Summary
There is increasing evidence that inflammation in the synovium plays a major role in the progression of osteoarthritis (OA). However, the immunogenic properties of mesenchymal stromal cells (MSCs), which are considered to regulate immunity in various diseases, remain largely unknown in OA. The purpose of this study was to determine the influence of MSCs from OA patients on regulatory T cells (Tregs) in an allogeneic co-culture model. Bone marrow (BM) and synovial membrane (SM) were harvested from hip joints of OA patients and co-cultured with lymphocytes enriched in CD4+CD25+CD127– regulatory T cells (Treg+LC) from healthy donors. Treg proportions and MSC markers were assessed by flow cytometry. Cytokine levels were assessed after 2 and 5 days of co-cultivation. Additionally, Treg+LC cultures were analysed in the presence of interleukin (IL)-6 and MSC-supernatant complemented medium. B-MSCs and S-MSCs were able to retain the Treg proportion compared to lymphocyte monocultures. T cell–MSC co-cultures showed a significant increase of IL-6 compared to MSC cultures. S-MSCs produced higher amounts of IL-6 compared to B-MSCs, both in single and T cell co-cultures. The effect of retaining the Treg percentage could be reproduced partially by IL-6 addition to the medium, but could only be observed fully when using MSC culture supernatants. Our data demonstrate that retaining the Treg phenotype in MSC–T cell co-cultures can be mediated by MSC derived from OA patients. IL-6 plays an important role in mediating these processes. To our knowledge, this study is the first describing the interaction of MSCs from OA patients and Tregs in an allogeneic co-culture model.
Keywords: co-culture, IL-6, inflammation, mesenchymal stromal cells, osteoarthritis, regulatory T cells
Introduction
While, in the past, osteoarthritis (OA) has generally been considered a ‘non-inflammatory’ disease, numerous recent studies have suggested that inflammation may play an important role in its progression [1–3]. These inflammatory processes take place in the synovial membrane [4], and are characterized by lymphocyte and macrophage invasion [5,6] and elevated proinflammatory cytokines [7].
Because there are currently no therapeutic approaches to halt OA progression, much hope has been expressed regarding the development of new therapeutic strategies, including cell-based approaches. In this context, mesenchymal stem or stromal cells (MSCs) have been investigated extensively throughout the past two decades mainly for their regenerative potential [8–10]. Their immunosuppressive competence has, however, become another important field of research (overview in [11] and [12]). Therefore, MSCs have been investigated in animal models of multiple sclerosis [13], pulmonary fibrosis [14], renal failure [15] and myocardial infarction [16]. In a clinical setting, MSCs have been used successfully as an immunosuppressive treatment in patients with severe graft-versus-host disease [17].
MSCs were also identified to play a crucial role in modulating the inflammatory processes in rheumatoid arthritis [18]. In an animal model of collagen-induced arthritis, MSCs reduced inflammation significantly in the joints by reducing proliferation and modulating cytokine expression [19].
The mechanisms of MSC-mediated immunosuppression are unclear and still controversial [20,21], while representing a promising target of cell-based therapies in diseases with important inflammatory processes. MSCs have been proved to suppress T cell proliferation successfully both in vitro and in vivo [22,23]. Recent studies have also shown that MSCs regulate and recruit regulatory T cells (Tregs) in a co-culture approach [24–26]. Tregs themselves have been identified as key players in numerous diseases, among them rheumatoid arthritis [2,27]; however, until recently they have not been associated with OA pathogenesis [28,29].
Although an important number of Phase I/II studies using MSCs in OA have been started (overview on [30]) and these cells have already been used in small patient series [31], the underlying processes of both the regenerative properties and, more importantly, the immunosuppressive capacities of MSCs in OA, are only poorly understood.
The aim of this study, therefore, was to analyse the effect of human MSCs from OA patients on Tregs in an allogeneic lymphocyte co-culture model. We compared MSCs derived from the bone marrow of a joint-adjacent bone and from the synovium of the affected joint to investigate whether the synovial MSCs located within the tissue affected by inflammation exerted different immunomodulatory properties.
Methods
Patients
MSCs were isolated from bone marrow and synovial membrane of 34 patients (age 68 ± 12 years, 19 female and 15 male) that had been collected during total hip arthroplasty for primary OA Kellgren grades III and IV. Exclusion criteria for these patients were a history of cancer, rheumatic diseases and acute or chronic infections.
Lymphocytes were extracted from whole blood samples of 16 young healthy donors (28 ± 7 years, five female and 11 male). Exclusion criteria for these donors were a history of cancer, rheumatic diseases, acute and chronic infections, cartilage injury and OA.
