Abstract
B cells perform various immunological functions that include production of antibody, presentation of antigens, secretion of multiple cytokines and regulation of immune responses mainly via their secretion of interleukin (IL)-10. While the liver is regarded both as an important immune organ and a tolerogenic environment, little is known about the functional biology of hepatic B cells. In this study we demonstrate that, following lipopolysaccharide (LPS) stimulation in vivo, normal mouse hepatic B cells rapidly increase their surface expression of CD39, CD40, CD80 and CD86, and produce significantly elevated levels of proinflammatory interferon (IFN)-γ, IL-6 and tumour necrosis factor (TNF)-α compared with splenic B cells. Moreover, LPS-activated hepatic B cells produce very low levels of IL-10 compared with activated splenic B cells that produce comparatively high levels of this immunosuppressive cytokine. Splenic, but not hepatic, B cells inhibited the activation of liver conventional myeloid dendritic cells (mDCs). Furthermore, compared with the spleen, the liver exhibited significantly smaller proportions of B1a and marginal zone-like B cells, which have been shown to produce IL-10 upon LPS stimulation. These data suggest that, unlike in the spleen, IL-10-producing regulatory B cells in the liver are not a prominent cell type. Consistent with this, when compared with liver conventional mDCs from B cell-deficient mice, those from B cell-competent wild-type mice displayed enhanced expression of the cell surface co-stimulatory molecule CD86, greater production of proinflammatory cytokines (IFN-γ, IL-6, IL-12p40) and reduced secretion of IL-10. These findings suggest that hepatic B cells have the potential to initiate rather than regulate inflammatory responses.
Keywords: B cells, dendritic cells, IL-10, liver, TLR-4
Introduction
As a major lymphoid organ that extracts nutrients from the portal venous blood, the liver is under constant immunological challenge by gut-derived dietary and microbial commensal products, such as lipopolysaccharide (LPS) and other microbe-associated molecular products (MAMPs) [1, 2]. To prevent chronic inflammation, the liver must modulate innate and adaptive immune responses to these diverse antigens [1, 3]. Conversely, the liver is an important organ in host defence against parasitic and microbial infections [4]. Thus, immune responses can be initiated in the liver to eliminate microbial infection [5, 6]. Further understanding of the mechanisms that determine the balance between immunity to pathogens and tolerance to diverse dietary and other antigens will provide new insights into the design of therapeutic strategies to regulate immunity in liver infection, autoimmunity and transplantation.
Hepatic B cells comprise approximately 5% of intrahepatic lymphocytes [8, 9]. Limited studies have addressed the function of hepatic B cells in vitro [11] and in the regulation of experimental autoimmune biliary disease [12, 13]. It has been shown that LPS-treated hepatic B cells enhance the production of interferon (IFN)-γ by liver natural killer (NK)1·1+ cells [11] and promote liver inflammation in the non-obese diabetic (NOD).c3c4 mouse model of autoimmune cholangitis [13], suggesting that hepatic B cells can regulate hepatic immune responses positively. In contrast, the Toll-like receptor (TLR) ligands LPS (TLR-4) and cytosine–phosphate–guanosine (CpG) (TLR-9) can stimulate interleukin (IL)-10-producing regulatory B cells (Breg) (B10) and regulate immune responses negatively [15, 16]. Given that LPS is delivered continuously by the liver via the portal blood, we hypothesize that the ability of hepatic B cells to regulate immune responses positively might be due to a lack of LPS-activated Breg in the liver.
In this study we demonstrate that, unlike splenic B cells, hepatic B cells lack B10 cells and comprise significantly smaller proportions of B1a and marginal zone (MZ)-like B cells [16]. In addition, when compared with liver conventional myeloid (m)DCs from B cell-deficient mice, those from B cell-competent wild-type mice were more immunostimulatory, as evidenced by higher levels of maturation marker expression in response to in-vivo LPS stimulation, and by a greater production of proinflammatory cytokines following ex-vivo LPS stimulation.
Materials and methods
Mice
Male C57BL/6 (B6; H2b) and B6·129S2-Ighmtm1Cgn/J (μMT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). B6·129P2-IL-10tm1Cgn mice (IL-10 reporter) were kindly provided by Dr David Rothstein (University of Pittsburgh). They were housed under specific pathogen-free conditions at the University of Pittsburgh School of Medicine, with unlimited access to food and water. Experiments were conducted under an Institutional Animal Care and Use Committee-approved protocol, and in accordance with National Institutes of Health-approved guidelines.
