating antibodies (TSAb), also called thyroid-stimulating immunoglobulins (TSI), are the causative agent of autoimmune hyperthyroidism, known as Graves' disease (GD) [1]. Recently, the detection of TSAb with a Food and Drug Administration (FDA)-cleared bioassay has been shown to have clinical utility for the diagnosis of GD and assessment of the severity of extrathyroidal manifestations of GD [2–7]. Other types of anti-TSHR can antagonize or block the action of TSH, and in so doing can cause hypothyroidism in certain patients with autoimmune thyroiditis such as Hashimoto's thyroiditis (HT) [8–10].
Thyroid-blocking antibodies (TBAb), also referred to as thyroid-blocking immunoglobulins (TBI), have been less well studied compared to TSAb. While a number of reports have established the importance of these autoantibodies in the pathophysiology of hypothyroidism, the methods used to detect TBAb have been highly variable [11–13]. In order to harmonize the methodology for measuring TBAb, and to enable more effective comparisons among investigations, we developed a sensitive TBAb bioassay that complements our recently developed bioassay for TSAb. The availability of both bioassays will allow routine testing for both stimulatory and blocking antibodies in patients with AITD. In this study we report on the salient features of this TBAb bioassay and present some preliminary data on its performance using patient sera.
Materials and methods
The local Ethical Committee approval was received for the studies and the informed consent of all participating subjects was obtained.
Human TSHR and luciferase reporter gene constructs
The construction of a neomycin-selectable plasmid that contains the chimeric TSHR gene under control of the SV40 early promoter and a firefly luciferase gene under control of the glycoprotein hormone promoter has been described previously [5,14]. To construct the equivalent plasmid expressing a wild-type TSHR gene a synthetic DNA fragment from the wild-type gene and spanning the chimeric insertion was used to repair the mutation by insertion with convenient restriction enzymes. The nucleotide sequences of both the chimeric and wild-type TSHR genes were determined (Davis Sequencing Inc., Davis, CA, USA) and compared with the NCBI database.
Reporter cell lines
Chinese hamster ovary (CHO)-K1 cells (American Type Culture Collection number: CCL-61; Manassas, VA, USA) were transfected with linearized pchTSHR-GPH/Luc or pwtTSHR-GPH-Luc plasmid using HyFect (Denville Scientific, Metuchen, NJ, USA), according to the manufacturer's instructions. Twenty-four hours after transfection the cells were placed under antibiotic selection with 1·0 mg/ml geneticin (Sigma, St Louis, MO, USA). Clones were screened for luciferase activity in cell lysates 3 h following stimulation with either control serum or serum from GD patients known to contain TSAb. Following two rounds of cloning by limiting dilution, clones were re-isolated and tested further for their stability upon successive passages and freeze–thaw cycles. Single clones of chimeric CHO-Luc or wild-type CHO-Luc cells that had the highest average signal-to-background ratio were chosen for the development of the bioassay. All subsequent experimental values were obtained with the final selected clone.
Thyroid-blocking antibody bioassay
Chimeric CHO-Luc or wild-type CHO-Luc cells were induced with bovine TSH (bTSH) (Sigma Aldrich) mixed with blocking monoclonal antibody (mAb) K1-70, stimulating mAb, M22 (RSR, Cardiff, UK) or human sera. The cells were plated in the inner 48 wells of a 96-well plate with black-wall, clear-bottomed wells. Twelve serum samples and four controls, consisting of reference standard bTSH, normal serum, positive TBAb control and cells alone, were tested in triplicate. Before planting the cells, the wells were treated with cell attachment solution (CAS; Diagnostic Hybrids Inc., Athens, OH, USA). The frozen cells were thawed and seeded immediately at 6·7 × 104 cells/well and then incubated in growth medium (GM; Diagnostic Hybrids Inc.) at 37°C with 5% CO2 for 18 h before addition of the samples. After 3 h, the luciferase expression levels of cell lysates were measured directly in the wells following the addition of substrate and lysis reagent (Promega, Madison, WI, USA) using a multi-well plate luminometer (Veritas Microplate Luminometer; Turner BioSystems, Sunnyvale, CA, USA or Tecan Infinite M200; Tecan GmbH, Crailsheim, Germany). Blocking activity was defined as percentage inhibition of luciferase expression relative to induction with bTSH alone.
TSAb bioassay
The TSAb bioassay (Thyretain®; Diagnostic Hybrids Inc.) was performed according to the manufacturer's recommendations. Results are expressed as specimen-to-reference ratio as percentage (SRR%).
TSH receptor binding assays (TRAb)
TRAb kits were purchased from Kronus (Star, Idaho, USA) or Roche Diagnostics (Mannheim, Germany) and all TRAb assays were performed according to the manufacturer's instructions.
