Summary
Rheumatic heart disease (RHD) is characterized by the presence of anti-streptococcal group A antibodies and anti-endothelial cell antibodies (AECA). Molecular mimicry between streptococcal antigens and self proteins is a hallmark of the pathogenesis of rheumatic fever. We aimed to identify, in RHD patients, autoantibodies specific to endothelial autoantigens cross-reactive with streptococcal proteins and to evaluate their role in inducing endothelial damage. We used an immunoproteomic approach with endothelial cell-surface membrane proteins in order to identify autoantigens recognized by AECA of 140 RHD patients. Cross-reactivity of purified antibodies with streptococcal proteins was analysed. Homologous peptides recognized by serum cross-reactive antibodies were found through comparing the amino acid sequence of streptococcal antigens with human antigens. To investigate interleukin (IL)-1R-associated kinase (IRAK1) and nuclear factor-κB (NF-κB) activation, we performed a Western blot analysis of whole extracts proteins from unstimulated or stimulated human microvascular cardiac endothelial cells (HMVEC-C). Adhesion molecule expression and release of proinflammatory cytokines and growth factors were studied by multiplex bead based immunoassay kits. We observed anti-vimentin antibodies in sera from 49% RHD AECA-positive patients. Cross-reactivity of purified anti-vimentin antibodies with heat shock protein (HSP)70 and streptopain streptococcal proteins was shown. Comparing the amino acid sequence of streptococcal HSP70 and streptopain with human vimentin, we found two homologous peptides recognized by serum cross-reactive antibodies. These antibodies were able to stimulate HMVEC-C inducing IRAK and NF-κB activation, adhesion molecule expression and release of proinflammatory cytokines and growth factors. In conclusion, streptococcal–vimentin cross-reactive antibodies were able to activate microvascular cardiac endothelium by amplifying the inflammatory response in RHD.
Keywords: anti-endothelial cell autoantibodies, rheumatic heart disease, vimentin
Introduction
Acute rheumatic fever (ARF) is a multi-systemic disease caused by an abnormal immunological response after group A Streptococcus (GAS) pharyngitis in predisposed people [1]. In 30–50% of cases recurrent episodes of ARF may lead to chronic rheumatic heart disease (RHD), with progressive and permanent damage of the cardiac valves [2].
During the 20th century the improvement of living conditions and prevention policies have cut substantially the incidence and prevalence of ARF and RHD in industrialized countries. Nevertheless, RHD remains one of the major causes of morbidity and mortality in developing countries. It is estimated that there are more than 15 million cases of RHD worldwide, with 282 000 new cases and 233 000 deaths annually [3]. Moreover, a recent systematic echocardiographic screening revealed a prevalence of RHD that is approximately 10 times higher than that based on clinical screening [4].
The endocardial valve tissue is the main localization of cardiac damage, which begins when peripheral T lymphocytes, reacting with adhesion molecules (i.e. vascular cell adhesion molecule 1, VCAM-1), infiltrate a non-vascularized tissue. The presence of anti-GAS antibodies is one of the major features, and deposits of antibodies and complement have been found in the heart of RHD patients [5,6].
In a recent study, in collaboration with Sana'a (Yemen) University, we demonstrated the presence of anti-endothelial cell antibodies (AECA) in RHD patients [7]. These antibodies have been demonstrated to play pathogenic roles in numerous autoimmune diseases in which endothelial damage is predominant [8,9]. They have proinflammatory and procoagulant effects on endothelial cells, inducing up-regulation of adhesion molecule expression and increase of tissue factor (TF) and cytokine release [10,11]. Molecular mimicry between GAS antigens and self-proteins is a hallmark of the pathogenesis of rheumatic fever [5,6,12–14]. As rheumatic valve damage may begin on the surface of valvular endothelium, AECA, possibly using a mechanism of molecular mimicry, could contribute to this damage by promoting endothelial stress.
In the present study, using immunoproteomic analysis, we characterized the autoantibodies directed against endothelium in RHD patients and investigated the presence of cross-reactivity between endothelial antigens and streptococcal antigens. Finally, we evaluated the functional effects of cross-reactive antibodies on human microvascular cardiac endothelial cells (HMVEC-C).
Materials and methods
Patients and controls
The study enrolled 140 consecutive patients (58 men 82 women, age range 11–55 years) who were admitted to Al-Thawrah Hospital in Sana'a, Yemen, for RHD described previously [7]. All patients were diagnosed according to the modified Jones criteria [1]. One hundred and forty sex-and age-matched normal health subjects, enrolled as blood donors in Yemen served as controls. Informed consent was obtained from all the patients and controls in accordance with local laws.
Cellular cultures
The immortalized hybridoma cell line EAhy926 was cultured in Dulbecco's modified medium (high glucose) containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin and 1 mM HEPES (Invitrogen, Carlsbad, CA, USA). Clonetics™ HMVEC-C (Lonza Group Ltd, Basel, Switzerland) were cultured in endothelial cell basal medium (EBM)-2 containing 5% FBS, hydrocortisone, human recombinant epidermal growth factor (hFGF)-B, vascular endothelial growth factor (VEGF), human recombinant insulin-like growth factor (R3-IGF)-1, ascorbic acid, human recombinant epidermal growth factor (hEGF) and gentamicin/amphotericin-B (GA)-1000. Cellular cultures were maintained at 37°C in a humidified 5% CO2 atmosphere. Experiments were performed in cells grown to 60–70% confluence.
Isolation of endothelial cell surface membrane proteins
Cell-surface membrane proteins were purified from EAhy926 endothelial cells using the Pierce Cell Surface Protein Isolation Kit, according to the manufacturer's instructions, with slight modifications (Pierce, Rockford, IL, USA). In brief, 1 × 107 cells were incubated in 1 ml of sulpho-NHS-SS-biotin, a cleavable biotinylation reagent. After two washes with Tris-buffered saline (TBS), the cells were subjected to sonication and the biotinylated proteins were incubated with immobilized NeutrAvidin gel (Pierce). After extensive washing of the resin (nine times), the proteins were eluted according to the protocol.
Group A Streptococcus culture and protein extract
GAS strains were grown in 10 ml Todd–Hewitt broth at 37°C in modified atmosphere (5% CO2) overnight without agitation. Cells were harvested by centrifugation at 3000 g for 3 min, resuspended and washed three times in 1 ml sterile phosphate-buffered saline (PBS), pH 7·0. Resuspended cells were then sonicated three times for 1 min in ice with a microtip sonicator, using a power setting of approximately 300 W. Whole-cell sonic extracts were centrifuged at 10 000 g for 15 min and supernatant was stored at −20°C.