The study protocol was approved by the ethics committee of the University of Heidelberg, Germany. Both patients and blood donors provided informed consent. The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
MSCs
Mononuclear cells (MNCs) were isolated from bone marrow samples by Ficoll Paque plus (GE Healthcare, Uppsala, Sweden) gradient centrifugation. MNCs were then resuspended in culture medium at a density of 5 × 105 cells/cm2 (= 2·5 × 106 cells/ml). Culture medium contained Dulbecco's modified Eagle's medium low glucose (DMEM-LG; Invitrogen, Karlsruhe, Germany), supplemented with 10% fetal calf serum (FCS; Biochrom, Berlin, Germany) and 1% penicillin/streptomycin (Invitrogen). The cells were cultured in 175 cm2 cell culture flasks (Nunc, Roskilde, Denmark) at 37°C with 6% CO2 in a humidified atmosphere. After 24 h, with the first media exchange, non-adherent cells were discarded; afterwards, medium replacement was carried out every 72 h until the cells reached an 80% confluent layer. The cells were then detached with trypsin (Biochrom), washed with complete medium and counted (trypan blue 0·4%; Sigma-Aldrich, Steinheim, Germany). Afterwards, MSCs were replated and cultured under the conditions described above until reaching confluence at passage 2. The ability of MSC to differentiate into chondrogenic, adipogenic and osteogenic lineages was demonstrated according to protocols described previously [32].
MSCs were allogeneic to the lymphocytes in all co-culture experiments.
Lymphocytes
Peripheral blood mononuclear cells (PBMC) were collected from whole blood samples using Ficoll Paque plus (GE Healthcare, Uppsala, Sweden) gradient centrifugation. PBMC were then separated into a mixture of CD4+CD25– and CD4+CD25+CD127– cells using magnetic separation (CD4+CD25+CD127dim/– regulatory T cell Isolation Kit II, LS and LD columns, MidiMACS™ separator, all from Miltenyi Biotec, Bergisch Gladbach, Germany). The isolated cells were then analysed for CD4, CD25, CD127 and forkhead box protein 3 (FoxP3) (see below).
Co-culture assays
MSCs derived from bone marrow (B-MSCs) and synovium (S-MSCs) from 18 patients were co-cultured with CD4+ T cells enriched in Tregs for 5 days in DMEM-LG (Invitrogen) supplemented with 10% FCS (Biochrom) and 1% penicillin/streptomycin (Invitrogen). The cells were resuspended in 48-well plates, each well containing 1 ml of medium and cells in various concentrations: T cells/MSCs 4:3 (37 500 T cells/cm2 and 28 125 MSCs/cm2), 2:1 (37 500 T cells/cm2 and 18 750 MSCs/cm2) and 4:1 (37 500 T cells/cm2 and 9375 MSCs/cm2). Single-cell cultures of T cells (37 500 cells/cm2) and MSCs (28 125, 18 750, 9375 MSCs/cm2) were used as controls. At day 2, the well plates were centrifuged at 488 g for 10 min. Supernatants were collected for cytokine analysis (see below). For all cultures, the whole medium was then replaced. After 5 days of co-culture, supernatants were again collected as described above and analysed for cytokines (see below). The cells were then resuspended in phosphate-buffered saline (PBS; Invitrogen) with 0·5% FCS (Biochrom) and 2 mM ethylenediamine tetraacetic acid (EDTA) (Sigma-Aldrich). The lymphocytes were thus separated from the MSCs, washed and prepared for flow cytometry (see below). MSCs were detached with trypsin as described above, washed in whole medium and resuspended in PBS with 0·5% FCS and 2 mM EDTA. MSCs were then prepared for flow cytometry (see below).
Interleukin (IL)-6 and MSC supernatant supplemented lymphocyte cultures
CD4+ T cells enriched in Tregs were generated as described above by magnetic bead separation. The cells were resuspended in 48-well plates, each well containing 1 ml of medium (see above) and 50 000 T cells. In one group, the medium was supplemented with 5 ng/ml IL-6 (Miltenyi Biotec); in another, 10 ng/ml IL-6 was added to the medium. A third group was supplemented with supernatants from passage 2 bone marrow-derived MSCs cultured in DMEM-LG with 10% FCS and 1% penicillin/streptomycin. Cell cultures without supplementation to the media were used as controls. At day 2, the 48-well plates were centrifuged at 488 g for 10 min. Supernatants were collected and analysed for cytokines (see below). For all cultures, the whole medium was then replaced. After 5 days of culture, supernatants were collected as described above and analysed for cytokines (see below). The cells were then resuspended in PBS (Invitrogen) with 0·5% FCS and 2 mM EDTA (Sigma-Aldrich) and prepared for flow cytometry.
Flow cytometry analysis
One-colour cytometry (MSCs) and three-and four-colour cytometry (T cells) was performed using a MACS Quant™ analyser and MACS Quantify version 2.1 software (Miltenyi Biotec). Positive fluorescence was defined as any event above the background fluorescence, which was defined by a line where 99·5% of the events in isotype antibody-labelled cells were considered negative.
The following anti-human antibodies were used in the experiments: for T cell analysis, CD4 fluorescein isothiocyanate (FITC) mouse immunoglobulin (Ig)G1, CD25 phycoerythin (PE) or allophycocyanin (APC) mouse IgG2b (Miltenyi Biotec), CD127 APC or PE-Cy5 mouse IgG2a (BD Biosciences, Heidelberg, Germany). FoxP3 intracellular staining was performed with the FoxP3 staining buffer set and FoxP3-PE mouse IgG1 antibodies (BD Biosciences), according to the manufacturer's protocol. For MSC analysis, CD105 PE mouse IgG1 (Miltenyi Biotec), CD10 FITC mouse IgG1, CD13 PE mouse IgG1, CD14 FITC mouse IgG1, CD34 PE mouse IgG1, CD44 FITC mouse IgG2b, CD45 FITC mouse IgG1, CD49a PE mouse IgG1, CD90 FITC mouse IgG1, CD140b PE mouse IgG2a CD146 PE mouse IgG1, CD166 PE mouse IgG1 (BD Biosciences) and human leucocyte antigen (HLA)-ABC PE (Dako, Glostrup, Denmark) were used. Isotype-matched control antibodies were used for assessment of background fluorescence.
