Abstract
Natural killer T cells are a potent mediator of anti-viral immunity in mice, but little is known about the effects of manipulating NKT cells in non-human primates. We evaluated the delivery of the NKT cell ligand, α-galactosylceramide (α-GalCer), in 27 macaques by studying the effects of different dosing (1–100 μg), and delivery modes [directly intravenously (i.v.) or pulsed onto blood or peripheral blood mononuclear cells]. We found that peripheral NKT cells were depleted transiently from the periphery following α-GalCer administration across all delivery modes, particularly in doses of ≥10 μg. Furthermore, NKT cell numbers frequently remained depressed at i.v. α-GalCer doses of >10 μg. Levels of cytokine expression were also not enhanced after α-GalCer delivery to macaques. To evaluate the effects of α-GalCer administration on anti-viral immunity, we administered α-GalCer either together with live attenuated influenza virus infection or prior to simian immunodeficiency virus (SIV) infection of two macaques. There was no clear enhancement of influenza-specific T or B cell immunity following α-GalCer delivery. Further, there was no modulation of pathogenic SIVmac251 infection following α-GalCer delivery to a further two macaques in a pilot study. Accordingly, although macaque peripheral NKT cells are modulated by α-GalCer in vivo, at least for the dosing regimens tested in this study, this does not appear to have a significant impact on anti-viral immunity in macaque models.
Keywords: activation, α-galactosylceramide, α-GalCer, influenza virus, macaques, NKT cells, SIV infection
Introduction
NKT cells are a small subset of T cells that provide an immune defence against viral infections and cancer. In human and macaque peripheral blood, NKT cells typically comprise 0·01–0·1% of the lymphocyte population [1]. NKT cells respond to glycolipid antigens presented by CD1d, a non-classical major histocompatibility complex (MHC)-like molecule, localized on antigen-presenting cells (APC) such as dendritic cells (DC), monocytes and B cells [2]. Type I NKT cells are referred to as semi-invariant NKT cells due to their common usage of the T cell receptor (TCR) chain Vα24-Jα18, paired with a limited repertoire of Vβ chains [2],[3]. They have a memory phenotype [5], and upon stimulation NKT cells produce large amounts of cytokines including interferon (IFN)-γ, tumour necrosis factor (TNF), interleukin (IL)-2, IL-4, IL-10, IL-13, IL-17, IL-21 and IL-22 [6, 7]. The cytokines produced by NKT cells lead to concomitant activation of other cells of the immune system such as NK cells, B cells and T cells, leading to a larger cascade of cytokine and chemokine production [2].
NKT cells are depleted during human immunodeficiency virus (HIV) infection of humans [8–10] or simian immunodeficiency virus (SIV) infection of macaques [11, 12]. Depletion of the CD4+ subset of NKT cells is most marked, and parallels depletion of the total conventional CD4+ T cells. Depletion of NKT cells correlates with disease progression in both HIV and SIV infection [12, 13]. Whether manipulation of NKT cell numbers or function could modulate HIV or SIV infection is not known.
The prototypical NKT cell ligand α-galactosylceramide (α-GalCer) has been used extensively in mice and human studies to demonstrate immunomodulatory effects in cancers [14–20], microbial infections [21–22] and autoimmunity [24–26]. NKT cells have also been shown to promote immunity to influenza virus [27, 28], and α-GalCer has been used successfully in mice to enhance immunity to influenza virus A [29],[30] or other viral infections [32, 33]. α-GalCer administration in combination with DNA vaccination of mice was protective in microbial infections or potent in augmenting T cell responses [34, 35]. Furthermore, mice immunized with α-GalCer together with herpes simplex virus-2 (HSV-2) glycoprotein D via the intranasal or intravaginal routes were protected against lethal HSV-2 intravaginal challenge [36].
Many previous studies have used autologous or syngeneic DC pulsed with NKT cell ligands to activate NKT cells in vivo, reviewed in [37]. Such systems require specialized facilities and will not be widely applicable. In previous studies our laboratory has demonstrated the effectiveness of ex-vivo pulsing of cytotoxic T lymphocyte (CTL) peptides onto macaque peripheral blood mononuclear cells (PBMC) or whole blood (WB) [38, 39]. Presumably, immature blood DC can effectively present the antigen following in-vivo maturation. Whether a simpler system of WB or PBMC delivery of NKT cell ligands is effective in vivo is unknown.