Peptides
We used multiple antigen peptide (MAP) containing four copies of the peptide (peptide A: AYFNDAQRQATKDA from streptococcal heat shock protein (HSP)70; peptide B: KKKLGVRLLSLLA from streptococcal streptopain), synthesized directly on a four-armed branched lysine core (Primm Labs, Cambridge, MA, USA), for enzyme-linked immunosorbent assay (ELISA) assay. Rabbits were immunized with peptides conjugated to BSA (peptides A and B) or plain BSA, and the production of polyclonal anti-serum followed standard immunization procedures.
Purification of specific autoantibodies from sera of patients and rabbits
Antibody purification was performed as described previously [15]. The antigen was spotted onto a nitrocellulose filter and incubated with the patients' sera. The antibodies were eluted with glycine 100 mM, pH 2·5 and neutralized immediately with Tris-HCl 1 M, pH 8. The antibody preparations were treated with Detoxi-Gel endotoxin removing columns gel (Pierce) and contained <0·00025 ng of endotoxin/μg of protein, as determined by the Limulus amebocyte lysate test (Associates of Cape Cod, Falmouth, MA, USA).
Two-dimensional electrophoresis and immunoblotting
Biotinylated endothelial proteins and GAS proteins were loaded onto two-dimensional electrophoresis (2DE). Isoelectrofocusing was performed on 7-cm immobilized pH gradient strips (range pH 3–10) using the IPGphor Isoelectric Focusing System (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The second dimension was determined on a 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) system after equilibrating the strips for 10 min in SDS equilibration buffer containing 50 mM Tris/HCl (pH 8·8), 6 M urea, 30% glycerol, 2% SDS, 2% dithiothreitol (DTT) and 2·5% iodoacetamide. Gels were then stained by colloidal Coomassie blue (Sigma-Aldrich, St Louis, MO, USA). For immunoblotting analysis, nitrocellulose membrane was incubated with human sera diluted 1:50. Peroxidase-conjugated goat anti-human immunoglobulin (Ig)G (Bio-Rad, Richmond, CA, USA) was used as secondary antibody, and the reaction was developed with 3-3′ diaminobenzidine (Sigma-Aldrich).
Mass spectrometry
For protein identification, spots of interest were excised from Coomassie-stained 2D gels, washed with 25 mM ammonium bicarbonate, dried with acetonitrile and digested with modified sequencing-grade trypsin (Promega Corporation, Madison, WI, USA), as described elsewhere [16]. Peptide mixtures were analysed by matrix-assisted laser desorption ionization time-of-flight mass spectrometer (MALDI-TOF MS) analysis using the dried droplet technique and α-cyano-4-hydroxycinnamic acid as matrix prepared in a concentration of 10 mg/ml in 40% acetonitrile, 0·1% v/v trifluoroacetic acid. The analyses were performed using a Voyager-DE STR (Applied Biosystems, Framingham, MA, USA) TOF MS operated in the delayed extraction mode. Peptides were measured in the mass range from 750 to 4000 Da; all spectra were calibrated internally and processed via the Data Explorer software to generate a peak list. Proteins were identified unambiguously by searching a comprehensive non-redundant protein database (Swiss-Prot) using the program Mascot (http://www.matrixscience.com). Search settings allowed one missed cleavage with the trypsin enzyme selected, with oxidation of methionine as variable modification, carboamidomethylation of cysteine as fixed modifications and peptide tolerance of 50 parts per million (ppm), all taxa. Sim+lalnview (Expasy server) was used to compare the amino acid sequence of human C-and N-ter vimentin fragments with streptococcal STRP1 and streptococcal HSP70 amino acid sequences to establish peptide homologies.
Cloning, expression and purification of the recombinant fragments of human vimentin
In order to obtain the N-and C-terminal regions of vimentin, cDNA from EAhy926 cells was amplified by polymerase chain reaction (PCR) using specific oligonucleotides as primer and cloned in pQE (Qiagen, Hilden, Germany) expression vector. The fusion protein was expressed in Escherichia coli SG130009 cells, purified by affinity of nickel-nitrilotriacetic acid (Ni-NTA) resin for the six-histidine tail and eluted under denaturing conditions [17]. Recombinant protein was electroeluted according to the manufacturer's instructions (Model 422 Electroeluter; Bio-Rad).
ELISA
After coating polystyrene plates (Maxisorp, Nunc, Roskilde, Denmark) with 5 μg/ml of the antigen (human vimentin; R&D Systems, Minneapolis, MN, USA; human C-and N-ter recombinant vimentin), ELISA was developed as described previously [18] and sera were diluted 1:100 in PBS, pH 7·4 containing 0·1% Tween 20 (PBS-T). ELISA with MAP4-peptides was performed as well as for detecting vimentin, except that plates were coated with 10 μg/ml of each peptide in PBS buffer. Positive reactions were defined for values over the cut-off at the mean optical density (OD) + 2 standard deviations (s.d.) of healthy controls. All assays were performed in quadruplicate.
For inhibition analysis, we incubated purified anti-vimentin antibodies (10 μg/ml) overnight with streptococcus protein extract (5, 10 and 20 μg/ml) in 100 μl PBS. We also incubated purified anti-peptides A and B antibodies (10 μg/ml) overnight with human vimentin (R&D Systems), 5–20 μg/ml.
Endothelial cell surface binding of anti-streptococcal peptide antibodies
HMVEC-C were fixed with 4% formaldehyde in PBS for 30 min at 4°C and were then incubated for 30 min at 25°C in the blocking buffer (2% BSA in PBS, containing 5% glycerol and 0·2% Tween-20). After washing three times with PBS, cells were incubated for 1 h at 4°C with 1 μg purified rabbit anti-streptococcal peptides A or B antibodies in PBS containing 1% BSA. Purified rabbit anti-streptococcal peptides A and B antibodies were also pre-absorbed overnight with 20 μg human vimentin (R&D Systems) or with 20 μg streptococcal protein extract. Fluorescein isothiocyanate-conjugated anti-rabbit IgG (γ-chain specific; Sigma-Aldrich) was then added and incubated at 4°C for 30 min. Acquisition was performed on a fluorescence activated cell sorter (FACS)Calibur cytometer (BD Immunocytometry Systems, San Jose, CA, USA), and 50 000 events/sample were run. Data were analysed using CellQuest Pro software (BD Immunocytometry Systems).