Results
Dose–response of TSH stimulation of luciferase activity
The determination of TBAb is based on their ability to block TSH-stimulation of the thyroid. For these studies we used bTSH. In order to determine the optimal dose of bTSH, we stimulated chimeric CHO-Luc and wild-type CHO-Luc cells with up to 800 mIU/l of bTSH. The bTSH was mixed with normal sera and added to the cells in reaction buffer (RB). After 3 h incubation at 37°C the cells were lysed and luciferase was measured in relative light units (RLU). Figure 1 shows signal-to-background versus concentration of bTSH for both cell lines. The wild-type CHO-Luc cells were much more sensitive to lower concentrations of bTSH, but they displayed a much narrower linear range. Similar results were obtained with recombinant human TSH (data not shown). We used these results to guide our choice of the optimal concentration of bTSH to use in the TBAb bioassay and used concentrations that were at the high end of the linear range.
Figure 1.
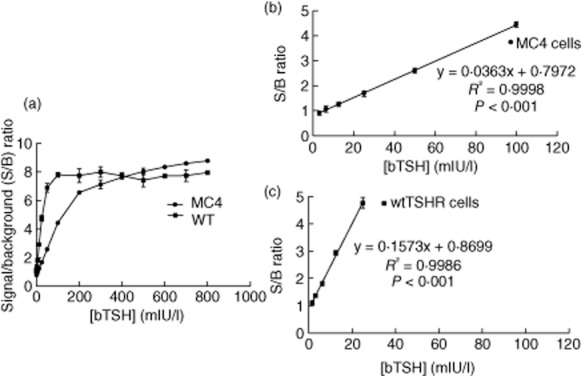
Dose–response of bovine thyrotrophin (bTSH). (a) The signal-to-background (S/B) ratio of luciferase activity of bTSH-treated chimeric Chinese hamster ovary (CHO)-Luc (circles) versus wild-type CHO-Luc cells (squares) is plotted versus bTSH concentration in milli-international units per litre (mIU/l). (b,c) The regions of the graph in (a) that are within the linear range are plotted for the chimeric CHO-Luc cells (b) and wild-type CHO-Luc cells (c).
Thyroid-blocking bioassay using chimeric CHO-Luc and wild-type CHO-Luc cells
We tested the thyroid-blocking activity of K1-70, a human mAb with known thyroid-blocking ability [15]. Figure 2 shows a dose–response of K1-70 using both cell lines. The cells were stimulated with 100 mIU/l bTSH and 25 mIU/l for the chimeric and wild-type CHO-Luc cells, respectively. The chimeric CHO-Luc cells were much more sensitive at detecting blocking activity of the K1-70, in that the 50% inhibitory concentration (IC50) was more than five-fold lower compared with the wild-type CHO-Luc cells. Similar results were obtained with concentrations of bTSH, ranging from 20–120 mIU/l for the chimeric CHO-Luc cells to 10–25 mIU/l for the wild-type CHO-Luc cells (data not shown). Based on these results, the chimeric CHO-Luc cells were chosen for further developmental studies on the TBAb bioassay.
Figure 2.
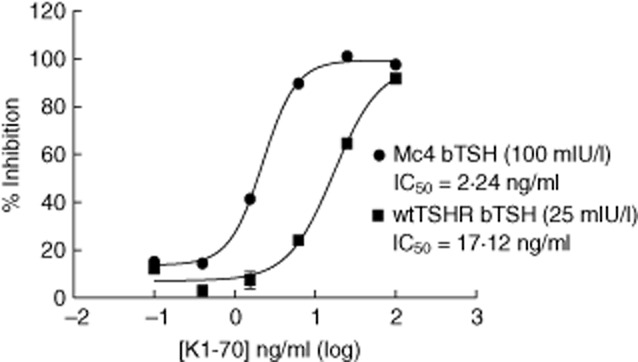
Blocking activity of K1-70 using chimeric Chinese hamster ovary (CHO)-Luc and wild-type CHO-Luc cells. A dose–response assay of the thyroid-blocking monoclonal antibody (mAb) K1-70 was performed in the thyroid-blocking antibody (TBAb) bioassay using chimeric CHO-Luc cells or wild-type CHO-Luc cells. The percentage (%) inhibition is plotted against the concentration of K1-70 in nanograms per millilitre (ng/ml). Each point represents the mean of six replicates ± standard deviation. The amount of bovine thyrotrophin (bTSH) used for each cell line was determined based on the linear range of the response to bTSH shown in Fig. 1b and c.
Detection of thyroid-blocking activity after stimulation with a stimulating mAb
We next compared the blocking activity of K1-70 following stimulation with a stimulating mAb, M22 [15]. The concentration of M22 used (0·20 ng/ml) was based on a previously run dose–response curve that defined the linear range of induction by M22 (data not shown). When the cells were stimulated with M22, the IC50 of K1-70 was within two-fold of the IC50 with bTSH-stimulated cells (data not shown). This result shows that the TBAb bioassay is not specific for bTSH.