Cytokine analysis
Multiple simultaneous cytokine detection for IL-2, IL-4, IL-6, IL-10, IL-17a, tumour necrosis factor (TNF)-α and interferon (IFN)-γ was performed using the human T helper type 1 (Th1)/Th2/Th17 cytokine kit (BD Biosciences) on a MACS Quant™ analyser and MACS Quantify version 2.1 software (Miltenyi Biotec) as well as the FCAP Array software, version 1.0.1 (BD Biosciences). The assays were performed with undiluted supernatants and with supernatants diluted to 1:10 with PBS (Invitrogen). In addition, enzyme-linked immunosorbent assays (ELISA, n = 5 per group) were performed with commercial kits for detection of IL-1ra (BioSource Europe SA, Nivelles, Belgium) and IL-1β and IL-8 (Invitrogen Corporation, Camarillo, CA, USA), according to the manufacturers' protocols.
Statistical analysis
Statistical analysis was performed using spss software (SPSS Inc., released 2009; PASW Statistics for Windows, version 18.0; SPSS Inc., Chicago, IL, USA). One-way analyses of variance (anova) followed by Bonferroni adjustment were performed to compare the different groups of lymphocyte cultures after creating interindividual differences for each patient. Differences were considered statistically significant for P-values smaller 0·05. Results are shown as means ± standard deviation (s.d.).
Results
B-MSC and S-MSC retain CD127 down-regulation and up-regulate FoxP3 in an allogeneic lymphocyte co-culture
An important variation could be observed of Treg percentages after magnetic separation (Fig. 1a). Because of the donor-associated varying baseline Treg percentages before co-culture, intraindividual differences between the Treg percentages after single-and co-cultures at day 5 and the initial Treg percentage (day 0) were calculated in each group. There were no significant differences in CD4 expression (P = 0·522 between the groups) and in the percentages of CD4+CD25+ cells (P = 0·258) between the groups.
Figure 1.
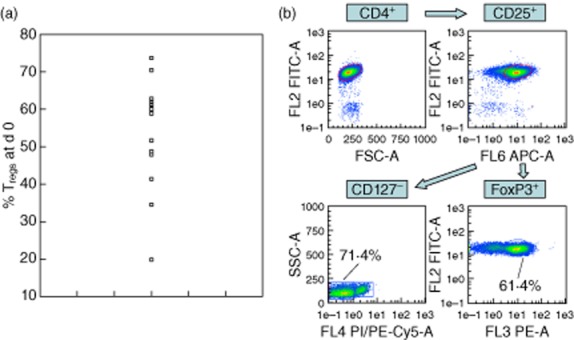
(a) CD4+CD25+CD127− Treg percentages of total CD4+ T cells at d0. Magnetic separation of CD4+CD25+CD127− cells was performed with each circle representing a single culture. Percentages varied from less than 20% to over 70%. (b) Gating strategy for regulatory T-cells (CD127−/FoxP3+).
Tregs were defined as CD4+CD25+CD127– or CD4+CD25+FoxP3 cells, respectively. The gating strategy is demonstrated in Fig. 1b. There was a negative correlation between CD127 and FoxP3 expression, the mean intraindividual difference between CD127– and FoxP3+ cells being 4·62 ± 6·31%.
Both B-MSC– and S-MSC–lymphocyte co-cultures showed no significant changes in the Treg proportion, while we observed a significant decrease in the proportion of Treg in T cell monoculture (Fig. 2). This was the case for CD4+CD25+CD127– cells (Fig. 2a, P < 0·001 for both T cell single-culture versus B-MSC/T cell co-culture and S-MSC/T cell co-culture) and CD4+FoxP3+ cells (Fig. 2b, P = 0·006 for T cell single-culture versus B-MSC/T cell and P = 0·005 versus S-MSC/T cell co-cultures). There were no statistical differences between S-MSC/T cell and B-MSC/T cell co-cultures regarding CD127 and FoxP3 expression. The MSC effect on Treg-enriched CD4+ lymphocyte culture was independent of the T cell : MSC ratio (Fig. 2c).
Figure 2.