In-vitro exposure of HMVEC-C to purified anti-streptococcal peptide antibodies
For in-vitro studies, HMVEC-C were incubated with purified anti-peptides A and B (200 μg/ml), anti-BSA antibodies (100 μg/ml) or LPS (100 ng/ml) as a positive control for different incubation times. Purified anti-peptides A and B antibodies (200 μg/ml) were also pre-absorbed with human vimentin (R&D System) (20 μg/ml) and streptococcal protein extract (50 μg/ml). All the materials contained less than 0·00025 ng endotoxin/μg protein, as determined by the Limulus amebocyte lysate test (Associates of Cape Cod).
Preparation of cell extracts
Unstimulated or 45-min-stimulated HMVEC-C cells were suspended in lysis buffer (20 mM HEPES, pH 7·2, 1% Nonidet P-40, 10% glycerol, 50 mM NaF, 1 mM Na3VO4, including protease inhibitors; Sigma-Aldrich). Protein content was determined by Bradford assay, using BSA as standard (Bio-Rad).
Western blot analysis of phospho-IL-1R-associated kinase (IRAK1) and phospho-nuclear factor-κB (NF-κB)
Equal amounts of whole extracts proteins were separated in 7·5% SDS-PAGE under unreducing conditions, blotted onto nitrocellulose membrane (Bio-Rad) and then probed with polyclonal anti-phospho-IRAK1 (Cell Signalling, Inc., Danvers, MA, USA) or anti-phospho-NF-κB p65 (Cell Signalling, Inc.). Bound antibodies were visualized with HRP-conjugated anti-rabbit IgG (Sigma-Aldrich) and immunoreactivity was assessed by the ECL Western blotting system (Amersham Pharmacia Biotech).
Analysis of cytokines, chemokines, adhesion molecules, growth factors and TF
In order to examine the levels of TF (American Diagnostica, Stamford, CT, USA) and VCAM-1 (Space Import, Milan, Italy) in the supernatant of endothelial cells after 1, 6, 12 and 24 h of in-vitro stimulation, ELISAs were performed following the manufacturer's instructions. Commercially available multiplex bead-based immunoassay kits (human 27-plex; Bio-Rad) were used to measure concentrations of cytokines, chemokines and growth factors. Assays were performed according to the manufacturer's instructions. Data were analysed with Bio-Plex manager software, version 4·1·1 (Bio-Rad) and reported as fluorescence intensity (FI). Values with a coefficient of variation >12% were excluded from the final data analysis.
Statistical analysis
The two-tailed Mann–Whitney U-test, unpaired t-test and χ2 test were performed. Statistical significance was set at P < 0·05. P values ≥ 0·05 were not significant.
Results
Vimentin is identified as an endothelial autoantigen in RHD patients
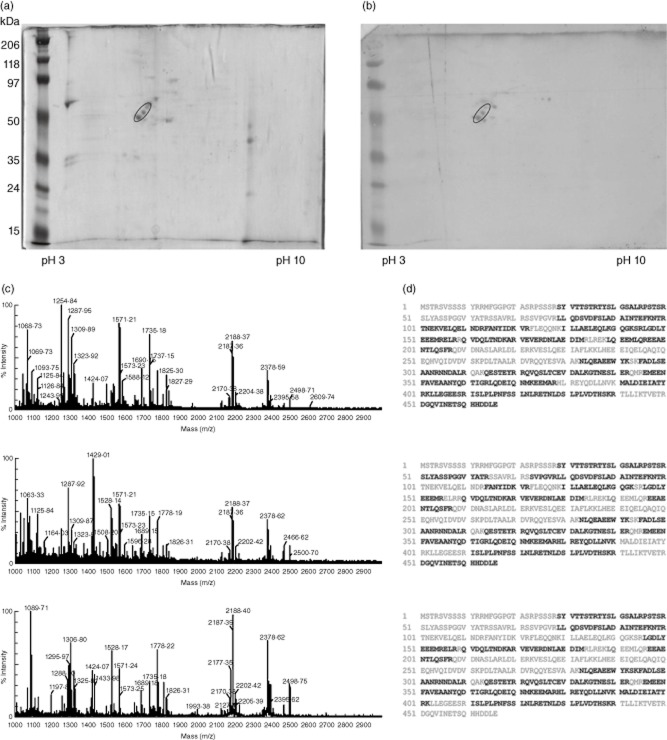
On the basis of our previous study suggesting a prominent role of AECA in the cardiac tissue damage associated with RHD [7], we performed an immunoproteomic analysis with sera from Yemeni patients with RHD in order to identify antigenic targets of AECA. Endothelial cell-surface membrane proteins from EAhy926 were separated by 2DE (Fig. 1a), transferred onto nitrocellulose membrane and analysed with sera from two RHD AECA-positive patients (Fig. 1b). The three spots identified were excised from 2DE gel, digested with trypsin and then analysed by MALDI-TOF MS. In Fig. 1c, MALDI-TOF MS spectra obtained from the tryptic digestion of each of the three spots are reported. The detected m/z peaks were searched against Swiss-Prot protein database. Results of the database search revealed that all three spectra match vimentin (NP_003371·2) with scores of 171, 130 and 104, respectively. Figure 1d shows the sequence of vimentin reported three times, but each one shows in bold the matched peptides for the relative spectrum reported on the left.
Figure 1.
Identification of vimentin as an endothelial autoantigen of rheumatic heart disease (RHD). (a) Endothelial cell-surface membrane proteins were separated by two-dimensional electrophoresis (2DE). (b) After transfer onto nitrocellulose membrane, immunoblotting was performed with sera [diluted 1:50 in phosphate-buffered saline (PBS)-T] from two patients with RHD that resulted anti-endothelial cell antibodies (AECA)-positive. Three spots with molecular weights of approximately 55 kDa, strongly reactive with serum immunoglobulin (Ig)G, were identified (circle). The three spots identified were excised from 2DE gel, digested with trypsin, and then analysed by matrix-assisted laser desorption ionization (MALDI time-of-flight mass spectrometry (MS). MALDI-MS spectra of the tryptic peptides mixtures obtained from the three spots are shown (c) and all of them match vimentin (NP_003371·2) with scores of 171, 130 and 104, respectively. (d) The vimentin sequence is reported three times, but each one shows, in bold, the matched peptides for the relative spectrum reported on the left.
