Abstract
Damage of target cells by cytotoxicity, either mediated by specific lymphocytes or via antibody-dependent reactions, may play a decisive role in causing the central nervous system (CNS) lesions seen in multiple sclerosis (MS). Relevant epitopes, antibodies towards these epitopes and a reliable assay are all mandatory parts in detection and evaluation of the pertinence of such cytotoxicity reactions. We have adapted a flow cytometry assay detecting CD107a expression on the surface of cytotoxic effector cells to be applicable for analyses of the effect on target cells from MS patients expressing increased amounts of human endogenous retrovirus antigens. MS patients also have increased antibody levels to these antigens. The target cells are spontaneously growing peripheral blood mononuclear cells (PBMCs) of B cell lineage, expressing human endogenous retrovirus HERV epitopes on their surface. Polyclonal antibodies against defined peptides in the Env-and Gag-regions of the HERVs were raised in rabbits and used in antibody-dependent cell-mediated cytotoxicity (ADCC)-assays. Rituximab® (Roche), a chimeric monoclonal antibody against CD20 expressed primarily on B cells, was used as control antibody. Without antibodies this system is suitable for analyses of natural killer cell activity. In optimization of the assay we have used effector lymphocytes from healthy donors. The most effective effector cells are CD56+ cells. CD8+ T cells also express CD107a in ADCC. Using the adapted assay, we demonstrate significant ADCC activity to target cells expressing HERV epitopes, and additionally a low level of NK activity.
Keywords: ADCC, CD107a, flow cytometry, HERV, NK cells
Introduction
The neurological disease multiple sclerosis (MS) is characterized by inflammation in different locations in the central nervous system (CNS), resulting in lesions with cell death and scar formation in both myelin sheaths and neurones. The initiating cause(s) of this process is unknown. The observed cell death could be caused by apoptosis (internal signals) or by external, possibly immune-mediated factors with cytotoxicity, caused by different effector cells and effector molecules, among the potential candidates.
We have shown previously that spontaneously growing cell cultures originating from peripheral blood mononuclear cells (PBMCs) from MS patients express human endogenous retroviruses (HERV)-H and HERV-W epitopes on their surface membranes [1]. These HERV epitopes are also expressed on the surfaces of PBMCs from MS patients with expression levels linked to different stages of the disease. These epitopes may trigger both natural killer (NK) cell activity and antibody production, the latter resulting in antibody-dependent cell-mediated cytotoxicity (ADCC). Activation of cytotoxic T cells (CD8+ and γδ T cells) may also occur, with a resulting continuum of HERV-related cytotoxic effector mechanisms that could play a role in development of the disease. The expressed epitopes could be the target, or part of the targets, for cytotoxic effectors, making testing of the different cytotoxic reactions highly relevant.
For many years, measuring of 51Cr-release from labelled target cells has been the gold standard for such assays, due particularly to the consistency and reproducibility of the results. However, some drawbacks are also built-in to this test, such as radiation, although at low levels, limited shelf life due to a short half-life and last, but not least, a tendency to high spontaneous release of the isotope from certain target cells, with the last-mentioned phenomenon making calculation of the cytotoxicity complicated, and perhaps even unreliable. Recently several methods, especially methods based on flow cytometry, have emerged, avoiding the use of radioactive isotopes. Several fluorochromes that can be integrated into the target cells have been used in a manner similar to 51Cr [2,3]. However, the spontaneous release of these fluorescent dyes can also be high, with possible labelling of other cells, thus preventing sufficient discrimination between target and effector populations [4].
In this study we present adaptions to an assay, described thoroughly by Bryceson et al. [5], by flow cytometric assessment of CD107a surface expression. This assay detects the amount of possible effector cell degranulation in response to recognition of antibodies bound to epitopes presented on the target cells, rather than measuring target cell lysis directly. Upon stimulation with appropriate target cells, the effector cells will release the assayed cytotoxic proteins by fusion of secretory lysosomes with the plasma membrane, thereby effecting target cell lysis [6]. This type of assay is used increasingly for measuring NK cell cytotoxicity [7], but it is also applicable for other types of cytotoxic effector mechanisms.
With the present optimized assay we analysed different aspects of cytotoxicity reactions and the potential consequences of HERV epitope expression on MS patient PBMCs.
Polyclonal antibodies against defined peptides, derived from specific sequences in the Env-and Gag-regions from HERV-H/F and HERV-W, were raised in rabbits. By including or excluding these antibodies in the test it is possible to assess the action of both antibody-dependent and-independent cytotoxic cell populations towards cells expressing these viral peptides/epitopes. Thus, the test contributes information about both the relevance of the constructed peptides/epitopes and also the pathogenic potential of these, when ‘seen’ by the cytotoxic cell populations. The results then lead to subsequent analysis of both the level of cytotoxic antibodies in MS patients and to the testing of possible pathogenic activation of cytotoxic cells in the patients, thereby gauging the potential of own lymphocytes in reactions against ‘self’ or ‘self with up-regulated HERV expression’.
Materials and methods
Effector cells
For the present study, PBMCs from 10 healthy donors [five females (aged 24–52 years), five males (aged 27–62 years)] were used as effector cells in NK and ADCC assays.
Venous blood was drawn and processed on the same day in our laboratory or the respective clinics. PBMCs were prepared by standard Isopaque-Ficoll centrifugation. The separated cells were aliquoted and cryopreserved in RPMI-1640 with the addition of 20% human serum (HS) and 10% dimethylsulphoxide (DMSO) at −135°C until use.
Before use, a portion of frozen PBMCs was thawed quickly (37°C), washed once in 10 ml RPMI-1640 media containing 10 mM HEPES, 0·03% w/v glutamine, 0·2 Mio IU/l penicillin–streptomycin and 10% heat-inactivated fetal calf serum (FCS), and resuspended to a concentration of 5 × 106 cells/ml. The PBMCs were placed in a humidified incubator overnight with 5% CO2 atmosphere at 37°C.
The yields and phenotypes of the 10 effector cells post-thaw were: total yields: 90–99%; CD3+ cells: 53–79%, CD3−CD56+ cells: 9–31%.
Target cells
The long-term, lymphoblastoid cell cultures (MS1533, MS1847, MS1874, MS1946), originating from the PBMCs of MS patients in different disease states, were cultured as described previously [8,9]. In brief, the cells were grown at 0·5 × 106 cells/ml of RPMI-1640 supplemented with 10% inactivated HS.
Cells were split three times a week and supplemented with fresh medium. Twenty-four h before use the cells were transferred to AIM-V serum-free medium (Gibco, Naerum, Denmark) containing 0·03% w/v glutamine, 10 mM HEPES and 0·1 Mio IU/l penicillin.