Comparison of the binding-inhibitory activity and the blocking activity of K1-70
We compared the blocking activity of K1-70 in our bioassay with its ability to inhibit binding of M22 to the TSHR in a TRAb immunoassay (Kronus). In this TRAb assay K1-70 was unable to achieve 100% inhibition at concentrations up to 100 ng/ml, so an accurate IC50 was not obtainable. However, it was estimated that the TBAb bioassay was approximately 20-fold more sensitive than this TRAb assay (Fig. 3).
Figure 3.
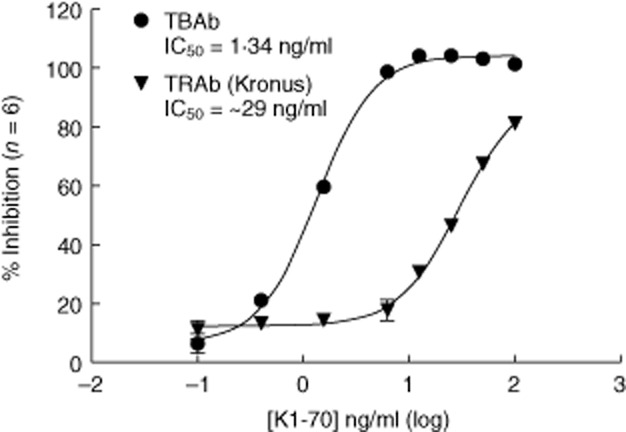
Comparison of the thyroid-blocking antibody (TBAb) bioassay and the TRAb assay using K1-70. Increasing concentrations of K1-70 were utilized in the TBAb bioassay (circles) and the TRAb assay (triangles). Results are plotted as percentage inhibition for both assays against the concentration of K1-70 in nanograms per millilitre (ng/ml). The 50% inhibitory concentration (IC)50 of the TBAb assay with Mc4 cells was 1·34 ng/ml, which was 22 times lower than the IC50 obtained with the TRAb assay.
Reproducibility
For the TBAb assay, reproducibility data were generated from 10 clinical sera with high (three sera with a mean of 96% inhibition), medium (four sera with a mean of 63% inhibition) and low (three sera with a mean of 46% inhibition) levels of blocking antibodies. These sera were tested multiple times within and between two lots and showed average coefficients of variation (CV) of 4·2% (range 4·1–4·5%), 9·2% (range 6·0–13·0%) and 25·4% (range 21·9–30·2%), respectively. The overall mean CV was 10·3%. The analytical performance of the TBAb assay was demonstrated by the low CV at high levels of serum TBAb.
Determination of dilution titres of thyroid-blocking activity in human sera
We performed serial two-fold dilutions of sera with high levels of thyroid-blocking activity.
Several serum samples with thyroid-blocking antibody were tested after two-fold serial dilution. We focused on diluting sera with 100% inhibitory activity in order to discriminate the activity for these sera, as 100% is the assay ceiling. Data from two diluted sera are shown in Fig. 4. As can be seen, the inhibition was below the cut-off of 40% at between 352-and 704-fold. These data show proof-of-principle that this type of analysis would allow a serum TBAb titre to be determined to differentiate the TBAb activity of sera with very high blocking activity (100% inhibition).
Figure 4.
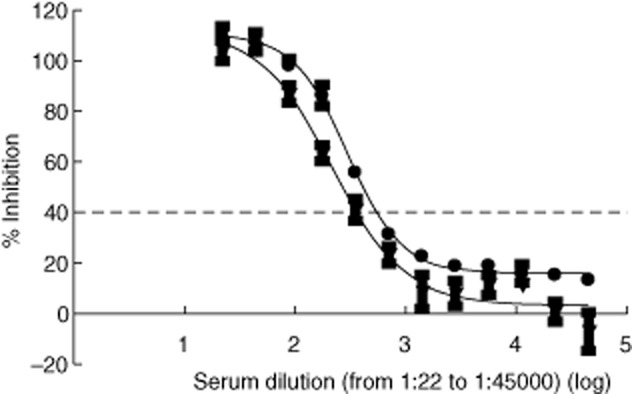
Dilution analysis of two sera with high levels of thyroid-blocking antibody (TBAb) activity. Sera were serially diluted two-fold into normal human sera. Each dilution was tested in the TBAb assay as described in the Methods section. The final dilutions shown ranged from 1:22 to 1:45 056. The dashed line indicates the preliminary cut-off of the TBAb assay (see text).