Serum anti-vimentin antibodies in patients with RHD
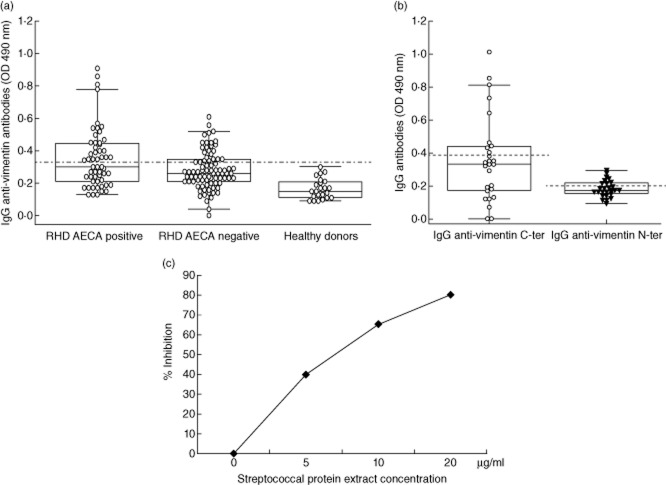
We analysed serum IgG immunoreactivity to vimentin by ELISA. We detected anti-vimentin antibodies in sera from 27 of 55 (49%) RHD AECA-positive patients and in 22 of 85 (25·8%) RHD AECA-negative patients (P < 0·001 by χ2 test). Immunoreactivity to vimentin (evaluated as mean OD) was significantly higher in AECA-positive RHD patients than in AECA-negative patients and in healthy donors (P < 0·001 by Mann–Whitney U-test). Clinical, serological and demographic characteristics of patients divided according to anti-vimentin positivity are shown in Table 1. To identify the immunoreactive regions of the protein, we cloned and expressed the N-and C-ter partially overlapping subunits. Using ELISA, we analysed the immunoreactivity against the two subunits (N-and C-ter) of vimentin in sera from the 27 RHD patients resulted AECA and vimentin positive. Both anti-N-ter vimentin and anti-C-ter vimentin antibodies were detected in nine of 27 sera (33·3% for each fragment) for a total of 18 positive sera (Fig. 2b); the immunoreactivity to the C-ter subunit appeared significantly higher than that to the N-ter subunit of vimentin (P < 0·001 by Mann–Whitney U-test).
Table 1.
Clinical, serological and demographic characteristic of serum anti-vimentin-positive and-negative patients with rheumatic heart disease.
| Patients with rheumatic heart disease | Serum anti-vimentin antibody-positive | Serum anti-vimentin antibody-negative |
|---|---|---|
| Male/female | 22/27 | 36/55 |
| Age (range/media) (years) | 11–55/27·52 | 23–50/28·27 |
| Anti-streptolysin titre +/− | 35/14 | 45/46 |
| State of heart disease acute/chronic | 2/47 | 8/83 |
Figure 2.
Anti-vimentin antibodies in patients and healthy donors. (a) Box-and-whisker plots of sera [diluted 1:100 in phosphate-buffered saline (PBS)-T] from anti-endothelial cell antibodies (AECA)-positive rheumatic heart disease (RHD) patients, AECA-negative RHD patients and healthy donors analysed by enzyme-linked immunosorbent assay (ELISA) for detection of immunoglobulin (Ig)G anti-vimentin antibodies. The occurrence of specific antibodies was significantly higher in AECA-positive RHD patients compared with those in AECA-negative and in healthy donors (P < 0·001). (b) To identify the immunoreactive epitopes of vimentin, the C-and N-terminal subunits were cloned and expressed, and analysed using ELISA with AECA and vimentin-positive sera (diluted 1:100 in PBS-T). Immunoreactive epitopes were present in both the subunits. The broken lines represent the cut-off (mean ± 2 standard deviations for healthy donors). Results are expressed as absorbance at 490 nm. The immunoreactivity to the C-ter subunit appeared significantly higher than that to the N-ter subunit of vimentin (P < 0·001 by Mann–Whitney U-test). (c) Inhibition of anti-vimentin ELISA reactivity by pre-absorption of purified human anti-vimentin antibodies (1 μg), with streptococcal protein extract. The y-axis represents percentage of inhibition and the x-axis indicates inhibitor concentrations.
Analysis of cross-reactivity between endothelial vimentin and streptococcal proteins
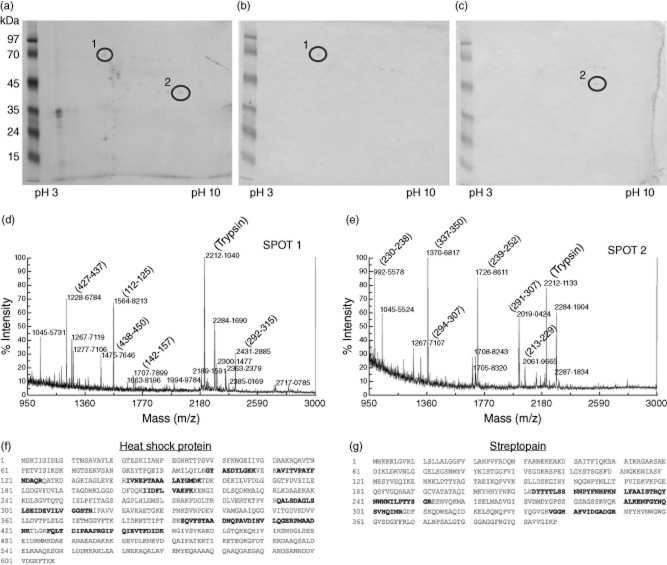
To demonstrate the cross-reactivity of purified anti-vimentin antibodies with streptococcal protein, we analysed their reactivity in ELISA after pre-absorption overnight with streptococcal protein extract (5, 10 and 20 μg/ml). We observed a dose-dependent inhibition of reactivity (Fig. 2c). To identify shared epitopes between human vimentin and streptococcal antigens, we performed an immunoproteomic analysis. Streptococcal protein extract was run in 2DE (Fig. 3a) and immunoblotting was performed with 20 μg/ml of purified anti C-ter and anti N-ter vimentin antibodies (Fig. 3b,c). The spots identified by anti-C-ter and anti-N-ter vimentin antibodies, analysed by MALDI-TOF MS, corresponded to the streptococcal HSP70 (Fig. 3d,f) and streptococcal streptopain (STRP1) (Fig. 3e,g), respectively.
Figure 3.