Macaques are a useful primate model for a variety of infectious diseases. However, there is no information on effective conditions to activate or expand NKT cells in macaques and thereby modulate virus infections. We conducted a study of 27 SIV-uninfected macaques to determine conditions suitable for in-vivo activation of NKT cells. We subsequently conducted studies to investigate the efficacy of α-GalCer administration in macaques to augment live-attenuated influenza virus immunity. We then investigated SIV disease progression in macaques administered α-GalCer just prior to SIV infection.
Methods
Study animals
We studied a total of 42 pigtail macaques (Macaca nemestrina) for changes in peripheral NKT cell frequencies in response to α-GalCer administration combined with or without SIVmac251 and/or influenza A virus inoculations. Twenty-seven animals were administered with α-GalCer and NKT cell frequencies and function were observed. Two animals were administered α-GalCer at the same time as live attenuated influenza virus infection, along with 13 control animals that received influenza only, and observed for anti-influenza virus immunity (Table 1). In another study, two animals were administered α-GalCer prior to SIV infection and observed for SIV infection along with five controls that received SIV only (Table 1). All animals expressed the MHC class I allele Mane-A1*08401 (previously named Mane-A*10) [40]. Prior to any procedure, macaques were sedated intramuscularly with ketamine (10 mg/kg). All animals were monitored for any significant changes in weight or behaviour or toxic side effects during the respective studies. All studies were approved by the relevant institutional ethics committees.
Table 1.
Study animals and procedures.
| Study | No. of animals | Macaque ID | Details |
|---|---|---|---|
| α-GalCer delivery (27 macaques in total) | 2 | 7448, C3752 | 1 μg α-GalCer pulsed onto PBMC for 1 h |
| 2 | C3751, C3767 | 10 μg α-GalCer pulsed onto PBMC for 1 h | |
| 3 | 5878, C3763, C3765 | 10 μg α-GalCer pulsed onto PBMC for 3 h | |
| 4 | 5873, 18869, 26300, C3749 | 10 μg α-GalCer pulsed onto PBMC for 12 h | |
| 3 | 7467, 15798, B0429 | 1 μg α-GalCer pulsed onto WB for 1 h | |
| 2 | 35414, 36142 | 1 μg α-GalCer pulsed onto WB for 3 h | |
| 2 | 36271, 45610 | 10 μg α-GalCer pulsed onto WB for 1 h | |
| 2 | 19340, 26121 | 10 μg α-GalCer pulsed onto WB for 3 h | |
| 3 | 26301, 26783, B0519 | i.v. 1 μg α-GalCer | |
| 2 | 16570, 35377 | i.v. 10 μg α-GalCer | |
| 2 | C0942, 36121 | i.v. 100 μg α-GalCer | |
| α-GalCer administered at the same time as live attenuated influenza A virus (28 macaques in total) [42] | 2 | B0440, B0547 | 5 μg α-GalCer pulsed onto WB for 2 h; delivered at the same time as the first inoculation of live-attenuated flu encoding SIV epitopes |
| 8 | 25377, 26359, 45418, B0443, B0517, B0526, B0527, C0933 | Live-attenuated flu encoding SIV epitopes | |
| 5 | 19341, 19351, 19530, B0508, C3754 | Control flu, not encoding SIV epitopes | |
| 13 | *5873, *5878, 18862, *19340, *26121, *35414, *36142, 36300, *45610, *C3749, *C3751, *C3763, *C3767 | Control animals, naive for flu | |
| α-GalCer administered 2 days prior to SIVmac251 infection (7 animals) | 2 | *5873, *C3751 | 5 μg α-GalCer pulsed onto WB for 2 h, 2 days prior to SIV infection |
| 5 | ‡19341, ‡19351, ‡19530, ‡B0508, ‡C3754 | Control animals not given α-GalCer, and infected with SIV at the same time as above |
Animals enrolled previously into the ‘α-galactosylceramide (α-GalCer) delivery’ study;
animals enrolled previously into the ‘control flu, not encoding simian immunodeficiency virus (SIV) epitopes’ group. PBMC: peripheral blood mononuclear cells; WB: whole blood; i.v.: intravenous.