Measurement of thyroid-blocking activity in human sera
We measured the thyroid-blocking activity of sera from euthyroid controls (n = 301). We used these data to establish a cut-off for the assay of 40% based on the mean percentage inhibition of these controls (−4%) plus 2 standard deviations (s.d.) (21%) from this mean. We next performed a small study in which we tested sera from 50 well-characterized, healthy euthyroid controls and 50 patients with either GD or HT. The diagnoses were based on a combination of clinical criteria (typical symptoms and signs of either hypo-or hyperthyroidism), imaging studies (typical ultrasound echo pattern with markedly enhanced vascularization of the thyroid gland) and the presence of anti-TPO and/or anti-TSHR autoantibodies. Figure 5 shows the results of this study. The control sera ranged from −16% to +37% with a mean of 6% ± 11%. Sera from patients with AITD had results that ranged from −157% to +108%. Seven sera that were positive in the TSAb bioassay had negative percentage inhibition of −43% to −157% in the TBAb bioassay. Fifteen of 50 (30%) patients (seven hypothyroid, two with serum baseline TSH above 100 mU/l, 13 with HT and two with GD) had positive inhibition > 40%, 10 of whom were > 80%. Of these 15 TBAb-positive patients, 10 were positive for TRAb (Elecsys anti-TSHR, ECLIA, Cobas E411; Roche Diagnostics).
Figure 5.
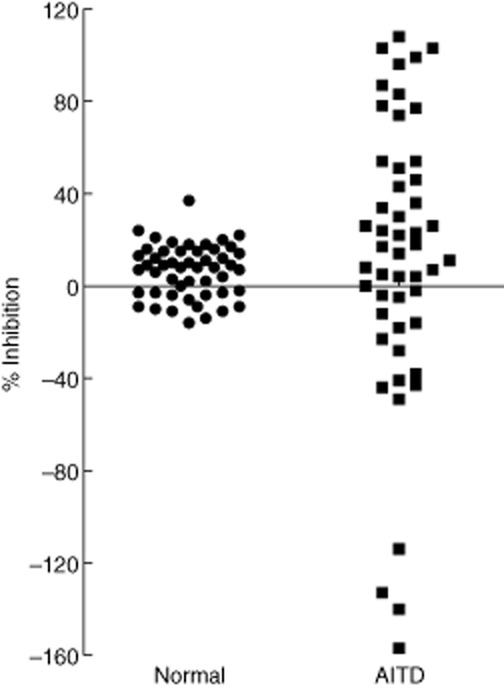
Thyroid-blocking activity of sera from normal controls and patients with AITD. Scatterplot of 50 control sera from well-characterized, healthy euthyroid individuals with neither thyroid nor autoimmune disease (circles) and 50 patients with autoimmune thyroid disease (AITD, squares) were tested in the thyroid-blocking antibody (TBAb) bioassay.
Detection of stimulatory activity in the TBAb bioassay
Sera from GD patients exhibited negative inhibition (i.e. luciferase levels above the bTSH stimulation) in the TBAb bioassay (Fig. 5). Based on the mean minus 2 s.d. the cut-off for this stimulatory activity in this assay would be negative (46%). Five sera had exhibited values of negative (46% or greater) and all these sera were TSAb-positive in the TSAb bioassay (data not shown). To assess this phenomenon further we performed a dose–response assay of the thyroid-stimulating mAb M22 on the TSAb and the TBAb bioassays. M22 had equivalent 50% effective concentration (EC50) results in each assay (data not shown). We next compared the stimulatory activity in 171 sera from patients with GD as measured in both assays. As shown in Fig. 6 there was a high correlation between the results in these assays, with a correlation coefficient of almost 0·9 between the SRR% results of the TSAb assay and the negative percentage inhibition of the TBAb assay. It is worth noting, however, that low levels of TSAb were not detectable in the TBAb bioassay.
Figure 6.
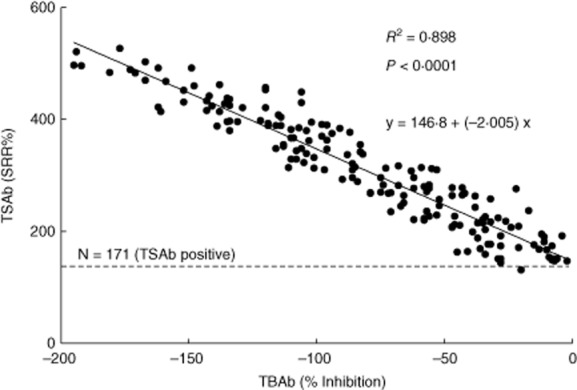
Correlation of the activity of thyroid-stimulating antibody (TSAb)-positive sera measured in the TSAb bioassay and the TBAb bioassay. One-hundred and seventy-one sera from Graves' disease patients were tested in the thyroid-stimulating (TSAb) bioassay and in the thyroid-blocking (TBAb) bioassay. Results are plotted as specimen-to-reference ratio percentage (SRR%) for the TSAb bioassay and percentage (%) inhibition for the TBAb bioassay. The linear fit equation is y = 146·8 + (–2·005) x. The slope = −2·005. The interception is = 146·8 (SRR%).