Identification of streptococcal proteins cross-reactive with human vimentin. Streptococcal extracts run in two-dimensional electrophoresis (2DE) (a) were immunoblotted and analysed by 20 μg/ml of purified anti C-ter vimentin antibodies (spot 1, b) and anti N-ter vimentin antibodies (spot 2, c). Spot 1 of 70 kDa and spot 2 of 45 kDa were excised by gel and analysed by matrix-assisted laser desorption ionization (MALDI) time-of-flight mass spectrometry (d,e). The analysis of the spectra allowed the identification, respectively, of the streptococcal heat shock protein 70 (HSP70, NP_665335) with score 102, and the streptococcal streptopain (STRP1, P0C0J1) with score 71: the matching peptides are shown in bold in the respective sequences (f,g).
Using bioinformatic software, we aligned the amino acid sequence of the C-and N-ter vimentin with the identified streptococcal proteins HSP70 and STRP1, respectively. These analyses showed one peptide from streptococcal HSP70 (peptide A) to present elevated homology with the amino acid sequence of the C-ter vimentin and one peptide from STRP1 (peptide B) highly homologous to N-ter vimentin (Fig. 4a).
Figure 4.
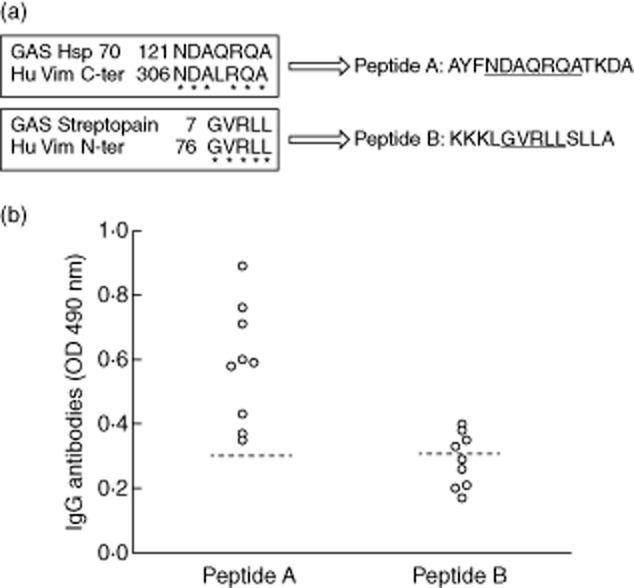
Identification and immunological characterization of the streptococcal peptides homologous to human vimentin. (a) Sequence homology between streptococcal heat shock protein (HSP)70 and C-ter human vimentin and between streptococcal STRP1 and N-ter human vimentin revealed two peptides named, respectively, peptides A and B (*identical amino acid). (b) Enzyme-linked immunosorbent assay (ELISA) using peptides as antigens with sera diluted 1:100 revealed that all nine sera from rheumatic heart disease (RHD) patients, positive to the C-ter vimentin, reacted with peptide A and four of nine sera from RHD patients reacted positive to the N-ter vimentin resulted positive to peptide B. The broken line represents the cut-off (mean ± 2 standard deviations for healthy donors). Results are expressed as absorbance at 490 nm.
To analyse the presence of serum cross-reactive antibodies, these two peptides were used as antigens in ELISA. All nine sera from RHD patients, positive to the C-ter vimentin, reacted with peptide A, and four of nine sera from RHD patients positive to the N-ter vimentin resulted positive to peptide B (Fig. 4b). Anti-peptides A and B antibodies were obtained from rabbit sera after immunization with the two peptides conjugated with BSA as molecular carrier. To confirm further the antibody cross-reactivity between streptococcal peptides and human vimentin, we pre-absorbed purified anti-peptides antibodies with human recombinant vimentin at different concentrations (5–20 μg/ml). We observed dose-dependent inhibition in ELISA (data not shown), reaching 100% with the maximal concentration of vimentin.
Effects of the anti-streptococcal peptide antibodies on microvascular cardiac endothelial cells
Purified anti-peptides A and B antibodies, obtained from rabbit sera by immunization with the two peptides conjugated with BSA as molecular carrier, were used for endothelial cell treatment. The cell-surface binding of anti-peptide antibodies on the endothelial cells was confirmed by FACS analysis (Supplementary Fig. S1). Pre-absorption of these antibodies with human vimentin or streptococcal protein extract completely prevents their reactivity.
Cell lysates from HMVEC-C cells, either unstimulated or stimulated with anti-peptides A and B, anti-BSA antibodies or LPS, were studied for IRAK1 phosphorylation upon Western blot analysis. Anti-peptides A and B antibodies, as well as LPS, were able to induce IRAK1 phosphorylation, as revealed by anti-phospho-IRAK1 reactivity (Fig. 5a). The pre-absorption of anti-peptide antibodies with human vimentin or streptococcal protein extract inhibited IRAK1 phosphorylation. As IRAK phosphorylation leads to NF-κB activation [19], we investigated the effects of anti-peptides A and B antibodies on p65 NF-κB in HMVEC-C cells. As expected, anti-peptides A and B antibodies, as well as LPS, induced NF-κB phosphorylation, as revealed by anti-phospho-p65 NF-κB reactivity (Fig. 5b). Conversely, rabbit anti-BSA antibodies showed no effect on stimulated cells.
Figure 5.