Modes of delivery of α-GalCer
α-GalCer (KRN7000; Sapphire Biosciences, Waterloo, Australia) was prepared as described previously [41]. For studies on optimization of expansion of in-vivo NKT cell levels we used a total of 27 healthy, SIV-uninfected macaques. Macaques were assigned randomly into three groups and administered α-GalCer intravenously (i.v.), pulsed onto autologous ex-vivo peripheral WB or pulsed onto autologous freshly prepared PBMC. Seven macaques were given α-GalCer IV at doses of 1, 10 or 100 μg each (Table 1 and Fig. 1). Nine macaques were given α-GalCer pulsed onto WB. Peripheral blood (9 ml) was drawn into Na-heparin vacuette tubes from each macaque, incubated with 1 or 10 μg α-GalCer for 1 or 3 h at 37°C with mixing every 15 min, and reinfused into the respective animal. Eleven macaques were assigned to the PBMC group. PBMC, typically 10–20 × 106 cells, were prepared from ex-vivo blood of the respective animal by density gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and incubated with 1 or 10 μg α-GalCer for 1, 3 or 12 h as above in 2 ml serum-free, RPMI-1640 media. Following α-GalCer administration, sequential peripheral blood was drawn from each macaque according to a schedule shown in Fig. 1 and monitored for NKT cell frequencies, as described previously [12, 41].
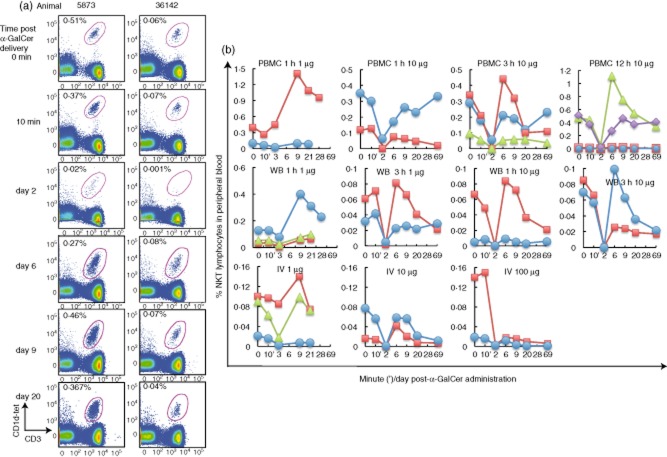
Figure 1.
Transient depletion of peripheral natural killer T cells (NKT cells) upon in-vivo α-galactosylceramide (α-GalCer) delivery. (a) Representative plots of flow cytometry analysis of pigtail macaque NKT cells within the lymphocyte population of peripheral blood stained ex vivo at different times following α-GalCer delivery; animal 5873 was administered 10 μg α-GalCer pulsed onto peripheral blood mononuclear cells (PBMC) for 12 h, animal 36142 was administered 1 μg α-GalCer pulsed onto WB for 3 h. NKT cell levels are enumerated as cells double-positive for CD1d tetramers loaded with the α-GalCer analogue, PBS-44, and CD3 as a proportion of gated lymphocytes. (b) Sequential blood samples were taken prior to delivery (0 min) and at 10 min, days 3, 9, 20 and 28 following 1 μg α-GalCer administration (n = 8) (b, left column) or at 0 min, 10 min, days 2, 6, 9, 20, 69, following 1–100 μg α-GalCer delivery (n = 19) (b, columns 2–4). Each line represents the peripheral NKT cell frequency of one animal. Dose and mode of administration of α-GalCer are indicated above each individual graph.
PMA/ionomycin activation of NKT cells and intracellular cytokine expression
Ex-vivo NKT cells were activated for 4 h with phorbol myristate acetate (PMA) (10 ng/ml) in combination with ionomycin (3 uM) in WB assays at days 0, 9 and 20 post-α-GalCer administration, as reported previously [41], with the addition of monensin (2 uM) for the last 2 h of the activation. Unstimulated controls, containing 0·41% dimethylsulphoxide (DMSO), contained the same percentage of DMSO as stimulated samples and were treated as above. Intracellular IFN-γ expression was enumerated as described [41].
Recombinant influenza SIV vaccination
Construction of three separate live-attenuated influenza A (flu) virus each encoding an SIV epitope restricted by Mane-A1*08401 has been described previously [42, 43]. Animals (n = 15) unexposed previously to α-GalCer or flu were enrolled into this study. Flu-SIV (n = 8), flu-SIV in combination with a single dose of α-GalCer (5 μg) pulsed onto WB for 2 h (n = 2) and control flu (n = 5) inoculations were given four times over 18 weeks (Fig. 4). Flu-specific antibody response was measured by haemagglutination inhibition (HI) assays, as described by Drs Karen Laurie and Aeron Hurt [42]. A flu-specific CD8+ T cell response to a nucleoprotein epitope was assayed with the Mane-A*10-RA9 tetramer reagent on thawed PBMC of the above 15 animals as well as those from flu-naive macaques (n = 13), as described previously [42].