Rabbit anti-HERV antibodies
Polyclonal antibodies against Env and Gag from HERV-H/F and Env from HERV-W were raised in New Zealand white rabbits. The antibodies were raised against 16-mer peptide epitopes localized at equivalent positions in open reading frames (ORFs) of the respective endogenous retroviruses. Both the peptides and the anti-sera were prepared by Sigma Genosys (Haverhill, UK). The polyclonal anti-sera were: anti-HERV-H/F Gag [the peptide translated from the long putative gag ORF of the HERV-Fc1 sequence (aa380-395) (GenBank AL354685)] in a region with very high similarity to the gag sequences of known HERV-H copies with complete Env ORFs: HERV-H env62/H19, HERV-H env60 and HERV-H env59 [10], anti-HERV-H Env (1–3) and anti-HERV-W Env (1–3) (these peptides were derived from equivalent positions in the Env ORFs of HERV-H env62/H19 (Env H1TM: aa489–505; Env H3SU: aa 370–386 (10) and syncytin 1 (Env W1TM: aa415–431, Env W3SU: aa301–317) [11], respectively. All peptide sequences fulfil the criteria of immunogenicity, and are localized at equivalent positions in the HERV-H and HERV-W Envs, while having highly dissimilar amino acid sequences.
Preimmune sera were collected from all rabbits before immunization. Rabbits were immunized with the peptides, boosted three times, and after the final boost peripheral blood was collected for subsequent measuring of anti-peptide antibodies. The specificity and cross-reactivity of the anti-HERV anti-sera were analysed by enzyme-linked immunosorbent assay (ELISA) and time-resolved immunofluorimetic assay (TRIFMA) assays. The anti-sera were at least 1000 times more reactive towards their relevant peptide antigens than towards non-relevant peptides (data not shown). The polyclonal anti-HERV antibodies were prepared for ADCC by thawing, dilution × 10 in AIM-V medium (Gibco), supplemented as described above, heat-inactivation for 30 min at 56°C and refreezing at −20°C. Immediately before use each diluted serum sample was thawed and added to the prepared target cells.
Monoclonal antibodies
Rituximab® (Roche, Welwyn Garden City, UK), which is a chimeric monoclonal antibody against CD20 expressed primarily on B cells, was used as a positive control. Rituximab® was used in the concentration 0·1 μg/ 0·2 × 106 target cells.
Cytotoxicity reactions
After counting and centrifugation (200 g, 10 min) the target cells were adjusted to 2 × 106 cells/ml AIM-V medium. Ten μl antibodies were added to 0·2 × 106 target cells (0·1 ml) and incubated for 15 min at room temperature.
The effector cells were counted and resuspended in AIM-V to a final concentration of 2 × 106 cells/ml; 0·2 × 106 of these cells were added to the antibody-coated target cells and after centrifugation (30 g for 3 min) the cells were incubated in a humidified incubator with 5% CO2 at 37°C for 2 h. After one wash in phosphate-buffered saline (PBS) the cells were ready for staining with the monoclonal antibodies given below and subsequent flow cytometry.
Flow cytometry
Samples were labelled with monoclonal antibodies for 30 min in the dark at 4°C, washed once in PBS (pH 7·4) and finally resuspended in PBS.
The following monoclonal mouse antibodies and other markers were used: anti-CD3 fluorescein isothiocyanate (FITC) (clone UCHT1, IgG1, F0818; Dako, Glostrup, Denmark), anti-CD56 phycoerythrin (PE) [clone c5·9, immunoglobulin (Ig)G2b, R7251; Dako], anti-CD107a Alexa 647 (clone eBio H4A3, IgG1, #51-1079; eBioscience, San Diego, CA, USA), anti-CD8 PC7 (clone SFCI21Thy2D3, IgG1, #737661; Beckman Coulter, Indianapolis, IN, USA), CD2/CD2R (CD2 clone L303·1,CD2R clone L304·1; #340366; BD Pharmingen, San Jose, CA, USA), AlexaFluor 647 mouse IgG1k isotype control (clone MOPC-21, #557714; BD Pharmingen) and 7-aminoactinomycin D (7-AAD) (# 555816; BD Via Probe, BD Pharmingen).
Flow cytometric analyses were performed using a Cytomics FC500 five-colour flow cytometer (Beckman Coulter) equipped with two lasers, an argon laser (488 nm) and a HeNe laser (633 nm).
FlowJo software version 9·3 (Tree Star, Inc., Ashland, OR, USA) was used for data analysis. A total of 20 000 events were collected for further analysis. NK cells were defined as CD3−/CD56+ lymphocytes.
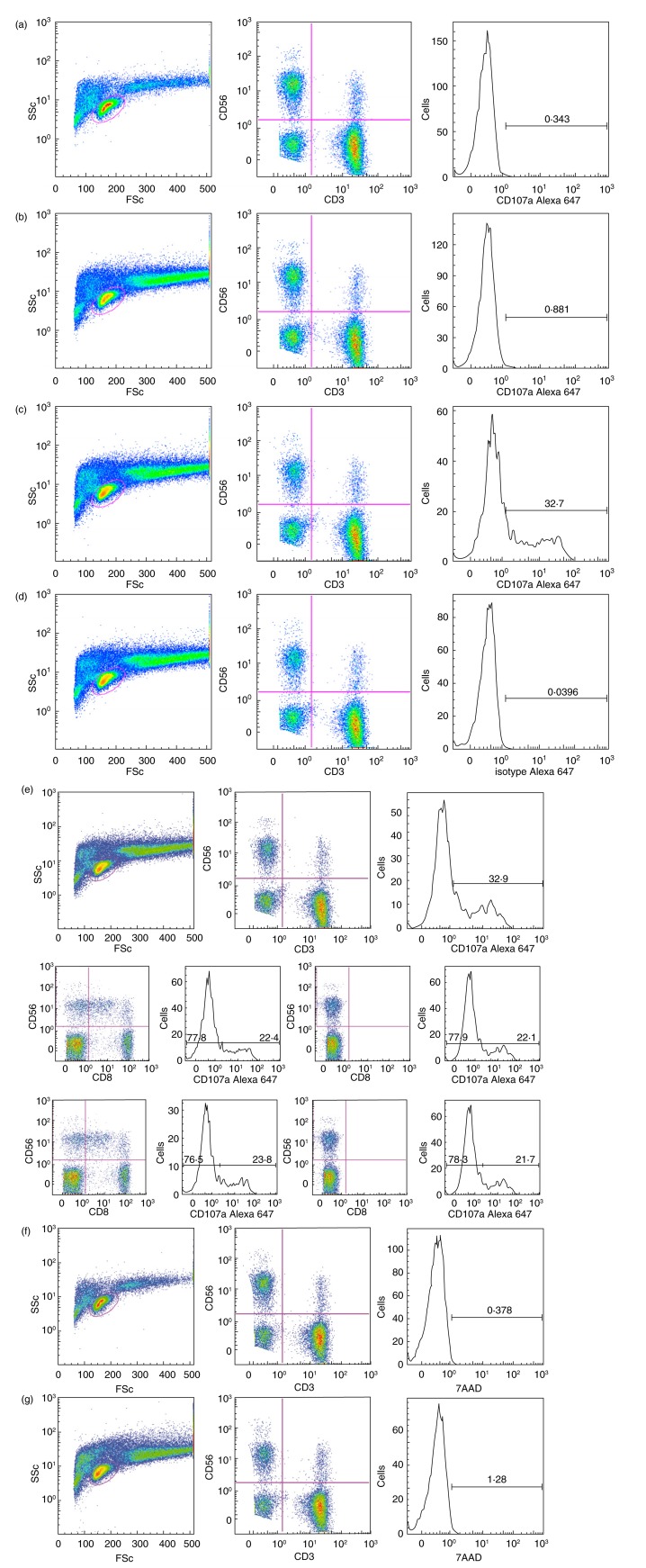
Effector cells alone were used to define the initial CD107a level of positive NK cells or CD8+ cells. In Fig. 1, we present examples of spontaneous up-regulation of CD107a on effector cells, as well as FMO (fluorescence-minus one), an isotype antibody control for CD107a and 7AAD viability staining.