Mixtures of TSAb and TBAb
There is strong evidence that the serum of certain patients can contain both blocking and stimulating antibodies [15]. We simulated this scenario by mixing K1-70 and M22 mAbs in different ratios. Thyroid-blocking mAb K1-70 (5 ng/ml) and thyroid-stimulating mAb M22 (0·8 ng/ml) were mixed in 10% increments from 0 to 100%. Each mixture was then tested in the TSAb (Fig. 7a) and the TBAb bioassays (Fig. 7b). As shown in Fig. 7, unlike the TSAb assay, which only detects stimulating activity, the TBAb bioassay can detect both the blocking and stimulating activity of the mixtures. In addition, neither blocking nor stimulating activity was detectable at M22 : K1-70 ratios of between 40:60 and 60:40. This suggests that the net activity can be negative when patients contain relatively equal activities of both types of autoantibodies.
Figure 7.
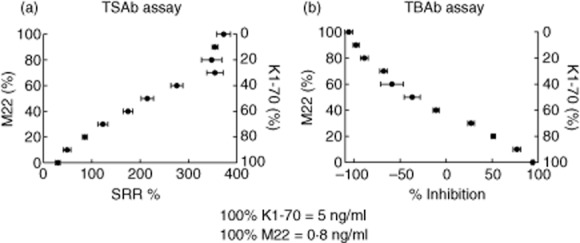
Activity of mixtures of M22 and K1-70 in the TSAb and the TBAb bioassays. A concentration of K1-70 (5 ng/ml) shown previously to give maximal blocking activity in the TBAb bioassay and a concentration of M22 (0·8 ng/ml) shown previously to give maximal stimulatory activity in the TSAb bioassay were mixed in 10% incremental proportions from 100:0% to 0:100%. An aliquot of each of these 11 mixtures was then tested in the TSAb bioassay (a) and the TBAb bioassay (b). Results are plotted as specimen-to-reference ratio percentage (SRR%) for the TSAb bioassay and percentage (%) inhibition for the TBAb bioassay. Each point represents the mean of six replicates ± standard deviation.
Discussion
In this report we describe the development of a new bioassay for measuring autoantibodies with thyroid-blocking activity. We compared a cell line that expresses a wild-type TSHR with a cell line that expresses a previously described chimeric TSHR. We performed a dose–response assay of bTSH in both cell lines and determined the linear range of luciferase induction. We found that the chimeric CHO-Luc cells displayed two features that were advantageous for a thyroid-blocking bioassay. First, the chimeric CHO-Luc cells had a wide linear range, and secondly, they were less responsive to bTSH than the wild-type TSHR-expressing cells. This latter feature is important, because it can blunt the effect of high TSH levels in the sera of hypothyroid patients that might interfere with the assay.
We used well-characterized mAbs to characterize the performance of our assay. Such analytical studies are difficult with poorly characterized polyclonal sera. Using a pure blocking human mAb, K1-70, we showed that the chimeric CHO-Luc cells performed better than wild-type cells as assessed by the IC50, which was five-fold lower when measured with the chimeric CHO-Luc cells. This result was somewhat unexpected, as previous work has suggested that the chimeric TSHR was unresponsive to blocking antibody [16]. Recent evidence, however, that blocking and stimulating mAb binds to very similar regions of the TSHR that are intact in the chimeric receptor, are consistent with the findings in this study [17]. We also tested our TBAb bioassay using the chimeric CHO-Luc cells on healthy controls and a small set of sera from patients with AITD. The detection of blocking activity in 15 of 50 of these candidate AITD sera demonstrated the potential clinical utility of the bioassay. The large collective of more than 300 euthyroid controls used to establish an assay cut-off was chosen randomly. In the subsequent step, performing the comparative study evaluating the TBAb assay in both 50 patients with autoimmune thyroid diseases and suspected serum TBAb as well as in 50 well-characterized, euthyroid healthy individuals, the latter were selected carefully according to a negative history for autoimmune and/or chronic diseases in the family, negative history of smoking or regular medication intake, completely normal sera for thyroid-related hormones and autoantibodies as well as a completely normal ultrasound evaluation of the thyroid gland. This extremely carefully performed selection of our healthy population might explain the differences in blocking activity.
While the role of TSAb in the aetiology of autoimmune hyperthyroidism (GD) is well established, the correlation between TBAb and autoimmune hypothyroidism has not been correlated as highly. Many reports have studied the occurrence of TBAb in various autoimmune diseases associated with hypothyroidism such as Hashimoto's, atrophic thyroiditis, etc. [8,10,11,18–23]. Other investigations have focused on the role of TBAb in congenital and neonatal hypothyroidism associated with mothers with autoimmune thyroid disease [19,24–32]. The importance of TBAb in congenital hypothyroidism, however, has been questioned [28]. Our goal was to develop a TBAb bioassay that would be useful diagnostically, but would also provide investigators with a reliable, reproducible and accurate assay to help answer important questions about the pathophysiology of autoimmune hypothyroidism.