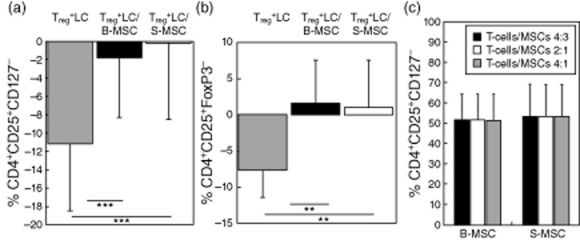
(a) Intraindividual differences between the baseline CD4+CD25+CD127− Treg percentage in Treg-enriched CD4+ lymphocyte cultures and the Treg percentages at d5. Treg+LC: CD4+ lymphocytes enriched in Tregs cultured alone. Treg+LC/B-MSC: CD4+ lymphocytes enriched in Tregs cultured with bone-marrow-derived MSC. Treg+LC/S-MSC: CD4+ lymphocytes enriched in Tregs cultured with synovium-derived MSC. Results are displayed as mean-SEM. ***P < 0.001. (b) Intraindividual differences between the baseline Treg (CD4+CD25+FoxP3+) percentage in Treg-enriched, CD4+ lymphocyte cultures and the Treg percentages at d5. Treg+LC: CD4+ lymphocytes enriched in Tregs cultured alone. Treg+LC/B-MSC: CD4+ lymphocytes enriched in Tregs cultured with bone-marrow-derived MSC. Treg+LC/S-MSC: CD4+ lymphocytes enriched in Tregs cultured with synovium-derived MSC. Results are displayed as mean-SD. **P < 0.01. (c) MSC effects on Treg percentages in cocultures with Treg enriched CD4+ lymhocytes are independent of MSC/lymphocyte ratio (4:3, 2:1, 4:1) in both B-MSC and S-MSC.
S-MSC and B-MSC express typical stem cell markers before and after allogeneic lymphocyte co-culture
Both S-MSCs and B-MSCs matched all criteria for MSCs according to the International Society for Cellular Therapy [33]; they were plastic-adherent and could be differentiated successfully into chondrogenic, osteogenic and adipogenic lineage (data not shown). Flow cytometry revealed the typical expression of mesenchymal stromal cell markers, MSCs being positive for CD90, CD105, CD73 and negative for CD45, CD34, CD14, among others. The surface marker profile of MSCs used in our experiments is shown in Table 1. There were no significant differences in surface profiles between B-MSC and S-MSC before co-culture, except for CD146, which showed very low expression levels on S-MSCs and was highly donor-dependent in B-MSCs.
Table 1.
Surface markers on bone marrow-and synovium-derived mesenchymal stromal cells before co-culture
| B-MSC | S-MSC | |
|---|---|---|
| CD90 | + | + |
| CD105 | + | + |
| CD73 | + | + |
| CD34 | − | − |
| CD45 | − | − |
| CD14 | − | − |
| CD10 | − | − |
| CD13 | + | + |
| CD44 | + | + |
| CD49a | + | + |
| CD140b | + | + |
| CD166 | + | + |
| HLA-ABC | + | + |
| CD146 | +/− | − |
B-MSC: bone marrow mesenchymal stromal cells; S-MSC: synovium mesenchymal stromal cells.
IL-6 release is increased in MSC–lymphocyte co-cultures
Cytometric bead array for several cytokines (n = 10 for day 2 and n = 5 for day 5) revealed high levels of IL-6 in cultures with MSCs, while IL-2, 4, TNF-α and IFN-γ were not detectable both in diluted and undiluted supernatants; IL-10 and IL-17a could be detected only sporadically in some supernatants without differences among the groups (data not shown). Neither IL-1ra, IL-1β nor IL-8 were detectable in the supernatants.
CD4+ T cells enriched in Tregs showed no significant IL-6 production when compared to co-cultures of S-MSCs and T cells and S-MSC single cultures (P < 0·001 for comparison with S-MSC single-cell and T cell co-cultures at day 2, P < 0·05 for comparison of S-MSC single-cell cultures and P < 0·001 for comparison of S-MSC/T cell co-cultures at day 5, Fig. 3a,b). IL-6 production in S-MSCs was significantly higher than in B-MSC cultures at day 2 (P < 0·001, Fig. 3a) and significantly higher in S-MSC/T cell co-cultures than in S-MSCs cultured alone (P = 0·01). At day 5, we observed an important decrease of IL-6 production in all groups, while the IL-6 quantity remained significantly higher in S-MSC/T cell co-cultures when compared to B-MSC/T cell co-cultures (P = 0·006; Fig. 3b).
Figure 3.
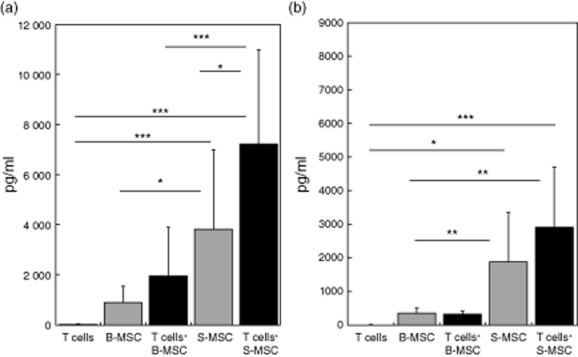
(a) IL-6 levels in the different groups at d2 of culture. T-cells in single culture showed no significant IL-6 production. S-MSC cultures showed significantly higher IL-6 levels compared to B-MSC at d2 and d5. *P < 0.05, ***P < 0.001. (b) IL-6 levels in the different groups at d5 of culture. IL-6 production was drastically reduced at d5 compared to d2. **P < 0.01.