Isolation of B cells from spleen and liver and mDCs from the liver
Livers were perfused with 10 ml of phosphate-buffered saline (PBS) via the portal vein to remove circulating lymphocytes. Liver and spleen single-cell suspensions were prepared from whole tissue by mechanical disruption in RPMI-1640/2% (v/v) fetal bovine serum (FBS). Bulk liver non-parenchymal cells (NPC) were enriched by density centrifugation using Histodenz (Sigma, St Louis, MO, USA). B cells were purified by CD19-positive selection using the magnetic affinity cell sorting (MACS) system (Miltenyi Biotec, Auburn, CA, USA). mDCs were purified as described [18]. Briefly, liver and spleen cells were depleted of NK1·1+, CD3+, CD19+ and/or plasmacytoid dendritic cell antigen-1 (PDCA-1)+ cells, followed by positive selection of CD11c+ cells using the MACS system (Miltenyi Biotec). B cells were isolated from wild-type mice 18 h after LPS [100 μg/kg intraperitoneally (i.p.); Alexis Biochemistry, San Diego, CA, USA] or PBS administration. In some experiments, mice were given poly I:C (4 mg/kg, i.p.) for 18 h. The purity of mDCs and B cells was consistently > 90%. mDCs were isolated from wild-type and B cell-deficient μMT mice given the endogenous DC poietin fms-like tyrosine kinase 3 ligand (Flt3L) (10 μg/mouse/day; i.p. for 10 days; Amgen, Thousand Oaks, CA, USA), with either PBS or LPS (100 μg/kg, i.p.) treatment for the last 18 h.
In-vitro stimulation of liver mDCs
B cell-depleted liver NPCs were stimulated with LPS (10 ug/ml) for 48 h in the presence or absence of liver or spleen B cells. Activation of mDCs was determined by the level of expression of CD80, CD86 and programmed cell death 1 ligand 1 (PD-L1) (B7-H1; CD274) on CD19–B220–CD11c+ cells.
Flow cytometry
Single-cell suspensions were blocked for 10–15 min with anti-CD16/32 followed by staining with a fluorescent-tagged antibody mixture directed against the cell surface markers CD1d, CD3, CD5, CD19, CD23, CD24, CD39, CD40, CD80, CD86, PD-L1, B220, CR1/2, immunoglobulin (Ig)M and IgD (BD PharMingen, Franklin Lakes, NJ, USA or BioLegend, San Diego, CA, USA). Data were acquired on a LSR II or LSR Fortessa (BD Bioscience, San Jose, CA, USA) and analysed with FlowJo software (Tree Star, Ashland, OR, USA).
Cytokine quantitation
Purified B cells were cultured with or without 500 ng/ml phorbol myristate acetate (PMA), 1 μM ionomycin and 10 μg/ml LPS; purified mDCs were cultured with or without 10 μg/ml LPS. The cells were maintained for 48 h at 37°C in RPMI-1640 supplemented with 50 μM 2-mercaptoethanol (ME), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Supernatants were collected and cytokine production measured using a cytometric bead assay (CBA) Flex Set system (BD Bioscience) and analysed using FCAP Array Software (BD Bioscience).
Intracellular IL-10 staining
Bulk splenocytes and liver non-parenchymal cells (NPC) were activated for 5 h with 10 μg/ml LPS, 500 ng/ml PMA (Sigma) and 1 μM ionomycin (Sigma) in the presence of GolgiStop (BD Bioscience), followed by staining with fluorescent-labelled CD19 monoclonal antibody (mAb). Intracellular IL-10 was stained according to the BD intracellular cytokine staining protocol.
Immunofluorescence staining of liver tissue
Liver tissue samples were snap-frozen in Optimal Cutting Temperature compound (OCT) and cryostat sections (5 μm) stained for B cells (CD19; green), DCs (CD11c; red) and nuclei (DRAQ5; blue). Fluorescent images were captured with an Olympus Fluoview 1000 confocal microscope (software version 1·7a).
Statistics
Differences in levels of cytokine production and surface marker expression between the various groups were analysed by unpaired Student's t-test. P < 0·05 was considered significant.