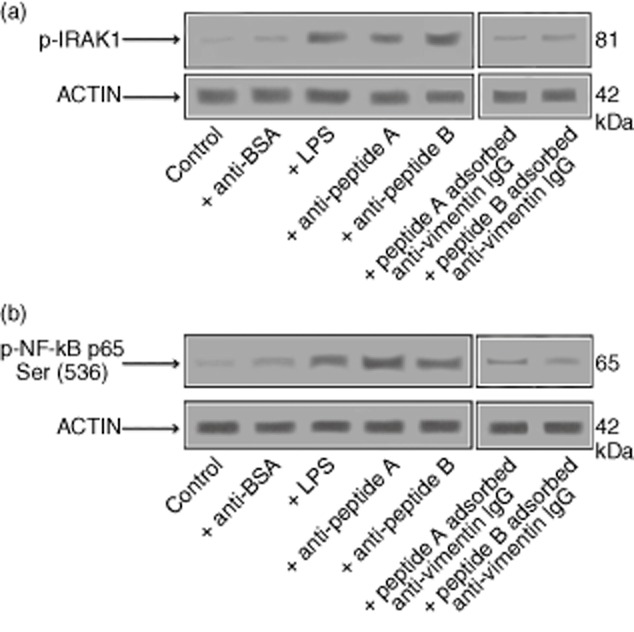
Anti-streptococcal peptides antibodies activate interleukin (IL)-1R-associated kinase (IRAK)1 and nuclear factor (NF)-κB in endothelial cells. Human microvascular cardiac endothelial cells (HMVEC)-C cells either unstimulated or stimulated with purified anti-peptides A and B (200 μg/ml), anti-bovine serum albumin (BSA) antibodies (100 μg/ml) or lipopolysaccharide (LPS) (100 ng/ml) were analysed by Western blot for IRAK1 phosphorylation and NF-κB activation. (a) Phosphorylated levels of IRAK1 (p-IRAK1) were analysed in whole cell extracts by Western blot with anti-phospho-IRAK1 antibodies; for control, the blotted membranes were reprobed with anti-actin antibodies. Bound antibodies were visualized with horseradish peroxidase (HRP)-conjugated immunoglobulin (Ig)G and immunoreactivity was assessed by enhanced chemiluminescence (ECL). Inhibition of IRAK1 phosphorylation by treating HMVEC-C cells with purified anti-peptides A and B (200 μg/ml) pre-absorbed with human vimentin (20 μg/ml) is also shown; (b) NF-κB activation was analysed in whole cell extracts by Western blot with anti-phospho-NF-κB p65 Ser antibodies; as control, the blotted membranes were reprobed with anti-actin antibodies. Bound antibodies were visualized with HRP-conjugated IgG and immunoreactivity was assessed by ECL. Inhibition of NF-κB activation by treating HMVEC-C cells with purified anti-peptide A and anti-peptide B (200 μg/ml) pre-absorbed with human vimentin (20 μg/ml) is also shown.
To analyse cytokines, chemokines, adhesion molecules, growth factors and TF production, we analysed cell culture supernatants after 1, 6, 12 and 24 h of exposure to anti-peptides A and B antibodies. The comparison with the corresponding values (optical density or fluorescence intensity) obtained after anti-BSA stimulus revealed a significant increase of IL-6, IL-8, platelet-derived growth factor (PDGF), monocyte chemoattractant protein-1 (MCP-1), VCAM-1 and TF in response to anti-peptide A antibody stimulus and a significant increase of IL-8, VCAM-1 and TF in response to anti-peptide B antibody (Fig. 6).
Figure 6.
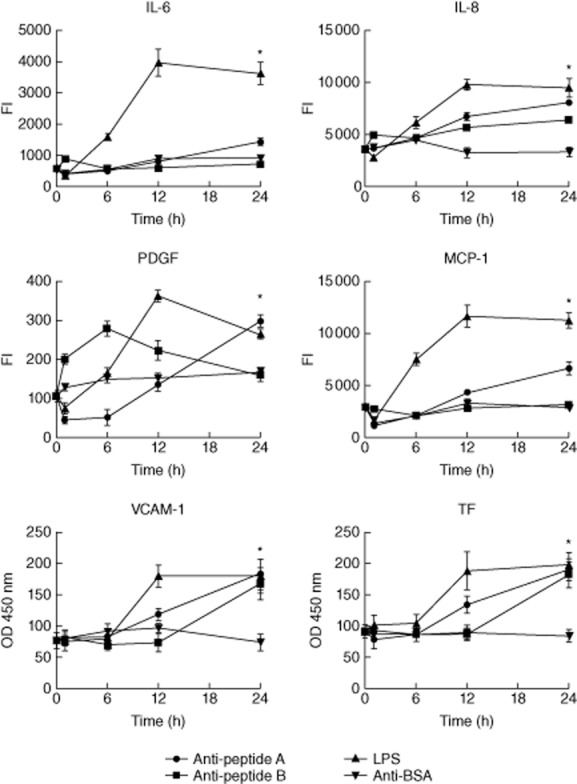
Anti-streptococcal peptide antibodies induce cytokines, chemokines, growth factors, adhesion molecules and tissue factor (TF) production in endothelial cells. In order to determine concentration of a panel of cytokines, chemokines, growth factor adhesion molecules and TF, enzyme-linked immunosorbent assay (ELISA) or multiplex analysis of culture supernatants from culture supernatants of human microvascular cardiac endothelial cells (HMVEC)-C cells either unstimulated or stimulated with purified anti-peptides A and B (200 μg/ml), anti-bovine serum albumin (BSA) antibodies (100 μg/ml) or lipopolysaccharide (LPS) (100 ng/ml) was performed. The supernatants were harvested and analysed after 1, 6, 12 and 24 h. The comparison with the corresponding values (optical density or fluorescence intensity) obtained after anti-BSA stimulus revealed a significant increase of interleukin (IL)-6, IL-8, platelet-derived growth factor (PDGF), monocyte chemoattractant protein (MCP)-1, vascular cell adhesion molecule (VCAM)-1 and TF in response to anti-peptide A antibody stimulus and a significant increase of IL-8, VCAM-1 and TF in response to anti-peptide B antibody. *P < 0·05.
Discussion
It is well known that molecular mimicry between cardiac tissues and GAS antigens might play a key role in the pathogenesis of RHD [5,12–14,20]. Recently, we documented evidence of the presence of AECA in patients with RHD [7]. In the present study we identified vimentin as a strongly immunoreactive autoantigen recognized by AECA in RHD patients. In particular, we detected anti-vimentin antibodies in sera from 49% RHD AECA-positive patients, whereas no anti-vimentin antibodies were found in sera from healthy donors. Vimentin is a cytoskeleton intermediate filament protein expressed ubiquitously. Surface-expressed forms of vimentin have been observed on several cell types, including vascular endothelial cells, and anti-endothelial vimentin antibodies have been demonstrated in several diseases [21,22]. Previous studies showed the presence of antibody cross-reactivity between streptococcal protein M and structural α-helix coiled-coil domain shared by many proteins of cardiac valvular tissue, including vimentin [6]. In particular, antibodies to N-acetyl-beta-D-glucosamine (GlcNAc) reacted with cytoskeletal and heart proteins (actin, keratin, myosin and vimentin), as well as with streptococcal recombinant M5 and M6 proteins [23]. Moreover, infiltrating autoreactive T lymphocytes specific to vimentin have been observed in valvular tissue [24].