Figure 1.
Flow cytometry gating strategy and controls. Fluorescence activated cell sorter (FACS) profiles illustrating gating strategy and controls – in this case with effector A and the target cell culture MS1946. The samples are: a: effector A alone: from left to right left: ungated [side scatter (SSc) versus forward scatter (FSc]; centre: lymphocyte gate (CD56 versus CD3); right: CD3−CD56+ gate (CD107a); b: effector A with target cells: left: ungated (SSc versus FSc); centre: lymphocyte gate (CD56 versus CD3); right: CD3−CD56+ gate (CD107a); c: effector A with target cells and Rituximab®: left: ungated (SSc versus FSc); centre: lymphocyte gate (CD56 versus CD3); right: CD3−CD56+ gate (CD107a); d: effector A with target cells and Rituximab® (isotype control): left: ungated (SSc versus FSc); centre: lymphocyte gate (CD56 versus CD3); right: CD3−CD56+ gate (isotype control for CD107a); e: effector A with target cells and Rituximab® (FMO control): (e), top row: left: ungated (SSc versus FSc); centre: fluorescence-minus one (FMO)CD8 lymphocyte gate (CD56 versus CD3); right: CD3−CD56+ gate (CD107a); (e), continued, mid-row: far left: lymphocyte gate (CD56 versus CD8); left: CD56+ (includes CD56+CD8+ and CD56+CD8−)(CD107a); right: FMOCD8 lymphocyte gate (CD56 versus CD8); far right: FMOCD8 CD56+(CD107a); (e), continued, bottom row: far left: lymphocyte gate (CD56 versus CD8); left: CD56+ (CD56+CD8− only) (CD107a); right: FMOCD8 lymphocyte gate (CD56 versus CD8); far right: FMOCD8 CD56+ (CD107a). Note that (e), continued, mid-row and bottom row present the same analysis with two different gating strategies. (f) Effector A alone, left: ungated (SSc versus FSc); centre: lymphocyte gate (CD56 versus CD3); right: natural killer (NK) gate with the viability marker 7-aminoactinomycin D (7AAD) (CD3−CD56+) (7AAD); (g) effector A with target cells and Rituximab, left: ungated (SSc versus FSc); centre: lymphocyte gate (CD56 versus CD3); right: NK gate with the viability marker 7AAD (CD3−CD56+)(7AAD). Anti-CD8 antibodies were also added to all samples, except for (e) FMOCD8 controls (f,g): viability marker.
Using the CD2/CD2R system, we also performed positive effector cell control experiments, confirming the activation potential of the effector cells (data not shown).
Cytotoxicity calculations
In 51Cr cytotoxicity assays results are given normally as percentages of cell killing, with the maximum killing as a basic value. In assays measuring granularity by CD107a this is not meaningful, as a maximum value is difficult, if not impossible, to give. The results are therefore given as increments, where either the NK value or the value with preimmune serum is subtracted from the value with immune serum. The increase can also be given as a ratio between, for example, immune sera and preimmune sera.
Ethical approval
All human materials were obtained with informed consent and the study was approved by the local ethical committee.
Statistics
Statistical evaluations were performed as either a t-test or a Mann–Whitney test using the GraphPad InStat version 3 program (San Diego, CA, USA).
Results
Effector cell reactivity against target cells combined with Rituximab®
All the long-term cultured MS target cells express B cell markers on their surfaces, and as Rituximab® is an anti-B cell antibody, a combination of target cells and this antibody comprises a possible control system for ADCC, assessed as effector cell granularity expressed as CD107a expression.
Three different target cell cultures, MS 1533, MS 1874 and MS 1946, were tested with effector cells from a total of 10 different donors.
As seen in Table 1, all target cells express sufficient amounts of B cell epitopes for the antibody to elicit CD107a expression on the effector cells. Results are given both with and without Rituximab®; the latter are to be considered as NK cell activity.
Table 1.
Flow cytometric analysis of CD107a as a functional marker for natural killer (NK)-mediated antibody-dependent cell-mediated cytotoxicity (ADCC) activity.
| MS1533 | MS 1874 | MS 1946 | |
|---|---|---|---|
| Effector A | |||
| NK* | 3·8 ± 1·7 | 1·8 ± 0·4 | 1·9 ± 1·2 |
| NK-ADCC† | 19·5 ± 4·3 | 31·70 ± 4·22 | 33·5 ± 7·3 |
| Effector B | |||
| NK* | 4·8 ± 2·6 | n.d. | 2·6 ± 1·9 |
| NK-ADCC† | 7·6 ± 3·4 | n.d. | 15·3 ± 7·3 |
| Effector C | |||
| NK* | 0·9 ± 0·2 | 3·3 ± 1·8 | 1·7 ± 0·4 |
| NK-ADCC† | 17·0 ± 1·6 | 25·5 ± 1·7 | 23·4 ± 4·7 |
| Effector D | |||
| NK* | 0·5 ± 0·2 | 1·0 ± 0·4 | 0·6 ± 0·3 |
| NK-ADCC† | 13·3 ± 1·9 | 15·0 ± 3·0 | 18·4 ± 6·6 |
| Effector E | |||
| NK* | 1·2 ± 0·4 | 2·2 ± 0·7 | 1·2 ± 0·2 |
| NK-ADCC† | 8·7 ± 0·7 | 24·9 ± 8·0 | 16·1 ± 2·6 |
| Effector F | |||
| NK* | 2·1 ± 0·6 | 2·1 ± 0·3 | 1·8 ± 0·5 |
| NK-ADCC† | 39·5 ± 2·4 | 25·3 ± 3·8 | 37·1 ± 6·7 |
| Effector G | |||
| NK* | 2·3 ± 0·9 | 3·4 ± 1·5 | 1·5 ± 0·3 |
| NK-ADCC† | 33·9 ± 4·9 | 32·9 ± 4·3 | 37·3 ± 5·9 |
| Effector H | |||
| NK* | 1·0 ± 0·2 | 1·3 ± 0·9 | 0·8 ± 0·2 |
| NK-ADCC† | 16·3 ± 1·6 | 12·7 ± 3·8 | 19·4 ± 3·0 |
| Effector I | |||
| NK* | 1·2 ± 0·2 | 1·4 ± 0·3 | 1·7 ± 0·9 |
| NK-ADCC† | 13·2 ± 1·2 | 15·5 ± 1·8 | 18·7 ± 3·1 |
| Effector J | |||
| NK* | 3·0 ± 0·1 | 2·2 ± 0·4 | 2·1 ± 2·9 |
| NK-ADCC† | 16·1 ± 2·3 | 20·3.0 ± 1·3 | 20·2 ± 1·9 |
Ten different sources of NK cells (effectors A–J) with three different target B lymphoblastoid cell cultures ± anti-CD20 antibodies (Rituximab®). Results represent percentages of the NK cell gate from at least three independent experiments. NK: (no Rituximab®) % CD107a+ CD56+ cells ± standard deviation (s.d.); NK-ADCC: (with Rituximab®) % CD107a+ CD56+ cells ± s.d.; n.d.: not done.