Results
In response to LPS stimulation, hepatic B cells produce more IFN-γ, IL-6 and tumour necrosis factor (TNF)-α, but less IL-10 than those from secondary lymphoid tissue
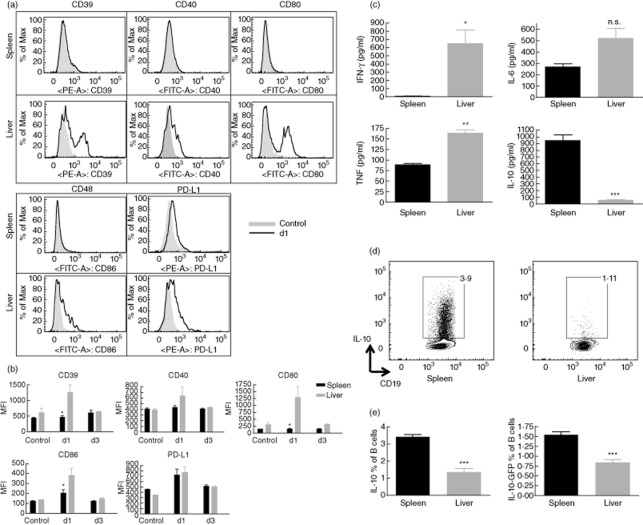
TLRs are the best-defined innate immune sensors that detect MAMPs. Recent evidence supports a role of TLRs in B cell activation and function [19]. We thus determined the expression of activation markers on B6 mouse freshly isolated liver versus splenic B cells from either LPS (TLR-4 ligand)-treated or untreated wild-type mice. As shown in Fig. 1a,b, hepatic but not splenic B cells up-regulated their cell surface expression of CD39, CD40, CD80 and CD86 within 24 h of LPS administration. By day 3, expression levels had returned to the normal steady-state level. This suggests that hepatic B cells respond in situ to systemic TLR-4 stimulation more strongly than splenic B cells. Because it has been reported that LPS and poly I:C (TLR-3 ligand) may have different effects on B cells [16], we next examined B lymphocytes isolated from either poly I:C-treated or untreated wild-type mice. As shown in Supplementary Fig. S1, both hepatic and splenic B cells up-regulated their expression of CD39, CD40, CD80, CD86 and PD-L1. This suggests that hepatic and splenic B cells respond in situ to systemic TLR-3 stimulation in a similar manner.
Figure 1.
In response to lipopolysaccharide (LPS) stimulation, hepatic B cells exhibit greater expression of activation markers and produce more interferon (IFN)-γ, interleukin (IL)-6 and tumour necrosis factor (TNF)-α, but less IL-10 than those from secondary lymphoid tissue. (a,b) Expression of cell surface activation markers on murine B cells following in-vivo LPS stimulation. C57BL/6 (B6) mice were injected intraperitoneally (i.p.) with LPS. On days 0, 1 and 3 post-injection, the mice were examined for the expression of the indicated cell surface molecules on spleen versus hepatic B cells; n = 3 mice per group. On day 1, liver but not splenic B cells up-regulated expression of CD39, CD40, CD80 and CD86. (a) Histogram overlay of day 1 and controls. (b) Data are graphed to compare the spleen and liver B cells on days 0 (control), 1 and 3; *P < 0·05. Data are representative of two independent experiments. (c) Spleen and hepatic B cells were purified from three individual mice using immunomagnetic beads, then cultured with 500 ng/ml phorbol myristate acetate (PMA), 1 μM ionomycin and 10 μg/ml LPS for 48 h before measuring the concentration of secreted IFN-γ, IL-6, TNF-α and IL-10 using cytometric bead assay (CBA) Flex Sets. Splenic B cells produced significantly greater amounts of IL-10 than hepatic B cells; ***P < 0·001; n = 3. Data are representative of two independent experiments. (d,e) Liver or spleen cells from normal B/6 or IL-10 reporter mice were cultured with 500 ng/ml PMA, 1 μM ionomycin and 10 μg/ml LPS in the presence of GolgiStop for 5 h. They were then tested for the frequency of B10 cells in normal B/6 mice by intracellular staining of IL-10 and surface staining of CD19 and the frequency of B10 cells in IL-10 reporter mice by green fluorescent protein (GFP)-IL-10 and surface staining of CD19. A greater proportion of splenic B cells than hepatic B cells produced IL-10; n = 3 mice. Data are representative of two independent experiments.