MSC supernatants retain the Treg proportions in Treg+ lymphocyte cultures
In order to determine whether or not the effects of MSCs on Tregs in co-culture could be reproduced by IL-6, CD4+ lymphocyte cultures enriched in Tregs were supplemented either with 5 ng/ml IL-6, 10 ng/ml IL-6 or supernatants from B-MSC cultures in passage 2. To assess the effective IL-6 concentrations in our supplemented media, IL-6 concentrations were analysed by cytometric bead array at days 2 and 5 of lymphocyte culture. The effective concentrations at both time-points were reduced to approximately a third of the initially administered concentrations (Table 2). However, in both the 5 ng/ml and the 10 ng/ml supplemented groups, the natural IL-6 level found in the B-MSC supernatants had been surmounted effectively.
Table 2.
Mean interleukin (IL)-6 concentrations after days 2 and 5 of regulatory T cell (Treg)+ lymphocyte culture
| T cells | T cells+ 5 ng/ml IL-6 | T cells+ 10 ng/ml IL-6 | T cells+ B-MSC supernatants | |
|---|---|---|---|---|
| Mean IL-6 concentration (pg/ml) day 2 | 31·21 ± 26·49 | 1851·5 ± 312·44 | 3117·34 ± 717·3 | 895·09 ± 118·9 |
| Mean IL-6 concentration (pg/ml) day 5 | 9·95 ± 15·81 | 1812·52 ± 416·45 | 3748·6 ± 923·12 | 694·32 ± 254·71 |
B-MSC: bone marrow mesenchymal stromal cells. The effective concentrations at these time-points were approximately three times lower than the originally administered concentration. Results are displayed as means ± standard deviation.
Figure 4a,b shows the effects of IL-6 and B-MSC supernatant supplementation on the CD4+ cultures. We could detect a significant decrease of the Treg proportion in non-supplemented T cell cultures compared to both the initial Treg percentage (P < 0·001, Fig. 4a) and T cell cultures supplemented with MSC supernatant (P = 0·003; Fig. 4a). There was no change in the CD4+ percentages between the groups (Fig. 4b).
Figure 4.
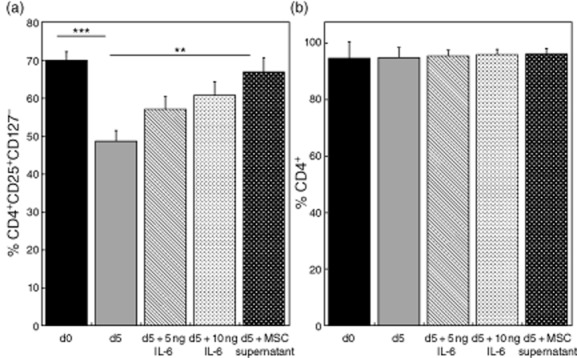
(a) Effects of IL-6 and B-MSC supernatants on CD4+ lymphocytes enriched in Tregs (CD4+CD25+CD127−). d0: baseline Treg percentage in CD4+ cultures before experiments. d5: Treg percentage in lymphocyte cultures without media supplementation. d5 + 5 ng/ml/d5 + 10 ng/ml: Treg percentage in lymphocyte cultures with 5 or 10 ng/ml IL-6 supplementation. d5 + MSC supernatant: Treg percentage in lymphocyte cultures with supplementation of B-MSC cultures derived supernatants. **P < 0.01, ***P < 0.001. (b) Effects of IL-6 and B-MSC supernatants on CD4+ lymphocytes enriched in Tregs. No significant differences in the percentage of CD4+ were observed between the groups.
This effect seemed to be partially reproducible by IL-6 at concentrations of 5 and 10 ng/ml; however, the full effect of retaining the Treg phenotype was observed only with MSC supernatants.
Discussion
Our findings show that mesenchymal stromal cells from OA patients modulate T cells effectively, maintaining a regulatory phenotype in an allogeneic co-culture approach with T cells from young and healthy donors. We chose to use this approach in order to attribute findings in the co-culture to MSCs connected to the disease rather than using Tregs from OA patients who also may have been preconditioned. Because of the unique ability of MSCs to escape allorecognition [34], allogeneic co-cultures are an adequate model for the investigation of MSC–lymphocyte interactions [24].
To our knowledge, this is the first study to report effective immunomodulatory capacities of MSCs from OA patients, and more specifically from OA synovium. MSC–Treg interactions have been reported in other contexts than OA, most importantly in transplantation immunology [35]; however, correlating these findings to OA remains a challenge in this early phase of research.
Interactions between MSCs and T cells
MSCs from healthy donors have been shown to recruit regulatory subsets from CD3+/CD45RA+ and CD3+/CD45RO+ fractions [24]. In these experiments, MSCs maintained FoxP3 expression and promoted CD127 down-regulation in purified Treg subsets. It is known that the suppressive effects of Tregs are lost when cultured ex vivo, and recent findings suggest that, with time, a shift of these cells will occur towards effector memory-like cells that produce IL-6, IL-17 and IFN-γ [36]. This effect can be prevented by co-culture with MSCs [25]. MSCs seem to not only promote CD4+ Treg generation, but also generation of CD8+ regulatory subsets [26].