No significant differences in % CD107a+ CD56+ cells between the effector cells or against the three target cell cultures;
Significant increase in % CD107a+ CD56+ cells in ADCC for all three target cells (for MS1533 P = 0·0002, for MS1874 P < 0·0001, for MS1946 P < 0·0001; Mann–Whitney rank sum test).
There is no difference in the relative number of CD56+ cells, and the CD107a expression is at similar levels for the NK activity, whereas ADCC activity with Rituximab® as the active antibody is increased significantly for all 10 effector cell donors. The ADCC activity against each of the three different target cells also differs with Rituximab® as the active antibody. CD56+ NK cells can be subdivided into two populations based on the relative expression of the surface marker CD56. These subsets, CD56bright and CD56dim, differ in their activity. CD56bright cells are a minor constituent of the NK population in PBMCs; they are active cytokine producers but are only weakly cytotoxic before activation, whereas the CD56dim cells are the cytotoxic killers [12,13]. As shown in Fig. 2, analyses of the distribution of CD56bright and CD56dim cells in the effector cell donors show certain variability in the relative proportions of the weakly cytotoxic CD56bright cells to CD56dim cells, which may have implications for the cytotoxic potential of the effector cells.
Figure 2.
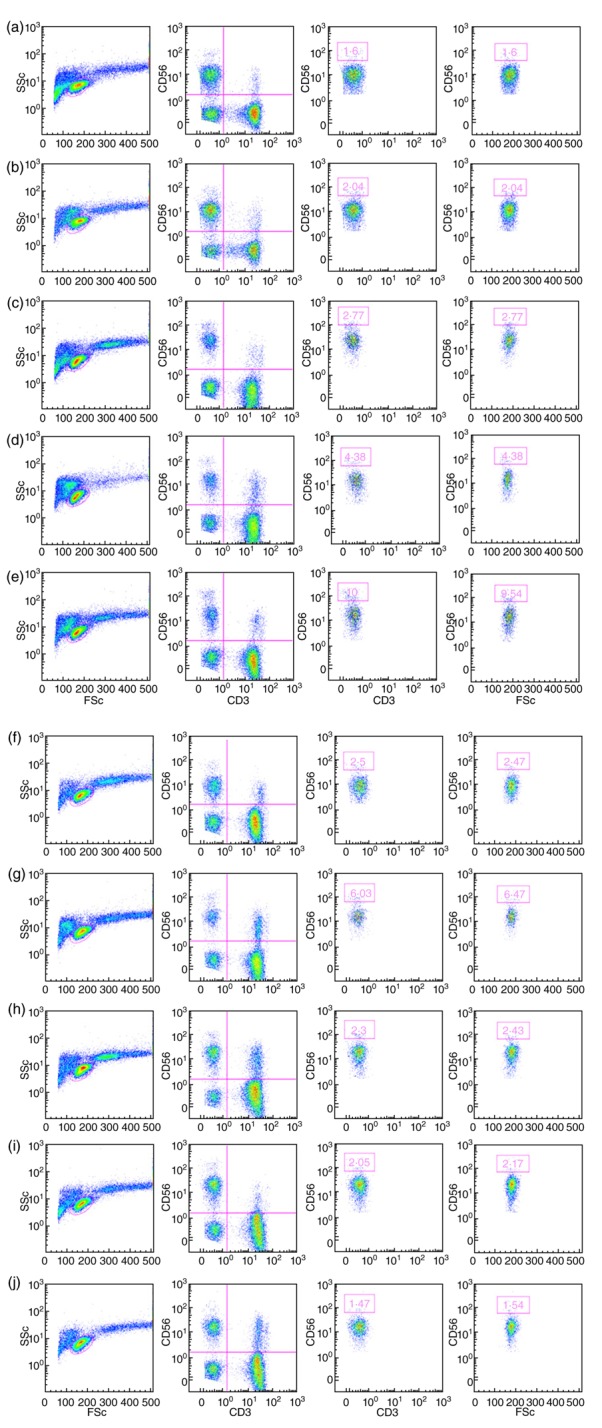
Differences in relative proportions of CD56bright and CD56dim natural killer (NK) cell populations in the effector cells. Fluorescence activated cell sorter (FACS) profiles shown as pseudo-colour plots illustrating the relative proportions of CD56bright and CD56dim NK cell populations in each of the 10 effector cells (a–j). For each effector cell the four panels are, from left to right: far left: ungated [side scatter (SSc) versus forward scatter (FSc]; left: lymphocyte gate (CD56 versus CD3); right, and far right: the populations representing CD56bright cells are boxed and depicted as both the CD3−CD56+ population (right) and in relation to FSc (far right).