Figure 4.
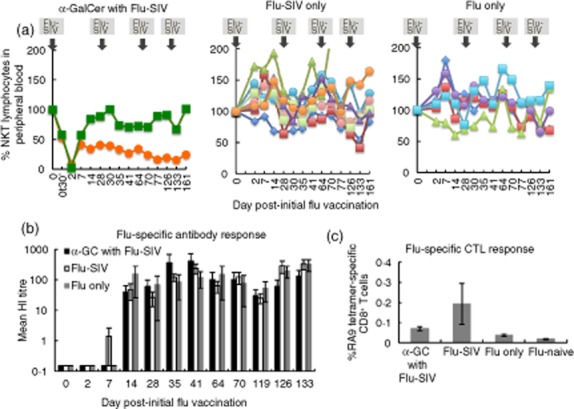
Effect of α-galactosylceramide (α-GalCer) on attenuated influenza vaccination. Live-attenuated influenza A virus encoding three distinct simian immunodeficiency virus (SIV) epitopes (flu-SIV) was administered on the same day (day 0) as 5 μg α-GalCer pulsed onto autologous peripheral blood (n = 2), flu-SIV without α-GalCer (n = 8) and control flu not containing exogenous antigens (flu-only; n = 5). Further flu vaccinations were given to the respective groups on days 28, 56 and 119 after the first flu vaccination, without additional delivery of α-GalCer. (a) Peripheral blood NKT cell levels normalized to pre-flu vaccination levels. (b) Serum antibodies to flu was measured with a haemagglutination inhibition (HI) assay using macaque red blood cells. HI titres of the groups were analysed by Kruskal–Wallis non-parametric test (c). A CD8 T cell response to a Mane-A1*08401 restricted flu nucleoprotein epitope, RA9, was assessed by tetramer staining. An additional group of flu-naive macaques (n = 13) as described previously [42] was included in the analysis. Data analysed by Kruskal–Wallis with Mann–Whitney post-hoc test. Error bars, standard error of the mean.
SIV infection of macaques
Macaques (n = 2) were given a single dose of α-GalCer (5 μg) pulsed onto WB for 2 h, 2 days prior to SIVmac251 infection with 104 TCID50 (Fig. 5). Five control animals were also infected with SIVmac251 at the same time. SIV viraemia and CD4 T cell counts were measured as described previously [43].
Figure 5.
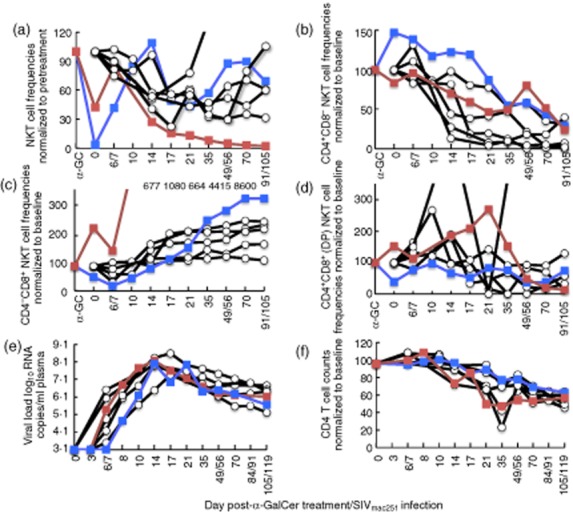
Effect of α-galactosylceramide (α-GalCer) on simian immunodeficiency virus (SIV) infection of macaques. Macaques were infected with SIVmac251 (control animals, n = 5, clear circles) at day 0 or administered 5 μg α-GalCer pulsed onto peripheral blood 2 days prior to SIVmac251 infection (day α-GC; n = 2, filled squares). (a) Peripheral blood NKT cell levels normalized to pre-α-GalCer NKT percentages (for animals given α-GalCer) or to peripheral NKT percentages prior to SIVmac251 infection (for control animals). (b,c,d) NKT cell subset frequencies for each subset, normalized to pre-α-GalCer or pre-infection subset frequencies, as indicated in axes. Values for red-filled squares are off-scale and are indicated above the graph. (e) SIV viraemia and (f) peripheral total CD4 T cells levels, normalized to pre-SIV infection levels were measured.