In our experiments, we found that both FoxP3 expression and absence of CD127 expression was maintained in CD4+ T cells enriched in Tregs when co-cultured with MSCs. Our data thus support previous findings that FoxP3 is correlated inversely with CD127 expression [24,37]. The synovial MSCs were able to effectively maintain the Treg proportions comparable to B-MSCs. These findings suggest that MSCs from OA patients effectively retain the Treg subpopulation, but do not recruit Tregs from the CD4+ fraction, as in the study by di Ianni et al. [24]. Whether this is related to the disease remains to be identified in future experiments; however, the differences observed may also be due to variations in the experimental setting.
OA, synovium and MSC regeneration and immunomodulation potential
There is discussion as to whether OA affects MSC ability to differentiate into various tissues. The chondrogenic potential of MSCs has been reported to be reduced in advanced OA [38]; however, other studies suggest that the chondrogenic potential of MSCs from OA or rheumatoid arthritis patients is equal to MSC from healthy donors [39,40]. To this day, whether or not OA affects MSC immunomodulatory potential is unknown. We believe that we are the first to report that synovial MSCs from an OA-affected joint effectively maintain the Treg proportions in a lymphocyte co-culture, thus suggesting that the inflammatory milieu has no deteriorating effects on MSC immunoregulatory potential.
There has been growing evidence of distinct properties of synovial membrane-derived human mesenchymal stromal cells [41–43]. Some studies suggest that S-MSC may be discriminated from B-MSC by their transcriptional profiles [44]. Apart from a negative CD146 expression in S-MSCs, there were no differences regarding the surface markers between B-MSC and S-MSC in our study. Also, no differences in plastic adherence or differentiation potential between these cells were observed in our experiments. While the synovium is located in the centre of joint inflammation associated with OA [5,7], this did not seem to have an influence on the immunomodulatory properties of MSCs in our experiments. The elevated IL-6 production, however, suggests that S-MSC from OA patients exert distinct properties in this particular setting. The question of whether or not the higher IL-6 secretion by S-MSCs is caused by the inflammatory conditions in the joint cannot be answered from our data, but it must be an aim of future experiments to link the degree of synovial inflammation to IL-6 secretion and MSC immunomodulatory potential.
We have used an in-vitro model in our experiments; thus, it is difficult to draw any conclusions regarding the in-vivo situation. However, these are only initial findings stating that the interaction of MSCs and regulatory T cells may play a role in the osteoarthritic joint in vivo, as has been suggested for numerous other diseases, including rheumatoid arthritis [2,27]. Future experiments will need to determine whether these findings will allow the application of some of the therapeutic strategies for rheumatoid arthritis locally to the OA-affected joint.
Influence of IL-6 in MSC-Treg interaction
IL-6 was the predominant cytokine in the co-cultures, which is why we chose to supplement Treg-enriched lymphocyte cultures with this cytokine. Our data suggest that IL-6 plays a role in S-MSC-and B-MSC-mediated immunomodulation, as supplementation of IL-6 to the culture media was shown to partially reproduce the MSC-mediated Treg maintenance. To our knowledge, this is the first study to report that MSC from OA patients may exert some of their effects via IL-6 and thus may play an important role in shifting the balance of regulatory and effector T cells in OA.
The full effect of Treg maintenance by MSCs, as seen in the MSC–lymphocyte co-cultures, was observed in the group supplemented with MSC supernatants. In our opinion, MSC–Treg interaction therefore seems to be based on paracrine effects rather than on cell–cell interaction. However, other soluble factors, that remain to be detected, appear to be involved in these processes, although none of the other cytokines analysed in our experiments seem to be of major importance in this particular in-vitro setting.
The role of IL-6 in immunomodulation is discussed most controversially. IL-6, along with IL-8, has been shown to promote Treg migration to certain tumours [45] and may therefore play a crucial role in Treg signalling. A recent study in type II diabetes has also shown that the percentage of CD4(+)CD25(hi)CD127(–) Tregs are correlated positively with plasma IL-6 [46].
Mesenchymal stem cells from mice were shown to promote the differentiation of uncommitted naive T cells to FoxP3+ regulatory T cells [47]. This study has shown that the major cytokines involved in these processes were transforming growth factor (TGF)-β and IL-6 and that immunomodulation could be blocked by administration of anti-TGF-β or anti-IL-6, suggesting paracrine mechanisms. In another study in mice, excessive IL-6 production caused an increase in natural Tregs, which maintained their immunosuppressive capacities both in vitro and in vivo [48]. This suggests that IL-6 may be a key mediator in effector/regulatory T cell balance.
However, there are also data that are in contrast to our findings. In another study, using Tregs derived from the rheumatic synovium, blocking IL-6 or TNF increased the suppressive immunomodulatory capacities of these cells [49]. This study suggests that Treg function varies importantly with the inflammatory milieu in the joint.