Effector cell reactivity against target cells combined with polyclonal anti-HERV antibodies
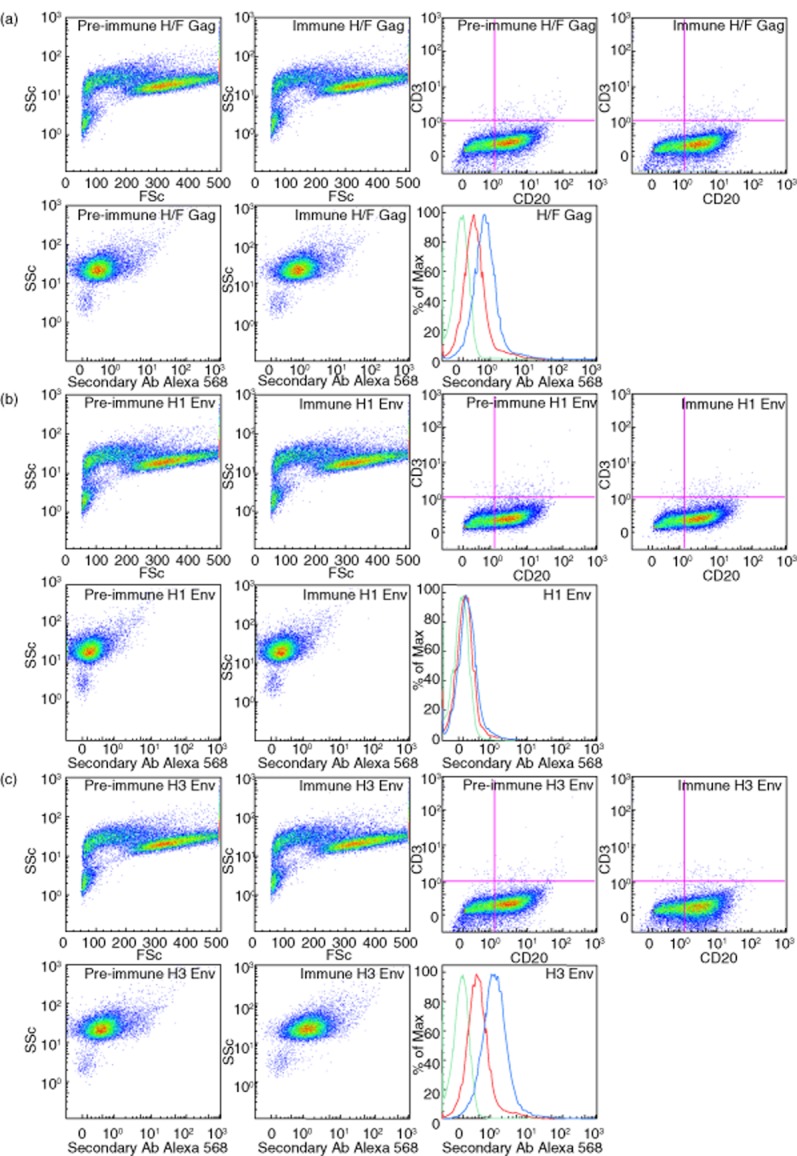
A panel of polyclonal rabbit antibodies has been raised against selected HERV epitopes. In Fig. 3 we illustrate the antibody reactivity by showing examples of HERV epitope expression and reactivity of anti-HERV H/F Gag-and anti-HERV-H antibodies on target cells. Figure 4 illustrates effector cell reactivity against target cells/anti-HERV antibodies, shown as flow cytometric profiles of induced changes in CD107a levels. Table 2 and 3 summarize data for all antibodies, examples of effector cells and target cells, with high CD107a expression in CD56+ cells when antibodies against HERV-H/F Gag and HERV-H Env H1 were added to the target cells. A somewhat lower reactivity was observed with anti-HERV-H Env H2, whereas activity was negligible for the remaining anti-sera in the panel.
Figure 3.
Flow cytometric profiles of typical examples of reactivity with both anti-human endogenous retroviruses (HERV)H/F Gag-and anti-HERV-H Env antibodies. Fluorescence activated cell sorter (FACS) profiles shown as pseudo-colour plots and overlay histograms after preincubation of target cells with preimmune or immune sera from the rabbits immunized with HERV H/F Gag (a); HERV-H Env H1 (b) or HERV-H Env H3 (c), respectively, illustrating anti-HERV antibody reactivity on the target cell MS1946. The seven panels for (a), (b) and (c) are in the same order from left to right: top row: far left: ungated cells [forward scatter (FSc) versus side scatter (SSc)] with preimmune serum, left: ungated cells (FSc versus SSc) with immune serum; right: ungated cells CD3 versus CD20 with preimmune serum; far right: ungated cells (CD3 versus CD20) with immune serum; second row: left: CD20+ gate with preimmune serum; centre: CD20+ gate with immune serum; right: summarized results in overlay histograms: green: control; red: target cells/preimmune sera; blue: target cells/anti-HERV-H/F Gag (a), anti-HERV-H Env H1 (b) or anti-HERV-H Env H3 (c), respectively.
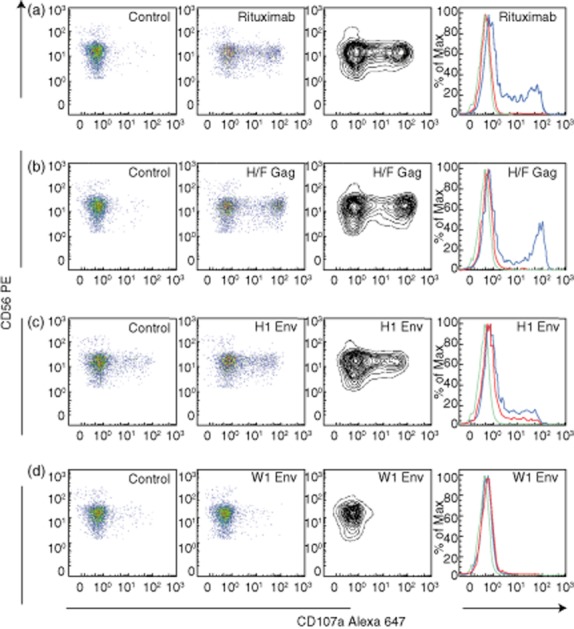
Figure 4.
Flow cytometric profiles of typical examples of changes in effector cell reactivity with both anti-human endogenous retroviruses (HERV) H/F Gag-and anti-HERV-H and HERV-W Env antibodies. Fluorescence activated cell sorter (FACS) profiles illustrating changes induced by anti-HERV antibodies in CD107a levels on CD3−/CD56+ gated lymphocytes from effector A incubated with target cells (the B lymphoblastoid MS1874 cell culture in these examples). Examples are either (a): for the anti-CD20 control (Rituximab®), (b) for anti-HERV H/F Gag; (c) for anti-HERV-H Env H1 or (d): for anti-HERV-W Env W1. The vertical panels show from left to right: far left: pseudo-colour plots of CD107a after preincubation of target cells with either AIM medium (a) or preimmune sera from the rabbits immunized with HERV H/F Gag (b); HERV-H Env H1 (c) or HERV-W Env W1 (d), respectively. Left panel: pseudo-colour plots of CD107a after preincubation of target cells with either Rituximab® (a) or immune sera from the rabbits immunized with HERV H/F Gag (b); HERV-H Env H1 (c) or HERV-W Env W1 (d). Right panel shows a contour plot of the data in the left panel; whereas the far right panel summarizes results in overlay histograms: green: Effector A (control); red: effector A/target cells/preimmune sera (control); blue: effector A/target cells/Rituximab® (a), anti-HERV-H/F Gag (b), anti-HERV-H Env H1 or anti-HERV-W Env W1, respectively. Increments for CD107a expression were: anti-CD20 (Rituximab®): 36·8; anti-HERV-H/F Gag: 37·4; anti-HERV-H Env H1: 15·4; anti-HERV-W Env W1: none.
Table 2.