Flow cytometry analyses absolute counts of NKT cells
Peripheral blood isolated from macaques was stained with CD1d tetramer (CD1dtet) loaded with an α-GalCer analogue, PBS-44 (kindly provided by Professor Paul Savage (Brigham Young University), CD3 (clone SP34-2; BD Biosciences, San Jose, CA, USA), CD4 (clone L200; BD Biosciences) and CD8 (clone SK1; BD Biosciences). NKT cells were identified by flow cytometry as doublet excluded lymphocytes that are double-positive for CD3 and CD1dtet.
NKT cell numbers were enumerated on a Coulter AC.T diff − Analyzer (Beckman Coulter, Indianapolis, IN, USA) by measuring total peripheral blood lymphocyte counts.
Statistical analyses
Statistical analyses were performed using spss version 20 (IBM, Chicago, IL, USA). Data were analysed by two-way analysis of variance (anova) in conjunction with Bonferroni's post-hoc test. Where necessary, log10 transformation before anova was performed for the data to pass or tend towards Levene's test for equal variances. Data that were not distributed normally were analysed by Kruskal–Wallis test (Fig. 4b) or Kruskal–Wallis with Mann–Whitney post-hoc test (Fig. 4c).
Results
α-GalCer administration modulates peripheral blood NKT cell frequencies
Enhancing NKT cell numbers or function is an important goal of immunotherapy strategies. Previous studies endeavouring to activate or expand peripheral blood NKT cell numbers in humans using α-GalCer directly have involved either i.v. injection of α-GalCer in solution [17], in-vitro expansion of autologous PBMC with α-GalCer in the presence of IL-2 and granulocyte–macrophage colony-stimulating factor (GM-CSF) [15, 16, 44, 45] or purified DCs pulsed with α-GalCer [46],[47]. Macaques are a useful primate model to study NKT cells [11, 12, 49, 50]; however, to our knowledge no studies have assessed NKT cell activation or expansion strategies using α-GalCer in macaques. Therefore, we sought to determine simple yet effective ways to achieve this with pigtail macaque NKT cells in vivo.
Twenty-seven healthy macaques were assigned randomly to be given 1–100 μg α-GalCer (KRN7000) by i.v. injection, pulsed onto ex-vivo WB or pulsed onto autologous PBMC for defined periods (Table 1, Figs 1 and 2). α-GalCer was well tolerated by all 27 macaques, as no systemic or local clinical manifestations were observed. CD1d tetramers loaded with the α-GalCer analogue, PBS-44, were used to identify NKT cells from these animals. PBS-44 is used as it loads more efficiently into CD1d tetramers, and we determined that these tetramers stained NKT cells comparably to CD1d tetramers loaded with KRN7000 (the form of this glycolipid that was administered into the animals) (Supporting information, Fig. S1). A pronounced reduced detection of NKT cell frequencies occurred at days 2/3 post-α-GalCer delivery, regardless of the mode of administration, in animals given 10–100 μg α-GalCer (mean 0·012% NKT cells within the lymphocyte population, range 0·00–0·06% for all animals in the 10–100 μg group; mean 0·02%, range 0·00–0·06% in the PBMC group; mean 0·001%, range 0·00–0·001% in the WB group and mean 0·002%, range 0·00–0·003% in the i.v. group). This effect was less pronounced when only 1 μg α-GalCer was given (mean 0·071% NKT cells of lymphocytes, range 0·002–0·45% for all animals within the 1 μg group; mean 0·24%, range 0·03–0·45% in the PBMC group; mean 0·02; range 0·002–0·08% in the WB group; mean 0·04%, range 0·003–0·09% in the i.v. group). In some animals, particularly those administered α-GalCer pulsed onto PBMC, there was even a slight decline in detection of NKT cell frequencies within the circulating lymphocyte population within 10 min of administration (range 3–43% reduced detection compared to baseline in 19 of 27 animals, Figs 1a,b, 2b). Although NKT cell levels illustrated variable patterns across the animals, after α-GalCer administration a common finding was that detectable NKT cell levels began to rise after the nadir at 2/3 days, reaching maximal levels between days 6–9, followed by a slight drop at days 20/21 before stabilizing. An interesting observation noted was that some animals that had higher baseline NKT cell frequencies achieved higher NKT cell expansion after α-GalCer delivery (Fig. 1b). For example, one animal in the PBMC 1 h 1 μg group (top panel, left-most graph) had a pre-administration NKT cell frequency of 0·4% that rose to 1·41% at day 9, while another animal in the WB 1h 1 μg group (centre panel, left-most graph) had a starting NKT cell frequency of 0·13% that rose to 0·4% after α-GalCer delivery. However, in 13 of 19 animals NKT cell levels did not return to pretreatment baseline levels at the end of the observation period of 69 days (range 4–140% of baseline numbers; median 34%). Pulsing either WB or PBMC for a longer duration (>1 h) did not increase NKT cell percentages perceptibly. Interestingly, NKT cell levels in the two animals administered 100 μg α-GalCer i.v. remained at 4–8% of baseline frequencies through to day 69, the lowest of all the animals studied.