In response to TLR stimulation, different mouse splenic B cell subsets exhibit different cytokine secretion profiles [19]. For instance, spleen B1 and marginal zone (MZ) B cells secrete more IL-10, while follicular B cells secrete more IFN-γ [19]. We next examined the pattern of in-vitro LPS-induced cytokine production by hepatic and splenic B cells. Compared with splenic B cells, hepatic B cells secreted significantly more IFN-γ, IL-6 and TNF-α (Fig. 1c). In contrast, splenic B cells comprised significantly more IL-10 producers (Fig. 1d,e) and secreted much larger amounts of IL-10 than hepatic B cells (Fig. 1c). Consistent with this finding, the spleen exhibited significantly higher percentages of B1a and MZ B cells and a lower incidence of follicular B cells than the liver (Fig. 2). As IL-10 appears to play a pivotal role in the suppressive function of Breg [20], our findings that the liver lacks B1a and MZ-like B cells, and that LPS-stimulated hepatic B cells secrete very low levels of IL-10, suggest that B10 cells are not a prominent regulatory cell subset in mouse liver.
Figure 2.
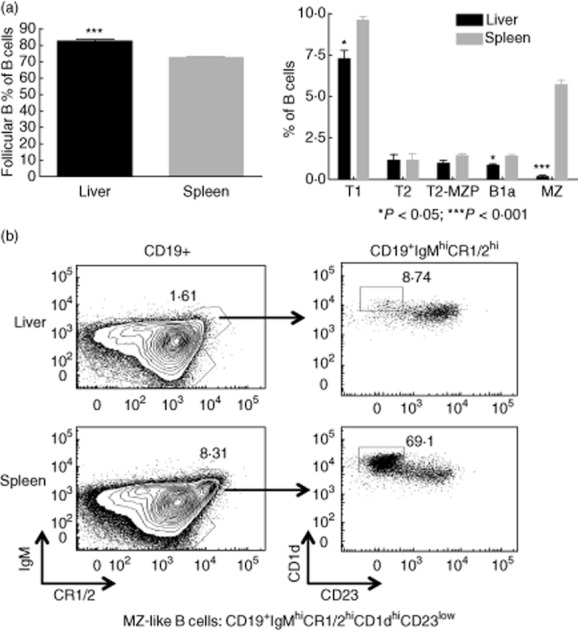
B cell subsets in the liver and spleen. Single cell suspensions of liver and spleen were blocked for 10–15 min with anti-CD16/32 and Normal Rabbit Serum (NRS) and stained with dead cell dye aqua, followed by staining with a fluorescent-tagged antibody mixture directed against the cell surface markers CD1d, CD3, CD5, CD19, CD23, CD24, CR1/2, IgM and IgD. B cells were gated on CD19+ cells after exclusion of dead cells and CD3+ cells. (a) Six immature and mature B cell subsets were identified as follows: transitional T1 B cells are CD19+CD24hiIgMlowCD23-; transitional T2 cells are CD19+CD23hiIgMhiCR1/2low; transitional T2-marginal zone-precursor (T2-MZP) B cells are CD19+IgMhiCD23hiCR1/2hi; follicular B cells are CD19+IgMlowCD5-CD23hiIgDhiCR1/2low; MZ-like cells are CD19+IgMhiCD5-CD23lowIgDlowCR1/2hi CD1dhi; B1a B cells are CD19+immunoglobulin (Ig)MhiCD5+CD23lowIgDlowCR1/2low. B1b B cells that are CD11b+ were too few to quantify in the spleen and liver. Data are plotted as % of CD19+ B cells. The liver exhibits a greater proportion of follicular B cells and a smaller proportion of T1, B1a and especially marginal zone (MZ)-like cells; *P < 0·05; ***P < 0·001; n = 4 mice. Data are representative of two independent experiments. (b) Representative flow plots for MZ-like B cells. Left panels are gated on CD19+ cells. Right panels are gated on CD19+ IgMhiCR1/2hi.
Liver mDCs from B cell-competent wild-type mice are more immunostimulatory than those from B cell-deficient mice
There is evidence that the tolerogenic milieu in the normal mouse liver inhibits hepatic mDC differentiation/maturation [3]. Our findings above suggest that hepatic B cells might resist this tolerogenic environment and play a proinflammatory role in liver homeostasis. It has been reported that hepatic B cells are not associated spatially with hepatic blood vessels [21]. In the current study, we confirmed (Supplementary Fig. S2) that hepatic B cells are located sparsely throughout the liver parenchyma and observed B cells in close proximity to DCs. This suggests a potential functional interaction between these cells.