These findings are not in accordance with our experiments, in which IL-6 maintained the percentage of Tregs. This discrepancy may be due to differences in the underlying pathologies, but also reflect the dual role of IL-6. However, the functional consequences remain to be identified in upcoming experiments. We have chosen to carry out our study on Tregs taken from healthy donors in order to provide information on the immunomodulatory capacities of MSCs and ruling out possible influences of OA on Tregs and thus on Treg–MSC interaction. An important question is whether or not Tregs taken from OA patients differ in their interaction with MSCs. Our findings therefore raise several questions that need to be verified by future research. Understanding the effects of MSCs in local joint inflammation in OA may pave the way for future cell-based approaches, as MSCs can be supposed to not only exert their regenerative potential when administered, but also intervene in local immunity.
Limitations
This study has several limitations. First, a comparison to MSCs taken from healthy donors would be useful to detect whether the inability to recruit Tregs from a CD4+ population, as has been shown by other groups, should be interpreted as a reduced capacity of immunomodulation in OA. This question has not yet been addressed due to ethical questions, as synovium from healthy individuals is not easily available. Taking synovium from a young population undergoing hip or knee arthroscopy might falsify the results, as it is known that cartilage injuries are found in up to 65% of the patients during arthroscopy, irrespective of the underlying pathology [50]. In our opinion, comparing healthy tissue to tissue derived from OA patients might reveal whether or not OA can be considered to be causing immunodepression in the joint synovium.
For this study, Tregs from healthy individuals were chosen in order to examine the effect of MSCs from OA patients on functional T lymphocytes. However, as stated above, it is necessary to conduct further research on how MSCs and Tregs taken from the same patients interact. In our experiments, blood volumes up to 150 ml were necessary to isolate a sufficient number of Tregs to conduct the co-culture experiments. While this is unproblematic for healthy individuals, in the context of a perioperative setting of a total hip arthroplasty with its high blood loss these volumes were considered too important to be taken from the OA patients. We are currently working on optimizing the isolation procedures as well as on methods that can provide Tregs from OA patients without taking important blood samples, such as collecting cells during the intraoperative autotransfusion procedure.
This study addressed only changes in phenotypical Treg properties and its important activation marker FoxP3. Our experiments cannot provide information on functional changes in Treg suppression potency. These experiments will need to be carried out in future to determine whether MSC immunomodulation has an effect on the functional properties of Tregs.
Joint inflammation may have differed among the patients recruited in this study; whether this has an effect on MSC immunomodulatory processes in vitro will need to be determined in future experiments. Therefore, it may be necessary to correlate inflammation in the synovium with the in-vitro immunomodulatory properties of MSCs.
We were able to detect IL-6 as an important factor in MSC–Treg interaction; however, future studies should focus upon other possible cytokines involved.
Acknowledgments
We would like to acknowledge Patrick Göthlich, Marc Hoffmann and Elena Tripel for their support. The study was carried out with internal funding by the Forschungsfond Orthopädische Universitätsklinik (F.200086). None of the authors received external funding in connection with the study presented in this publication.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Alvarez-Soria MA, Largo R, Santillana J, et al. Long term NSAID treatment inhibits COX-2 synthesis in the knee synovial membrane of patients with osteoarthritis: differential proinflammatory cytokine profile between celecoxib and aceclofenac. Ann Rheum Dis. 2006;65:998–1005. doi: 10.1136/ard.2005.046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benito-Miguel M, Garcia-Carmona Y, Balsa A, et al. A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+CD25+ regulatory T cells and CD4+CD25– responder T cells. J Immunol. 2009;183:8268–8279. doi: 10.4049/jimmunol.0900007. [DOI] [PubMed] [Google Scholar]
- 3.Young L, Katrib A, Cuello C, et al. Effects of intraarticular glucocorticoids on macrophage infiltration and mediators of joint damage in osteoarthritis synovial membranes: findings in a double-blind, placebo-controlled study. Arthritis Rheum. 2001;44:343–350. doi: 10.1002/1529-0131(200102)44:2<343::AID-ANR52>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Bullough P. Pathology of osteoarthritis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. Edinburgh: Mosby; 2003. pp. 1835–1845. [Google Scholar]
- 5.Myers SL, Brandt KD, Ehlich JW, et al. Synovial inflammation in patients with early osteoarthritis of the knee. J Rheumatol. 1990;17:1662–1669. [PubMed] [Google Scholar]
- 6.Revell PA, Mayston V, Lalor P, et al. The synovial membrane in osteoarthritis: a histological study including the characterisation of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann Rheum Dis. 1988;47:300–307. doi: 10.1136/ard.47.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MD, Triantafillou S, Parker A, et al. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 8.Guo X, Zheng Q, Yang S, et al. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor beta1 gene. Biomed Mater. 2006;1:206–215. doi: 10.1088/1748-6041/1/4/006. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 10.Lyons FG, Al-Munajjed AA, Kieran SM, et al. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials. 2010;31:9232–9243. doi: 10.1016/j.biomaterials.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 11.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao S, Wehner R, Bornhauser M, et al. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19:607–614. doi: 10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