Ever since anti-TSHR antibodies have been known to exhibit different functional activities it has been suspected that certain patients might contain both TSAb and TBAb, and that this might explain certain clinical presentations [33,34]. Recent evidence has proved that a patient can have both TSAb and TBAb by isolating monoclonal antibodies with stimulatory and blocking activity from the lymphocytes of a single patient [15]. In addition, there has been speculation that patients with GD may shift between stimulating and blocking antibodies as they transition from hyperthyroid to hypothyroid [35–37]. This is the basis for our goal to develop bioassays that can measure both activities. Therefore, the purpose of these experiments was to demonstrate that these assays detect net TSAb or TBAb activity. The way in which we performed the mixing (using different ratios of an EC100% of these monoclonal antibodies) allowed us to demonstrate this phenomenon. These results demonstrate clearly that sera may have both TSAb and TBAb, and that the results can appear as TSAb-positive, TBAb-positive or even negative, depending on how much of each antibody the sera contains.
Presumably, when patients have both stimulatory and blocking antibodies, bioassays measure the net activity present in the patient's serum. Our previously described TSAb assay detects only stimulatory activity and therefore, in a patient with both activities, only net stimulatory activity will be detected. Our TBAb assay can detect both activities, so either net stimulatory or net blocking activity can be detected. However, the assay cannot distinguish between pure stimulatory or pure blocking activity from mixed activity with net stimulatory or net blocking activity, respectively. In one report, mutant TSHR were used to develop assays that measure only stimulatory or only blocking activity [38]. Whether this can be reproduced and used routinely remains to be determined.
In summary, we have developed and characterized a reliable bioassay for autoantibodies that block thyroid activity. The combination of our TSAb and TBAb bioassays should provide excellent tools to assist clinicians in evaluating patients with the wide spectrum of different presentations of AITD.
Acknowledgments
We are grateful to M. Kanitz, laboratory technician, and E, Kolbe, study nurse (Molecular Thyroid Research Laboratory, Gutenberg University Medical Center, Mainz, Germany), as well as to L. Grippa, J. Houtz and A. Larrimer (DHI) for helpful suggestions and excellent technical assistance. Funding for this work was provided by Diagnostic Hybrids, Inc. (DHI, a Quidel Company).
Disclosure
G.J.K. consults for DHI. Y.L., J.K., R.K. and P.D.O. are DHI employees. T.D. has nothing to disclose.
References
- 1.Brent GA. Clinical practice. Graves' disease. N Engl J Med. 2008;358:2594–2605. doi: 10.1056/NEJMcp0801880. [DOI] [PubMed] [Google Scholar]
- 2.Kamijo K, Murayama H, Uzu T, Togashi K, Kahaly GJ. A novel bioreporter assay for thyrotropin receptor antibodies using a chimeric thyrotropin receptor (mc4) is more useful in differentiation of Graves' disease from painless thyroiditis than conventional thyrotropin-stimulating antibody assay using porcine thyroid cells. Thyroid. 2010;20:851–856. doi: 10.1089/thy.2010.0059. [DOI] [PubMed] [Google Scholar]
- 3.Kamijo K, Murayama H, Uzu T, Togashi K, Olivo PD, Kahaly GJ. Similar clinical performance of a novel chimeric thyroid-stimulating hormone receptor bioassay and an automated thyroid-stimulating hormone receptor binding assay in Graves' disease. Thyroid. 2011;21:1295–1299. doi: 10.1089/thy.2011.0056. [DOI] [PubMed] [Google Scholar]
- 4.Lytton SD, Kahaly GJ. Bioassays for TSH-receptor autoantibodies: an update. Autoimmun Rev. 2010;10:116–122. doi: 10.1016/j.autrev.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Lytton SD, Li Y, Olivo PD, Kohn LD, Kahaly GJ. Novel chimeric thyroid-stimulating hormone-receptor bioassay for thyroid-stimulating immunoglobulins. Clin Exp Immunol. 2010;162:438–446. doi: 10.1111/j.1365-2249.2010.04266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponto KA, Kanitz M, Olivo PD, Pitz S, Pfeiffer N, Kahaly GJ. Clinical relevance of thyroid-stimulating immunoglobulins in Graves' ophthalmopathy. Ophthalmology. 2011;118:2279–2285. doi: 10.1016/j.ophtha.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Leschik JJ, Diana T, Olivo PD, et al. Analytical performance and clinical utility of a bioassay for thyroid-stimulating immunoglobulins. Am J Clin Pathol. 2013;139:192–200. doi: 10.1309/AJCPZUT7CNUEU7OP. [DOI] [PubMed] [Google Scholar]