We next tested whether hepatic B cells could affect the maturation and function of liver mDCs. Flt3L-treated mice were stimulated with LPS for 18 h. Liver mDCs were then isolated and analysed. As shown in Fig. 3a, these liver mDCs displayed significantly greater levels of CD86 and major histocompatibility complex (MHC) II when isolated from LPS-treated wild-type compared with μMT mice. This suggests that, in the presence of B cells, liver mDCs are more responsive to LPS stimulation and display a more stimulatory phenotype. To test further the influence of hepatic B cells on liver mDC function, we isolated liver mDCs and analysed their pattern of cytokine secretion in response to ex-vivo LPS stimulation for 48 h. As shown in Fig. 3b, liver mDC from μMT mice showed markedly reduced secretion of proinflammatory IFN-γ, IL-6, IL-12p40 and TNF-α, while they produced significantly more IL-10. These data further suggest a stimulatory influence of hepatic B cells on liver mDC maturation and function.
Figure 3.
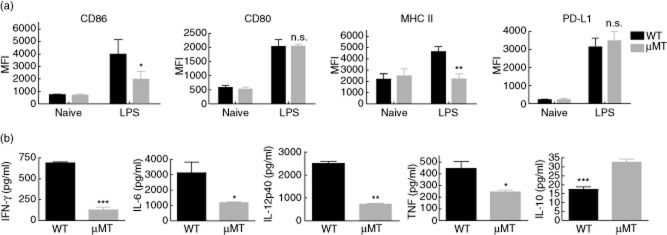
Liver myeloid dendritic cells (mDCs) from B cell-competent mice are more responsive to lipopolysaccharide (LPS) and secrete greater levels of proinflammatory cytokines but less interleukin (IL)-10 than those from B cell-deficient mice. (a) Liver non-parenchymal cells (NPC) were isolated from LPS-treated or control mice after 18 h and analysed for the expression of CD86 and programmed cell death 1 ligand 1 (PD-L1) on mDCs (B220–CD11chigh). In response to in-vivo LPS stimulation, B cell-deficient mice exhibited lower levels of CD86 and major histocompatibility complex (MHC) II, but not PD-L1 expression on liver mDCs when compared with wild-type mice. Data are pooled from two separate experiments, with three, three, two and three mice in groups of wild-type naive, wild-type LPS, μMT naive and μMT LPS, respectively. (b) Liver mDCs (CD3–CD19–NK1·1–plasmacytoid dendritic cell antigen-1 (PDCA-1)–CD11c+) were enriched using immunomagnetic beads, followed by in-vitro LPS stimulation for 48 h to measure levels of interferon (IFN)-γ, interleukin (I)L-6, IL-12p40,tumour necrosis factor (TNF)-α and IL-10 secretion. In response to in-vitro LPS stimulation, liver mDCs from B cell-deficient mice produced less proinflammatory IFN-γ, IL-6, IL-12p40 and TNF-α and more regulatory IL-10.
To test the direct influence of hepatic and splenic B cells on liver mDC maturation, we cultured B cell-depleted liver NPC with or without LPS in the presence or absence of hepatic or splenic B cells for 48 h to analyse the maturation of mDCs. As shown in Supplementary Fig. S3, hepatic B cells up-regulated the expression of CD86 and PD-L1, while splenic B cells down-regulated the expression of CD80 and CD86 on mDCs. This finding suggests that splenic, but not hepatic, B cells regulate liver mDC maturation negatively.
Discussion
Liver homeostasis is a complex process that involves maintaining tolerance to diverse dietary and other antigens, while retaining the capacity to mount effective immune responses against harmful pathogens [3]. In this report, we provide new evidence supporting a proinflammatory role of hepatic B cells, due probably to a lack of IL-10-producing B cells (B10). The first key observation is that hepatic B cells respond rapidly to LPS stimulation (Fig 1a,b) and secrete proinflammatory cytokines (Fig. 1c,d). Unlike splenic B cells, however, hepatic B cells produce very little, if any, anti-inflammatory IL-10 in response to LPS stimulation. In addition we demonstrate that, compared to splenic B cells, hepatic B cells comprise significantly lower proportions of B1a and MZ-like B cells (Fig. 2), that have been reported to secrete more IL-10 than follicular B cells [19]. Our observation suggests that B10 cells might not be prevalent immune regulatory cells in the liver. Consistent with this idea, in the presence of splenic but not hepatic B cells, liver mDCs are less responsive to LPS stimulation (Supplementary Fig. S3). This suggests that modulation of DCs by B10 cells observed in other tissue compartments [17] does not occur in the liver.