Representative example of flow cytometric analysis of antibody-dependent cell-mediated cytotoxicity (ADCC) activity mediated by CD3−CD56+ natural killer (NK) or CD3+CD8+ T effector cells and anti-human endogenous retroviruses (HERV) antibodies.
| HERV peptide epitope | CD56+ |
CD8+ |
||||
|---|---|---|---|---|---|---|
| PI | I | Increment | PI | I | Increment | |
| HERV-H/F Gag | 2·05 | 39·4 | 37·35 | 0·77 | 4·75 | 3·98 |
| HERV-F Gag | 2·77 | 3·05 | 0·28 | 0·41 | 0·68 | 0·27 |
| HERV-H H1 Env | 12·30 | 27·7 | 15·40 | 0·63 | 0·97 | 0·34 |
| HERV-H H2 Env | 6·06 | 8·83 | 2·74 | 0·55 | 1·57 | 1·02 |
| HERV-H H3 Env | 2·00 | 2·61 | 0·61 | 0·63 | 0·71 | 0·08 |
| HERV-F Env | 6·65 | 6·73 | 0·08 | 0·83 | 0·42 | – |
| HERV-W W1 Env | 3·73 | 2·64 | – | 0·52 | 0·38 | – |
| HERV-W W2 Env | 4·40 | 2·69 | – | 0·49 | 0·86 | 0·37 |
| HERV-W W3 Env | 4·98 | 2·52 | – | 0·39 | 0·56 | 0·17 |
Effector cells sourced from effector A: NK cells (CD56+) and cytotoxic T cells (CD8+), respectively. Target cells were the B lymphoblastoid cell culture MS1874. Results shown are % CD107a+ CD56+ cells and % CD107a+ CD8+ cells with added antibodies from preimmune (PI) or immune (I) serum after immunization with each specific HERV peptide.
Table 3.
Flow cytometric analyses of antibody-dependent cell-mediated cytotoxicity (ADCC) activity mediated by CD3−CD56+ natural killer (NK) or CD3+CD8+ T effector cells and anti-human endogenous retroviruses (HERV) antibodies.
| Effector A |
Effector B |
||||||
|---|---|---|---|---|---|---|---|
| CD56+ |
CD8+ |
CD56+ |
CD8+ |
CD56+ |
CD56+ |
CD56+ |
|
| MS1874 | MS1946 | MS1533 | MS1847 | MS1946 | |||
| HERV peptide epitope | |||||||
| HERV-H/F Gag | 37·35 | 3·98 | 31·27 | 3·64 | 17·94 | 18·66 | 20·15 |
| HERV-H H1 Env | 15·40 | 0·34 | 15·7 | 0·20 | 0·70 | 0·01 | 5·55 |
| HERV-H H2 Env | 2·74 | 1·02 | 1·61 | 1·08 | 1·97 | 1·55 | 1·04 |
| HERV-H H3 Env | 0·61 | 0·08 | 1·02 | 0·12 | 1·47 | – | 0·51 |
| HERV-W W1 Env | – | – | – | 0·10 | – | – | 0·75 |
| HERV-W W2 Env | – | 0·37 | 0·00 | 0·28 | – | – | – |
| HERV-W W3 Env | – | 0·17 | – | – | 0·57 | – | – |
Effector cells sourced from effector A or effector B: NK cells (CD56+) and cytotoxic T cells (CD8+), respectively. Target cells were the B lymphoblastoid cell cultures MS1533, MS1847, MS1874 or MS1946. Results shown are increments for % CD107a+ CD56+ cells and % CD107a+ CD8+ cells, respectively, with added antibodies from preimmune or immune serum after immunization with each specific HERV peptide.
The antibodies were tested against target cell cultures and effector cells as performed in the assays with Rituximab®. A similar reactivity pattern was seen against the target cells. Examples with two different effector cells are shown. A variation in reactivity levels was found, with the same effector cells (effector A) showing higher reactivity, as in the previous experiment. The results given are for the ADCC activity with NK values (reactivity without antibodies) subtracted.
CD8+ cells were also tested as effector cells and, as expected, the activity without antibodies was overall at a negligible level, although with low, yet detectable ADCC activity for effector A cells and anti-HERV-H/F Gag antibodies.
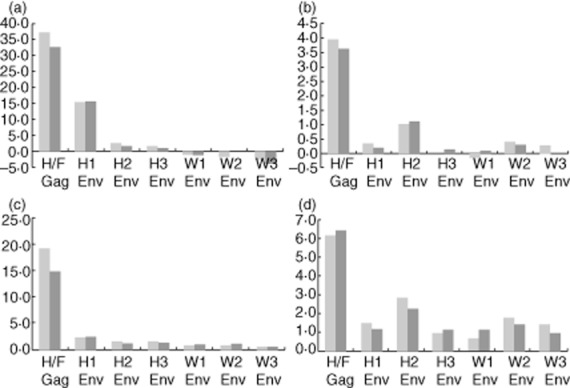
The results for both types of effector cells are shown in Fig. 5 both as increments where results with preimmune sera are subtracted from the results with immune sera and also as the value in folds (immune sera/preimmune sera). We find that increments are the most accurate and instructive values, as artificially increased values may result from calculating folds, when the denominator is below 1·0.
Figure 5.
Antibody-dependent cell-mediated cytotoxicity (ADCC) mediated by anti-human endogenous retroviruses (HERV) antibodies. ADCC mediated by anti-HERV antibodies with the two target cell cultures MS 1874 (blue) and MS 1946 (red) with effector A. This example illustrates increments for CD56+ natural killer (NK) cells (a) and for CD8+ T cells (b) as well as fold increase for CD56+ NK cells (c) and for CD8+ T cells (d). The peptide epitope for each polyclonal anti-serum is indicated below. The highest ADCC activity was mediated by anti-HERV-H/F Gag and anti-HERV-H Env.
Discussion
The causative agent(s) initiating MS continues to evade exposure of their nature. The processes leading to cell death are also incompletely understood, although parts of the process are known, thus offering possibilities for different types of intervention in the course or the symptoms of the disease.
Cytotoxicity reactions are not investigated greatly, either for the types of possible effector cells or for the antibodies/epitopes involved, although these reactions may play a significant role in MS pathogenesis by killing CNS cells expressing the epitopes. The type of effector cells gaining most attention recently have been CD8+ T cells rather than CD4+ T cells [14,15], which for several years were regarded as the main participants in the disease processes [16], due in part to extensive investigations based on the animal model of brain inflammation, experimental autoimmune encephalomyelitis (EAE). This model has some similarities but also significant differences from MS, illustrated markedly by the lack of efficacy of clinical MS trials targeting CD4+ T cells [17].
Different types of cytotoxic activities of possible significance are due to NK [18] or ADCC, both executed mainly by CD56+ cells. In particular, the latter type of cytotoxicity may be worthwhile studying, as increased production of oligoclonal antibodies against both known and unknown epitopes (including HERV and herpesvirus epitopes) is one of the characteristic and puzzling findings in MS [19–21].