Keeping in mind the goal of identifying the streptococcal antigenic target cross-reactive with vimentin, we analysed anti-vimentin antibodies purified from sera of RHD patients with GAS proteins by immunoproteomic analysis. These antibodies recognized two streptococcal proteins: HSP70 and STRP1. HSP70 is a ubiquitously expressed component of the chaperonine family involved in maturation or degradation of proteins [25]. STRP1 is a cysteine protease expressed by all GAS strains isolated from patients with RHD and is an important virulence factor in Streptococcus infection [26,27]. Hence, an immune response against these streptococcal antigens could trigger antibodies cross-reactive with human vimentin through the mechanism of molecular mimicry.
Furthermore, aligning the amino acid sequence of streptococcal HSP70 and STRP1 with human vimentin, we identified two highly homologous peptides that were recognized by serum antibodies of RHD patients. We stimulated HMVEC-C with the cross-reactive antibodies; in order to prove their pathogenic role, we immunized rabbits with streptococcal HSP70 and STRP1 peptides and stimulated HMVEC-C with the obtained antibodies. A proinflammatory activation of HMVEC-C through IRAK-mediated signal transduction and NF-κB activation was induced, supporting the pathogenetic role of the antibodies [19,28]. As a consequence of this activation, increased expression levels of VCAM-1, TF, PDGF, MCP-1, IL-8 and IL-6 were observed in supernatants of endothelial cells. VCAM-1, an adhesion molecule expressed on endothelial cells, can enable infiltration of leucocytes by rolling and diapedesis. As a consequence, inflammatory status leads to endothelial activation with up-regulation of VCAM-1 and its release as soluble form [29]. Interestingly, high levels of VCAM-1 have been demonstrated in valvular tissue of patients with RHD [30].
TF is a glycoprotein expressed on the surface of many cells and is involved in triggering coagulation [31]. Endothelial cells do not express TF except after endothelial perturbation. As TF is a transmembrane protein it is unlikely to be released into circulation, unless great damage takes place. PDGF has been implicated in cardiac fibrosis and in the hypertrophy of intima that occurs in atherosclerosis [32], whereas the proinflammatory IL-6 may promote an increase of endothelial permeability [33]; it is noteworthy that MCP-1 and IL-8 have a chemoattractive action on CD4+ T lymphocytes, monocytes, basophils and eosinophils [34–37]. Thus, the in-vitro demonstration that cross-reactive GAS-vimentin antibodies induce cardiac endothelial cells to release all the above-mentioned factors confirms further the proinflammatory role of these antibodies in the pathogenic scenario of RHD. Inhibition of the binding of anti-streptococcal peptides antibodies on the endothelial cell-surface, obtained by human vimentin pre-absorption, supported their specificity to this protein, even though we cannot exclude the possibility that there were other cross-reactive antigens on the surface of the endothelium.
In conclusion, in this study we have added new information to the mechanisms of molecular mimicry that induce cardiac failure in RHD by identifying new epitopes of streptococcal antigens that cross-react to endothelial vimentin. These GAS-vimentin cross-reactive antibodies are able to activate valvular endothelium, amplifying the autoinflammatory response which culminates in RHD. Stemming from these findings, further studies are necessary in order to investigate the potential use of anti-peptide antibodies as prognostic markers and therapeutic targets so that they can be translated into clinical practice.
Acknowledgments
The authors thank Dr R. Creti for the kind gift of group A Streptococcus samples. This work was supported by a research grant from the Italian Ministry of Health (to E.O.) and by grants from Sapienza Università di Roma and Fondazione Umberto di Mario ONLUS (to C.A and G.V.).
Disclosure
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Representative flow cytometry histograms showing the fluorescence intensity of fluorescein isothiocyanate (FITC)-labelled anti-peptide A (a) and anti-peptide B (b) antibodies compared to that of control staining pre-adsorbed or not with recombinant human vimentin (R&D Systems) (20 μg/ml). Dotted lines represent control staining; black lines represent anti-peptide A or anti-peptide B-labelled cells, and grey lines represent anti-peptide A or anti-peptide B-labelled cells pre-adsorbed with human vimentin.
References
- 1.Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on cardiovascular Disease in the Young of the American Heart Association. Guidelines for the diagnosis of rheumatic fever. Jones criteria, 1992 update. JAMA. 1992;268:2069–2073. [PubMed] [Google Scholar]
- 2.Smith MT, Zurynski Y, Lester-Smith D, Elliott E, Carapetis J. Rheumatic fever – identification, management and secondary prevention. Aust Fam Physician. 2012;41:31–35. [PubMed] [Google Scholar]
- 3.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 4.Marijon E, Ou P, Celermajer DS, et al. Prevalence of rheumatic heart disease detected by echocardiographicic screening. N Engl J Med. 2007;357:470–476. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guilherme L, Kalil J. Rheumatic fever and rheumatic heart disease: cellular mechanisms leading autoimmune reactivity and disease. J Clin Immunol. 2010;30:17–23. doi: 10.1007/s10875-009-9332-6. [DOI] [PubMed] [Google Scholar]
- 7.Scalzi V, Hadi HA, Alessandri C, et al. Anti-endothelial cell antibodies in rheumatic heart disease. Clin Exp Immunol. 2010;161:570–575. doi: 10.1111/j.1365-2249.2010.04207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renaudineau Y, Grunebaum E, Krause I, et al. Anti-endothelial cell antibodies (AECA) in systemic sclerosis – increased sensitivity using different endothelial cell substrates and association with other autoantibodies. Autoimmunity. 2001;33:171–179. doi: 10.3109/08916930109008045. [DOI] [PubMed] [Google Scholar]