Having demonstrated that hepatic B cells comprise fewer regulatory subsets than splenic B cells, a question not addressed in this study is why Bregs appear not to contribute to the overall tolerogenic liver environment. One possibility may be to prevent overinhibition of immune responses in the liver. As shown in this report and by others [15, 16], the TLR-4 ligand LPS, a normal constituent of portal venous blood, is a potent stimulator of B10 cells. The absence of B10 cells and the presence of B cells with proinflammatory potential in an overall tolerogenic liver environment could help to balance the hepatic capacities of immune tolerance and immune stimulation. Our data presented here show that the absence of hepatic B cells compromises further the capacity of mDCs to respond to LPS (Fig. 3). To obtain sufficient numbers of liver mDCs for analysis, Flt3L-treated mice were used in Fig. 3 and Supplementary Figs S2 and S3. We are aware of the caveat that Flt3L might modify the composition of mDC subsets as well as other cells. Extended experiments using animal models are needed to confirm the positive regulation of liver mDCs and liver immune responses by hepatic B cells. Future research to understand more clearly the mechanisms underlying hepatic B cell activation and function is merited, and may lead to improved understanding and therapy of different liver-related pathological conditions.
Acknowledgments
The authors thank Dr David Rothstein for the gift of IL-10 reporter mice and Thomson laboratory members for helpful discussion. The work was supported by NIH grant P01AI81678 (A.W.T.), grant (874279717) from the Roche Organ Transplantation Research Foundation (A.W.T.) and by an American Society of Transplantation Basic Science Fellowship awarded to Hong Zhang. Hong Zhang did most of the experiments and wrote the manuscript, Donna Beer Stoltz performed immunofluorescence, Geetha Chalasani provided direction for B cell subset analysis and Angus W. Thomson provided intellectual input and guided the preparation of the manuscript.
Disclosure
The authors declare no financial or commercial conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1. Expression of cell surface activation markers on murine B cells following in-vivo poly I:C administration. C57BL/6 (B6) mice were injected intraperitoneally (i.p.) with lipopolysaccharide (LPS). On days 0 and 1 post-injection, the mice were examined for the expression of the indicated surface molecules on spleen versus hepatic B cells; n = 4 mice per group. On day 1, both liver and splenic B cells up-regulated expression of CD39, CD40, CD80 and CD86; *P < 0·05. No significant difference was observed between the liver and spleen. Data are representative of two independent experiments.
Fig. S2. Close proximity of B cells (CD19+) and dendritic cells (DCs) (CD11c+) in liver parenchyma. Cryostat sections (5 μm) of liver tissue samples from fms-like tyrosine kinase 3 ligand (Flt3L)-treated B6 mice were stained for B cells (CD19; green), DCs (CD11c; red) and nuclei (DRAQ5; blue). Fluorescent images were captured with an Olympus Fluoview 1000 confocal microscope (software version 1·7a).
Fig. S3. Splenic, but not hepatic, B cells inhibit the activation of liver myeloid dendritic cells (mDCs) in response to lipopolysaccharide (LPS) in vitro. B cell-depleted liver non-parenchymal cells (NPC) isolated from fms-like tyrosine kinase 3 ligand (Flt3L)-treated B6 mice were cultured with or without LPS in the presence or absence of hepatic or splenic B cells for 48 h. Activation of mDCs was analysed by expression of CD80, CD86 and programmed cell death 1 ligand 1 (PD-L1) on CD19–B220–CD3–CD11c+ mDCs. Liver mDCs in the presence of B cells were compared with those in the absence of B cells; **P < 0·01.