For several years we have grown blood lymphocytes from MS patients in our laboratory [9]. Some of these lymphocytes, particularly when sourced from MS patients in relapse, have changed the growth pattern into continuously growing B lymphoblastoid cell cultures expressing and producing endogenous retroviruses, predominantly HERV-H/F, and also HERV-W, together with low amounts of Epstein–Barr virus proteins. B cells and their role in MS and other defined autoimmune diseases have gained increased attention recently [22], as various B cell-related mechanisms also display the ability to be effector cells, regulatory cells or target cells [17,23,24].
The suitability of these cells as target cells was tested originally in 51Cr-release, but the cells spontaneously leak too high amounts of the isotope to show reliable results in a cytotoxicity test. In a few pilot experiments, where target cells are labelled with fluorescent dye, comparable leakage of the dye, also reported by others [4], may also complicate the reading of the results, whereas in the present set-up the target cells are able to stimulate a significantly increased effector cell degranulation assessed as CD107a expression, when specific antibodies are added. The most effective effector cells are the CD56+ cells exhibiting only low amounts of NK activity against the target cells, no matter which of the four cell cultures are used as the target, whereas ADCC reactivity is significant for all target cells, indicating that these cells express HERV epitopes, and expose these epitopes on their surfaces thereby enabling the formation of antigen–antibody complexes that can activate the effector cells. These HERV epitopes may thus constitute a pathogenic potential in combination with specific antibodies, and also in conjunction with other molecules such as cytokines or complement [25]. Different levels of granularity/cytotoxicity of different effector cell donors are a general observation in cytotoxicity systems [26].
As expected, CD8+ T cells have low CD107a expression without antibodies added as their activity depends on major histocompatibility complex (MHC) matching. However, some ADCC activity can also be observed with these effector cells, but to a much lower degree than with the CD56+ cells.
We have demonstrated previously that the target cells also express HERV-H/F as HERV-W epitopes [1], and our main goal in the present study was to test the cells together with the appropriate antibodies in the cytotoxicity assay. In the present set-up, anti-HERV-H/F antibodies resulted in markedly increased granularity of the effector cells, whereas the anti-HERV-W Env antibodies elicited low to negligible activities. This difference in intensity is in accordance with our previous results demonstrating high expression of HERV-H/F Gag and Env epitopes [1,27], and may reflect the reported targeting of Gag proteins in particular to the plasma membrane for particle assembly [28]. The low level of anti-HERV-W Env-mediated activation of the effector cells was unexpected, as HERV-W epitopes have been found by others to be of great significance in MS pathogenesis [29,30]. Whether demographic/geographic differences in the epitope expression, as reported for HERV-W [31], may play a role for these differences is not currently known.
Having demonstrated that the cytotoxicity reactions with polyclonal anti-HERV anti-sera elicit appropriate reactions, and that the set-up is reasonably straightforward (results for different time-points not shown), further investigation could involve assessing the ADCC impact of the known, increased seroreactivity to HERV epitopes in MS patient sera [1] using singular target and effector cells, as well as assessing the efficacy of MS patient lymphocytes as effector cells in ADCC.
The relevance of ADCC as a pathogenic factor has been disputed for several years. However, the rapidly increasing use of antibodies in immunotherapy ought to increase the focus on this mechanism and the involved effector cells [32]. Previously reported activation of NK cells upon stimulation by HIV-specific antibodies also seems to be of relevance in this context [33].
An interesting set-up would be MHC matching of target and effector cells to elucidate the role of cytotoxic CD8+ T cells for which this type of assay seems extremely appropriate [34]. Finally, it could also be of interest to combine the present set-up with cytokine [35], lectin and complement parameters [36] to shed further light on processes that may damage the CNS cells. It may also be possible to test CD8+ T cell-mediated cytotoxicity in different MS disease states with patient lymphocytes as either target or effector cells [37]. The possibility that γδ T cells could be an active part in the pathogenesis [38,39] has not been considered here, but a recent review [40] comprising several of the mechanisms discussed above indicates that experiments including these cells could also add to the understanding of the different mechanisms possibly influencing the disease course.
Acknowledgments
This work was supported by The Danish MS Society, Aase and Einar Danielsen's Foundation; Fonden til Lægevidenskabens Fremme; Jascha Fonden; Direktør Jacob Madsens Fond; Torben og Alice Frimodts Fond; Wilhelm Bangs Fond; CC Klestrups Fond, Dagmar Marshalls Fond, Grosserer AV Lykfeldts Legat, Brdr Hartmanns Fond, Krista og Viggo Petersens Fond and Carl og Ellen Hertz' Legat.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Brudek T, Christensen T, Aagaard L, Petersen T, Hansen HJ, Møller-Larsen A. B cells and monocytes from patients with active multiple sclerosis exhibit increased surface expression of both HERV-H Env and HERV-W Env, accompanied by increased seroreactivity. Retrovirology. 2009;6:104. doi: 10.1186/1742-4690-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottley G, Cook GP, Blair GE. A flow cytometric assay for analysis of natural-killer cell-mediated cytolysis of adenovirus-transformed cells. Methods Mol Med. 2007;131:221–230. doi: 10.1007/978-1-59745-277-9_16. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann SY, Esser R, Rohrbach E, Klingebiel T, Koehl U. A novel four-colour flow cytometric assay to determine natural killer cell or T-cell-mediated cellular cytotoxicity against leukaemic cells in peripheral or bone marrow specimens containing greater than 20% of normal cells. J Immunol Methods. 2005;296:63–76. doi: 10.1016/j.jim.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Cholujová D, Jakubíková J, Kubes M, et al. Comparative study of four fluorescent probes for evaluation of natural killer cell cytotoxicity assays. Immunobiology. 2008;213:629–640. doi: 10.1016/j.imbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Bryceson YT, Fauriat C, Nunes JM, et al. Functional analysis of human NK cells by flow cytometry. Methods Mol Biol. 2010;612:335–352. doi: 10.1007/978-1-60761-362-6_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhrberg M. The CD107 mobilization assay: viable isolation and immunotherapeutic potential of tumor-cytolytic NK cells. Leukemia. 2005;19:707–709. doi: 10.1038/sj.leu.2403705. [DOI] [PubMed] [Google Scholar]