- 9.Frampton G, Jayne DR, Perry GJ, Lockwood CM, Cameron JS. Autoantibodies to endothelial cells and neutrophil cytoplasmic antigens in systemic vasculitis. Clin Exp Immunol. 1990;82:227–232. doi: 10.1111/j.1365-2249.1990.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alessandri C, Bombardieri M, Valesini G. Pathogenic mechanisms of anti-endothelial cell antibodies (AECA): their prevalence and clinical relevance. Adv Clin Chem. 2006;42:297–326. doi: 10.1016/S0065-2423(06)42008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horváthová M, Jahnová E, Nyulassy S. Detection of antiendothelial cell antibodies in patients with connective tissue disease by flow cytometry and their relation to endothelial cell activation. Physiol Res. 2002;51:613–617. [PubMed] [Google Scholar]
- 12.Luo YH, Chuang WJ, Wu JJ, et al. Mimicry of SPE B and endothelial cells. Lab Invest. 2010;90:1492–1506. doi: 10.1038/labinvest.2010.93. [DOI] [PubMed] [Google Scholar]
- 13.Galvin JE, Hemric ME, Ward K, Cunningham MW. Cytotoxic monoclonal antibody from rheumatic carditis reacts with human endothelium: implications in rheumatic heart disease. J Clin Invest. 2000;106:217–224. doi: 10.1172/JCI7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulizia JM, Cunningham MW, McManus BM. Immunoreactivity of anti-streptococcal monoclonal antibodies to human heart valves: evidence for multiple cross-reactive epitopes. Am J Pathol. 1990;138:285–301. [PMC free article] [PubMed] [Google Scholar]
- 15.Margutti P, Matarrese P, Conti F, et al. Autoantibodies to the C-terminal subunit of RLIP76 induce oxidative stress and endothelial cell apoptosis in immune-mediated vascular diseases and atherosclerosis. Blood. 2008;111:4559–4570. doi: 10.1182/blood-2007-05-092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 17.Margutti P, Delunardo F, Sorice M, et al. Screening of a HUAEC cDNA library identifies actin as a candidate autoantigen associated with carotid atherosclerosis. Clin Exp Immunol. 2004;137:209–215. doi: 10.1111/j.1365-2249.2004.02491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delunardo F, Conti F, Margutti P, et al. Identification and characterization of the carboxy-terminal region of Sip-1, a novel autoantigen in Behçet's disease. Arthritis Res Ther. 2006;8:R71. doi: 10.1186/ar1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raschi E, Testoni C, Bosisio D, et al. Role of the MyD88 transduction signalling pathway in endothelial activation by antiphospholipid antibodies. Blood. 2003;101:3495–3500. doi: 10.1182/blood-2002-08-2349. [DOI] [PubMed] [Google Scholar]
- 20.Guilherme L, Ramasawmy R, Kalil J. Rheumatic fever and rheumatic heart disease: genetics and pathogenesis. Scand J Immunol. 2007;66:199–207. doi: 10.1111/j.1365-3083.2007.01974.x. [DOI] [PubMed] [Google Scholar]
- 21.Xu B, deWaal RM, Mor-Vaknin N, Hibbard C, Markovitz DM, Kahn ML. The endothelial cell-specific antibody PAL-E identifies a secreted form of vimentin in the blood vasculature. Mol Cell Biol. 2004;24:9198–9206. doi: 10.1128/MCB.24.20.9198-9206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortona E, Capozzi A, Colasanti T, et al. Vimentin/cardiolipin complex as a new antigenic target of the antiphospholipid syndrome. Blood. 2010;116:2960–2967. doi: 10.1182/blood-2010-04-279208. [DOI] [PubMed] [Google Scholar]
- 23.Shikhman AR, Greenspan NA, Cunningham MW. A subset of murine monoclonal antibodies crossreactive with cytoskeletal proteins and group A streptococcal M protein recognize N-acetyl-β-D-glucosamine. J Immunol. 1993;151:3902–3913. [PubMed] [Google Scholar]
- 24.Faé KC, Diefenbach da Silva D, Bilate AM, et al. PDIA3, HSPA5 and vimentin, proteins identified by 2-DE in the valvular tissue, are the target antigens of peripheral and heart infiltrating T cells from chronic rheumatic heart disease patients. J Autoimmun. 2008;31:136–141. doi: 10.1016/j.jaut.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 26.Burns EH, Lukomski S, Jr, Rurangirwa J, Podbielski A, Musser JM. Genetic inactivation of the extracellular cysteine protease enhances in vitro internalization of group A streptococci by human epithelial and endothelial cells. Microb Pathog. 1998;24:333–339. doi: 10.1006/mpat.1998.0204. [DOI] [PubMed] [Google Scholar]
- 27.Ohara-Nemoto Y, Sasaki M, Kaneko M, Nemoto T, Ota M. Cysteine protease activity of streptococcal pyrogenic exotoxin B. Can J Microbiol. 1994;40:930–936. doi: 10.1139/m94-149. [DOI] [PubMed] [Google Scholar]
- 28.Colasanti T, Alessandri C, Capozzi A, et al. Autoantibodies specific to a peptide of β2-glycoprotein I cross-react with TLR4 inducing a pro-inflammatory phenotype in endothelial cells and monocyte. Blood. 2012;120:3360–3370. doi: 10.1182/blood-2011-09-378851. [DOI] [PubMed] [Google Scholar]
- 29.Golias C, Tsoutsi E, Matziridis A, Makridis P, Batistatou A, Charalabopoulos K. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. In Vivo. 2007;21:757–769. [PubMed] [Google Scholar]
- 30.Roberts S, Kosanke S, Terrence Dunn S, Jankelow D, Duran CM, Cunningham MW. Pathogenic mechanisms in rheumatic carditis: focus on valvular endothelium. J Infect Dis. 2001;183:507–511. doi: 10.1086/318076. [DOI] [PubMed] [Google Scholar]
- 31.Steffel J, Lüscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 32.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maruo N, Morita I, Shirao M, Murota S. IL-6 increases endothelial permeability in vitro. Endocrinology. 1992;131:710–714. doi: 10.1210/endo.131.2.1639018. [DOI] [PubMed] [Google Scholar]
- 34.Xia M, Sui Z. Recent developments in CCR2 antagonists. Exp Opin Ther Pat. 2009;19:295–303. doi: 10.1517/13543770902755129. [DOI] [PubMed] [Google Scholar]
- 35.Angiolillo AL, Sgadari C, Taub DD, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnaswamy G, Smith JK, Mukkamala R, et al. Multifunctional cytokine expression by human coronary endothelium and regulation by monokines and glucocorticoids. Microvasc Res. 1998;55:189–200. doi: 10.1006/mvre.1998.2079. [DOI] [PubMed] [Google Scholar]
- 37.Gerszten RE, Garcia-Zepeda EA, Lim YC, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative flow cytometry histograms showing the fluorescence intensity of fluorescein isothiocyanate (FITC)-labelled anti-peptide A (a) and anti-peptide B (b) antibodies compared to that of control staining pre-adsorbed or not with recombinant human vimentin (R&D Systems) (20 μg/ml). Dotted lines represent control staining; black lines represent anti-peptide A or anti-peptide B-labelled cells, and grey lines represent anti-peptide A or anti-peptide B-labelled cells pre-adsorbed with human vimentin.