- 8.Endo K, Kasagi K, Konishi J, et al. Detection and properties of TSH-binding inhibitor immunoglobulins in patients with Graves' disease and Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1978;46:734–739. doi: 10.1210/jcem-46-5-734. [DOI] [PubMed] [Google Scholar]
- 9.Drexhage HA, Bottazzo GF, Bitensky L, Chayen J, Doniach D. Thyroid growth-blocking antibodies in primary myxoedema. Nature. 1981;289:594–596. doi: 10.1038/289594a0. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson FA, Dahlberg PA, Ritzen EM. Thyroid blocking antibodies in thyroiditis. Acta Med Scand. 1984;215:461–466. doi: 10.1111/j.0954-6820.1984.tb17679.x. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka H, Yamauchi K, Takagi S, et al. Pathophysiological role of thyroid blocking antibody in patients with primary hypothyroidism. Endocrinol Japon. 1987;34:689–699. doi: 10.1507/endocrj1954.34.689. [DOI] [PubMed] [Google Scholar]
- 12.Chiovato L, Vitti P, Santini F, et al. Incidence of antibodies blocking thyrotropin effect in vitro in patients with euthyroid or hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab. 1990;71:40–45. doi: 10.1210/jcem-71-1-40. [DOI] [PubMed] [Google Scholar]
- 13.Vitti P, Chiovato L, Fiore E, Mammoli C, Rocchi R, Pinchera A. Use of cells expressing the human thyrotropin (TSH) receptor for the measurement of thyroid stimulating and TSH-blocking antibodies. Acta Med Austriaca. 1996;23:52–56. [PubMed] [Google Scholar]
- 14.Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves' orbitopathy. J Clin Endocrinol Metab. 2010;95:2123–2131. doi: 10.1210/jc.2009-2470. [DOI] [PubMed] [Google Scholar]
- 15.Evans M, Sanders J, Tagami T, et al. Monoclonal autoantibodies to the TSH receptor, one with stimulating activity and one with blocking activity, obtained from the same blood sample. Clin Endocrinol (Oxf) 2010;73:404–412. doi: 10.1111/j.1365-2265.2010.03831.x. [DOI] [PubMed] [Google Scholar]
- 16.Tahara K, Ishikawa N, Yamamoto K, et al. Epitopes for thyroid stimulating and blocking autoantibodies on the extracellular domain of the human thyrotropin receptor. Thyroid. 1997;7:867–877. doi: 10.1089/thy.1997.7.867. [DOI] [PubMed] [Google Scholar]
- 17.Sanders P, Young S, Sanders J, et al. Crystal structure of the TSH receptor (TSHR) bound to a blocking-type TSHR autoantibody. J Mol Endocrinol. 2011;46:81–99. doi: 10.1530/JME-10-0127. [DOI] [PubMed] [Google Scholar]
- 18.Takasu N, Naka M, Mori T, Yamada T. Two types of thyroid function-blocking antibodies in autoimmune atrophic thyroiditis and transient neonatal hypothyroidism due to maternal IgG. Clin Endocrinol (Oxf) 1984;21:345–355. doi: 10.1111/j.1365-2265.1984.tb03221.x. [DOI] [PubMed] [Google Scholar]
- 19.Arikawa K, Ichikawa Y, Yoshida T, et al. Blocking type antithyrotropin receptor antibody in patients with nongoitrous hypothyroidism: its incidence and characteristics of action. J Clin Endocrinol Metab. 1985;60:953–959. doi: 10.1210/jcem-60-5-953. [DOI] [PubMed] [Google Scholar]
- 20.Kraiem Z, Lahat N, Glaser B, Baron E, Sadeh O, Sheinfeld M. Thyrotrophin receptor blocking antibodies: incidence, characterization and in-vitro synthesis. Clin Endocrinol (Oxf) 1987;27:409–421. doi: 10.1111/j.1365-2265.1987.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 21.Feingold SB, Smith J, Houtz J, Popovsky E, Brown RS. Prevalence and functional significance of thyrotropin receptor blocking antibodies in children and adolescents with chronic lymphocytic thyroiditis. J Clin Endocrinol Metab. 2009;94:4742–4748. doi: 10.1210/jc.2009-1243. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama K, Okuda J, Saijo M, et al. Recombinant monoclonal thyrotropin-stimulation blocking antibody (TSBAb) established from peripheral lymphocytes of a hypothyroid patient with primary myxedema. J Endocrinol Invest. 2003;26:1076–1080. doi: 10.1007/BF03345253. [DOI] [PubMed] [Google Scholar]