- 13.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Lin GS, Bao CY, et al. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30:97–104. doi: 10.1007/s10753-007-9025-3. [DOI] [PubMed] [Google Scholar]
- 17.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 18.Jones E, Churchman SM, English A, et al. Mesenchymal stem cells in rheumatoid synovium: enumeration and functional assessment in relation to synovial inflammation level. Ann Rheum Dis. 2010;69:450–457. doi: 10.1136/ard.2008.106435. [DOI] [PubMed] [Google Scholar]
- 19.Augello A, Tasso R, Negrini SM, et al. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 20.Batten P, Sarathchandra P, Antoniw JW, et al. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 2006;12:2263–2273. doi: 10.1089/ten.2006.12.2263. [DOI] [PubMed] [Google Scholar]
- 21.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 22.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 23.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 24.Di Ianni M, Del Papa B, De Ioanni M, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–318. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 26.Prevosto C, Zancolli M, Canevali P, et al. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell–lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 27.Behrens F, Himsel A, Rehart S, et al. Imbalance in distribution of functional autologous regulatory T cells in rheumatoid arthritis. Ann Rheum Dis. 2007;66:1151–1156. doi: 10.1136/ard.2006.068320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagmann S, Moradi B, Müller T, et al. Interaction of human mesenchymal stromal cells and regulatory T-cells in osteoarthritis [Abstract] Osteoarthritis Cartilage. 2011;19:S30–31. [Google Scholar]
- 29.Moradi B, Rosshirt N, Hagmann S, et al. Osteoarthritis progression is accompanied by inflammatory CD4+ T-cell polarisation [Abstract] Osteoarthritis Cartilage. 2012;20:S232–233. [Google Scholar]
- 30.National Library of Medicine (NLM) at the National Institutes of Health (NIH) homepage. Available at: http://clinicaltrials.gov (accessed 10 December 2012)
- 31.Davatchi F, Abdollahi BS, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2010;14:211–215. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 32.Dexheimer V, Mueller S, Braatz F, et al. Reduced reactivation from dormancy but maintained lineage choice of human mesenchymal stem cells with donor age. PLoS ONE. 2011;6:e22980. doi: 10.1371/journal.pone.0022980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 34.Ryan JM, Barry FP, Murphy JM, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engela AU, Baan CC, Peeters AM, et al. Interaction between adipose-tissue derived mesenchymal stem cells and regulatory T cells. Cell Transplant. 2013;22:41–54. doi: 10.3727/096368912X636984. [DOI] [PubMed] [Google Scholar]
- 36.Marek N, Bieniaszewska M, Krzystyniak A, et al. The time is crucial for ex vivo expansion of T regulatory cells for therapy. Cell Transplant. 2011;20:1747–1758. doi: 10.3727/096368911X566217. [DOI] [PubMed] [Google Scholar]
- 37.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy JM, Dixon K, Beck S, et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 39.Dudics V, Kunstar A, Kovacs J, et al. Chondrogenic potential of mesenchymal stem cells from patients with rheumatoid arthritis and osteoarthritis: measurements in a microculture system. Cells Tissues Organs. 2009;189:307–316. doi: 10.1159/000140679. [DOI] [PubMed] [Google Scholar]
- 40.Scharstuhl A, Schewe B, Benz K, et al. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007;25:3244–3251. doi: 10.1634/stemcells.2007-0300. [DOI] [PubMed] [Google Scholar]
- 41.Arufe MC, De la Fuente A, Fuentes-Boquete I, et al. Differentiation of synovial CD-105(+) human mesenchymal stem cells into chondrocyte-like cells through spheroid formation. J Cell Biochem. 2009;108:145–155. doi: 10.1002/jcb.22238. [DOI] [PubMed] [Google Scholar]
- 42.Ju YJ, Muneta T, Yoshimura H, et al. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. 2008;332:469–478. doi: 10.1007/s00441-008-0610-z. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 44.Djouad F, Bony C, Haupl T, et al. Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal stem cells. Arthritis Res Ther. 2005;7:R1304–1315. doi: 10.1186/ar1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eikawa S, Ohue Y, Kitaoka K, et al. Enrichment of Foxp3+ CD4 regulatory T cells in migrated T cells to IL-6-and IL-8-expressing tumors through predominant induction of CXCR1 by IL-6. J Immunol. 2010;185:6734–6740. doi: 10.4049/jimmunol.1000225. [DOI] [PubMed] [Google Scholar]
- 46.Zeng C, Shi X, Zhang B, et al. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl) 2012;90:175–186. doi: 10.1007/s00109-011-0816-5. [DOI] [PubMed] [Google Scholar]
- 47.Svobodova E, Krulova M, Zajicova A, et al. The role of mouse mesenchymal stem cells in differentiation of naive T-cells into anti-inflammatory regulatory T-cell or proinflammatory helper T-cell 17 population. Stem Cells Dev. 2012;21:901–910. doi: 10.1089/scd.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujimoto M, Nakano M, Terabe F, et al. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol. 2011;186:32–40. doi: 10.4049/jimmunol.0903314. [DOI] [PubMed] [Google Scholar]
- 49.Herrath J, Muller M, Amoudruz P, et al. The inflammatory milieu in the rheumatic joint reduces regulatory T-cell function. Eur J Immunol. 2011;41:2279–2290. doi: 10.1002/eji.201041004. [DOI] [PubMed] [Google Scholar]
- 50.Aroen A, Loken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211–215. doi: 10.1177/0363546503259345. [DOI] [PubMed] [Google Scholar]