- 7.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Munch M, Møller-Larsen A, Christensen T, Morling N, Hansen HJ, Haahr S. B-lymphoblastoid cell lines from multiple sclerosis patients and a healthy control producing a putative new human retrovirus and Epstein–Barr virus. Mult Scler. 1995;1:78–81. doi: 10.1177/135245859500100204. [DOI] [PubMed] [Google Scholar]
- 9.Christensen T, Jensen AW, Munch M, et al. Characterization of retroviruses from patients with multiple sclerosis. Acta Neurol Scand. 1997;S169:49–58. doi: 10.1111/j.1600-0404.1997.tb08150.x. [DOI] [PubMed] [Google Scholar]
- 10.de Parseval N, Casella J, Gressin L, Heidmann T. Characterization of the three HERV-H proviruses with an open envelope reading frame encompassing the immunosuppressive domain and evolutionary history in primates. Virology. 2001;279:558–569. doi: 10.1006/viro.2000.0737. [DOI] [PubMed] [Google Scholar]
- 11.Mallet F, Bouton O, Prudhomme S, et al. The endogenous retroviral locus ERVWE1 is a bona fide gene involved in hominoid placental physiology. Proc Natl Acad Sci USA. 2004;101:1731–1736. doi: 10.1073/pnas.0305763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson TA, Evans BL, Durafourt BA, et al. Reduction of the peripheral blood CD56(bright) NK lymphocyte subset in FTY720-treated multiple sclerosis patients. J Immunol. 2011;187:570–579. doi: 10.4049/jimmunol.1003823. [DOI] [PubMed] [Google Scholar]
- 14.Neumann H, Medana IM, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- 15.Friese MA, Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol. 2009;66:132–141. doi: 10.1002/ana.21744. [DOI] [PubMed] [Google Scholar]
- 16.Sobottka B, Harrer MD, Ziegler U, et al. Collateral bystander damage by myelin-directed CD8+ T cells causes axonal loss. Am J Pathol. 2009;175:1160–1166. doi: 10.2353/ajpath.2009.090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasper LH, Shoemaker J. Multiple sclerosis immunology: the healthy immune system vs the MS immune system. Neurology. 2010;74(Suppl. 1):S 2–8. doi: 10.1212/WNL.0b013e3181c97c8f. [DOI] [PubMed] [Google Scholar]
- 18.Flodström-Tullberg M, Bryceson YT, Shi FD, Höglund P, Ljunggren HG. Natural killer cells in human autoimmunity. Curr Opin Immunol. 2009;21:634–640. doi: 10.1016/j.coi.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Christensen T. Association of human endogenous retroviruses with multiple sclerosis and possible interactions with herpes viruses. Rev Med Virol. 2005;15:179–211. doi: 10.1002/rmv.465. [DOI] [PubMed] [Google Scholar]
- 20.Jolivet-Reynaud C, Perron H, Ferrante P, Becquart L, Dalbon P, Mandrand B. Specificities of multiple sclerosis cerebrospinal fluid and serum antibodies against mimotopes. Clin Immunol. 1999;93:283–293. doi: 10.1006/clim.1999.4789. [DOI] [PubMed] [Google Scholar]
- 21.Reiber H, Ungefehr S, Jacobi C. The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult Scler. 1998;4:111–117. doi: 10.1177/135245859800400304. [DOI] [PubMed] [Google Scholar]
- 22.Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol. 2008;7:852–858. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- 23.Cepok S, Rosche B, Grummel V, et al. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128:1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- 24.Stüve O, Cepok S, Elias B, et al. Clinical stabilization and effective B-lymphocyte depletion in the cerebrospinal fluid and peripheral blood of a patient with fulminant relapsing–remitting multiple sclerosis. Arch Neurol. 2005;62:1620–1623. doi: 10.1001/archneur.62.10.1620. [DOI] [PubMed] [Google Scholar]
- 25.Hafler DA, Slavik JM, Anderson DE, O'Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis. Immunol Rev. 2005;204:208–3. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 26.Motzer SA, Tsuji J, Hertig V, Johnston SK, Scanlan J. Natural killer cell cytotoxicity: a methods analysis of 51chromium release versus flow cytometry. Biol Res Nurs. 2003;5:142–152. doi: 10.1177/1099800403257196. [DOI] [PubMed] [Google Scholar]
- 27.Laska M, Brudek T, Nissen K, et al. Expression of HERV-Fc1, a human endogenous retrovirus, is increased in patients with active multiple sclerosis. J Virol. 2012;86:3713–3722. doi: 10.1128/JVI.06723-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorweiler IJ, Ruone SJ, Wang H, Burry RW, Mansky LM. Role of the human T-cell leukemia virus type 1 PTAP motif in Gag targeting and particle release. J Virol. 2006;80:3634–3643. doi: 10.1128/JVI.80.7.3634-3643.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perron H, Germi R, Bernard C, et al. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler. 2012;18:1721–1736. doi: 10.1177/1352458512441381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antony JM, Izad M, Bar-Or A, et al. Quantitative analysis of human endogenous retrovirus-W env in neuroinflammatory diseases. AIDS Res Hum Retroviruses. 2006;22:1253–1259. doi: 10.1089/aid.2006.22.1253. [DOI] [PubMed] [Google Scholar]
- 31.Serra C, Sotgiu S, Mameli G, Pugliatti M, Rosati G, Dolei A. Multiple sclerosis and multiple sclerosis-associated retrovirus in Sardinia. Neurol Sci. 2001;22:171–173. doi: 10.1007/s100720170019. [DOI] [PubMed] [Google Scholar]
- 32.Fischer L, Penack O, Gentilini C, et al. The anti-lymphoma effect of antibody-mediated immunotherapy is based on an increased degranulation of peripheral blood natural killer (NK) cells. Exp Hematol. 2006;34:753–759. doi: 10.1016/j.exphem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Chung AW, Rollman E, Center RJ, Kent SJ, Stratov I. Rapid degranulation of NK cells following activation by HIV-specific antibodies. J Immunol. 2009;182:1202–1210. doi: 10.4049/jimmunol.182.2.1202. [DOI] [PubMed] [Google Scholar]
- 34.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Evaluation of the CD107 cytotoxicity assay for the detection of cytolytic CD8+ cells recognizing HER2/neu vaccine peptides. Breast Cancer Res Treat. 2005;92:85–93. doi: 10.1007/s10549-005-0988-1. [DOI] [PubMed] [Google Scholar]
- 35.Shacklett BL. Beyond 51Cr release: new methods for assessing HIV-1-specific CD8+ T cell responses in peripheral blood and mucosal tissues. Clin Exp Immunol. 2002;130:172–182. doi: 10.1046/j.1365-2249.2002.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen T, Petersen T, Thiel S, Brudek T, Ellermann-Eriksen S, Møller-Larsen A. Gene–environment interactions in multiple sclerosis: innate and adaptive immune responses to human endogenous retrovirus and herpesvirus antigens and the lectin complement activation pathway. J Neuroimmunol. 2007;183:175–188. doi: 10.1016/j.jneuroim.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z, Freedman MS. Correlation of specialized CD16(+) gammadelta T cells with disease course and severity in multiple sclerosis. J Neuroimmunol. 2008;194:147–152. doi: 10.1016/j.jneuroim.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Freedman MS. γδ T cells and multiple sclerosis: friends, foes, or both? Autoimmun Rev. 2011;10:364–367. doi: 10.1016/j.autrev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Gandhi R, Laroni A, Weiner HL. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2010;221:7–14. doi: 10.1016/j.jneuroim.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]