References
- 1.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 2.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 3.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 4.Seki S, Habu Y, Kawamura T, et al. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1·1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 5.Pradel G, Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology. 2001;33:1154–1165. doi: 10.1053/jhep.2001.24237. [DOI] [PubMed] [Google Scholar]
- 6.Cormier EG, Durso RJ, Tsamis F, et al. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc Natl Acad Sci USA. 2004;101:14067–14072. doi: 10.1073/pnas.0405695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breiner KM, Schaller H, Knolle PA. Endothelial cell-mediated uptake of a hepatitis B virus: a new concept of liver targeting of hepatotropic microorganisms. Hepatology. 2001;34:803–808. doi: 10.1053/jhep.2001.27810. [DOI] [PubMed] [Google Scholar]
- 8.Mehal WZ, Azzaroli F, Crispe IN. Immunology of the healthy liver: old questions and new insights. Gastroenterology. 2001;120:250–260. doi: 10.1053/gast.2001.20947. [DOI] [PubMed] [Google Scholar]
- 9.Mackay IR. Hepatoimmunology: a perspective. Immunol Cell Biol. 2002;80:36–44. doi: 10.1046/j.1440-1711.2002.01063.x. [DOI] [PubMed] [Google Scholar]
- 10.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto A, Kinoshita M, Ono S, et al. Cooperative IFN-gamma production of mouse liver B cells and natural killer cells stimulated with lipopolysaccharide. J Hepatol. 2006;45:290–298. doi: 10.1016/j.jhep.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Moritoki Y, Zhang W, Tsuneyama K, et al. B cells suppress the inflammatory response in a mouse model of primary biliary cirrhosis. Gastroenterology. 2009;136:1037–1047. doi: 10.1053/j.gastro.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 13.Moritoki Y, Tsuda M, Tsuneyama K, et al. B cells promote hepatic inflammation, biliary cyst formation, and salivary gland inflammation in the NOD.c3c4 model of autoimmune cholangitis. Cell Immunol. 2011;268:16–23. doi: 10.1016/j.cellimm.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhirapong A, Lleo A, Yang GX, et al. B cell depletion therapy exacerbates murine primary biliary cirrhosis. Hepatology. 2011;53:527–535. doi: 10.1002/hep.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lampropoulou V, Hoehlig K, Roch T, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 16.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest. 2011;121:4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morva A, Lemoine S, Achour A, Pers JO, Youinou P, Jamin C. Maturation and function of human dendritic cells are regulated by B lymphocytes. Blood. 2012;119:106–114. doi: 10.1182/blood-2011-06-360768. [DOI] [PubMed] [Google Scholar]
- 18.Sumpter TL, Packiam V, Turnquist HR, Castellaneta A, Yoshida O, Thomson AW. DAP12 promotes IRAK-M expression and IL-10 production by liver myeloid dendritic cells and restrains their T cell allostimulatory ability. J Immunol. 2011;186:1970–1980. doi: 10.4049/jimmunol.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browne EP. Regulation of B-cell responses by Toll-like receptors. Immunology. 2012;136:370–379. doi: 10.1111/j.1365-2567.2012.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 21.Inman CF, Murray TZ, Bailey M, Cose S. Most B cells in non-lymphoid tissues are naive. Immunol Cell Biol. 2012;90:235–242. doi: 10.1038/icb.2011.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of cell surface activation markers on murine B cells following in-vivo poly I:C administration. C57BL/6 (B6) mice were injected intraperitoneally (i.p.) with lipopolysaccharide (LPS). On days 0 and 1 post-injection, the mice were examined for the expression of the indicated surface molecules on spleen versus hepatic B cells; n = 4 mice per group. On day 1, both liver and splenic B cells up-regulated expression of CD39, CD40, CD80 and CD86; *P < 0·05. No significant difference was observed between the liver and spleen. Data are representative of two independent experiments.
Fig. S2. Close proximity of B cells (CD19+) and dendritic cells (DCs) (CD11c+) in liver parenchyma. Cryostat sections (5 μm) of liver tissue samples from fms-like tyrosine kinase 3 ligand (Flt3L)-treated B6 mice were stained for B cells (CD19; green), DCs (CD11c; red) and nuclei (DRAQ5; blue). Fluorescent images were captured with an Olympus Fluoview 1000 confocal microscope (software version 1·7a).
Fig. S3. Splenic, but not hepatic, B cells inhibit the activation of liver myeloid dendritic cells (mDCs) in response to lipopolysaccharide (LPS) in vitro. B cell-depleted liver non-parenchymal cells (NPC) isolated from fms-like tyrosine kinase 3 ligand (Flt3L)-treated B6 mice were cultured with or without LPS in the presence or absence of hepatic or splenic B cells for 48 h. Activation of mDCs was analysed by expression of CD80, CD86 and programmed cell death 1 ligand 1 (PD-L1) on CD19–B220–CD3–CD11c+ mDCs. Liver mDCs in the presence of B cells were compared with those in the absence of B cells; **P < 0·01.