- 23.Tamai H, Nozaki T, Mukuta T, et al. The incidence of thyroid stimulating blocking antibodies during the hypothyroid phase in patients with subacute thyroiditis. J Clin Endocrinol Metab. 1991;73:245–250. doi: 10.1210/jcem-73-2-245. [DOI] [PubMed] [Google Scholar]
- 24.Iseki M, Shimizu M, Oikawa T, et al. Sequential serum measurements of thyrotropin binding inhibitor immunoglobulin G in transient familial neonatal hypothyroidism. J Clin Endocrinol Metab. 1983;57:384–387. doi: 10.1210/jcem-57-2-384. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura N, Konishi J. Transient hypothyroidism in infants born to mothers with chronic thyroiditis – a nationwide study of twenty-three cases. The Transient Hypothyroidism Study Group. Endocrinol Japon. 1990;37:369–379. doi: 10.1507/endocrj1954.37.369. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura N, Yamada Y, Nohara Y, et al. Familial neonatal transient hypothyroidism due to maternal TSH-binding inhibitor immunoglobulins. N Engl J Med. 1980;303:738–741. doi: 10.1056/NEJM198009253031306. [DOI] [PubMed] [Google Scholar]
- 27.Schwingshandl J, Donaghue K, Luttrell B, Cowell C, Ward P, Silink M. Transient congenital hypothyroidism due to maternal thyrotrophin binding inhibiting immunoglobulin. J Paediatr Child Health. 1993;29:315–318. doi: 10.1111/j.1440-1754.1993.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 28.Kung AW, Low LC. Thyrotrophin-blocking antibodies in congenital hypothyroidism. J Paediatr Child Health. 1992;28:50–53. doi: 10.1111/j.1440-1754.1992.tb02617.x. [DOI] [PubMed] [Google Scholar]
- 29.Ishihara T, Waseda N, Ikekubo K, Kuroda K, Akamizu T, Mori T. A predicted case with neonatal transient hypothyroidism due to blocking type thyrotropin binding inhibitor immunoglobulins (TBII) Endocrinol Japon. 1985;32:189–194. doi: 10.1507/endocrj1954.32.189. [DOI] [PubMed] [Google Scholar]
- 30.Evans C, Gregory JW, Barton J, et al. Transient congenital hypothyroidism due to thyroid-stimulating hormone receptor blocking antibodies: a case series. Ann Clin Biochem. 2011;48:386–390. doi: 10.1258/acb.2011.011007. [DOI] [PubMed] [Google Scholar]
- 31.Ginsberg J, Walfish PG, Rafter DJ, Westarp C, Ehrlich RM. Thyrotrophin blocking antibodies in the sera of mothers with congenitally hypothyroid infants. Clin Endocrinol (Oxf) 1986;25:189–194. doi: 10.1111/j.1365-2265.1986.tb01681.x. [DOI] [PubMed] [Google Scholar]
- 32.Notsu K, Ito Y, Hasegawa A, et al. Clinical courses and thyroid conditions in three infants born to a mother with thyroid stimulating-blocking antibodies. Endocr J. 1997;44:233–237. doi: 10.1507/endocrj.44.233. [DOI] [PubMed] [Google Scholar]
- 33.Miyauchi A, Amino N, Tamaki H, Kuma K. Coexistence of thyroid-stimulating and thyroid-blocking antibodies in a patient with Graves' disease who had transient hypothyroidism. Am J Med. 1988;85:418–420. doi: 10.1016/0002-9343(88)90598-0. [DOI] [PubMed] [Google Scholar]
- 34.Lenzner C, Morgenthaler NG. The effect of thyrotropin-receptor blocking antibodies on stimulating autoantibodies from patients with Graves' disease. Thyroid. 2003;13:1153–1161. doi: 10.1089/10507250360731569. [DOI] [PubMed] [Google Scholar]
- 35.Inoue A, Koizumi S, Matsuda A, et al. Graves' hyperthyroidism showing transient hypothyroidism during interferon therapy for chronic hepatitis type C. Endocr J. 2005;52:293–298. doi: 10.1507/endocrj.52.293. [DOI] [PubMed] [Google Scholar]
- 36.Cherif L, Ben Abdallah N, Khairi K, Hadj Ali I, Turki S, Ben Maiz H. [Particular evolution of the thyroid state in Grave's disease: two cases] Tunis Med. 2003;81:747–750. [PubMed] [Google Scholar]
- 37.Yoshida K, Aizawa Y, Kaise N, et al. Role of thyroid-stimulating blocking antibody in patients who developed hypothyroidism within one year after 131I treatment for Graves' disease. Clin Endocrinol (Oxf) 1998;48:17–22. doi: 10.1046/j.1365-2265.1998.00330.x. [DOI] [PubMed] [Google Scholar]
- 38.Kosugi S, Ban T, Akamizu T, Valente W, Kohn LD. Use of thyrotropin receptor (TSHR) mutants to detect stimulating TSHR antibodies in hypothyroid patients with idiopathic myxedema, who have blocking TSHR antibodies. J Clin Endocrinol Metab. 1993;77:19–24. doi: 10.1210/jcem.77.1.8100829. [DOI] [PubMed] [Google Scholar]