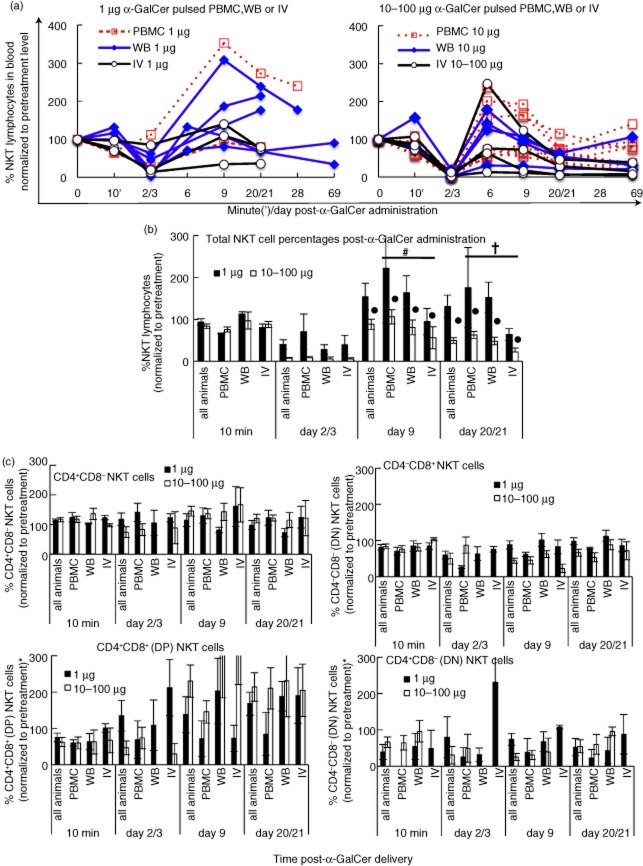
Figure 2.
Impact of dose and mode of delivery of α-galactosylceramide (α-GalCer) and impact on peripheral NKT cell subsets. (a). NKT cell levels were normalized to baseline levels and animals were segregated according to the dose of α-GalCer (a) and dose and mode of delivery (b). Groups contained variable numbers of animals: all animals/1 μg (n = 10), peripheral blood mononuclear cells (PBMC)/1 μg (n = 2), whole blood (WB)/1 μg (n = 5), IV/1 μg (n = 3); all animals/10 μg (n = 17), PBMC/10 μg (n = 9), WB/10 μg (n = 4), intravenous (i.v.)/10–100 μg (10 μg, n = 2; 100 μg, n = 2). Two-way analysis of variance (anova) was carried out on data prior to normalization to baseline NKT cell frequencies. (c) NKT cell subsets defined as CD4+CD8–, CD4–CD8+, CD4+CD8+ double-positive (DP) or CD4–CD8– double-negative (DN) populations, as indicated above each graph. Graphs show data normalized to the respective subset frequencies measured at day 0 with some exceptions in the DP and DN graphs, where no DP or DN NKT cell subsets were detected at day 0, as follows: four animals shown in the DP graph were normalized to DP NKT cell frequencies measured at day 6, while one animal had no DP cells at any time-point; eight animals in the DN graph were normalized to DN NKT cell frequencies detected between the 10 min – day 20/21 time-point, while two animals had no DN NKT cells at any time-point. Two-way anova carried out on subset frequencies prior to baseline normalization (P ≥ 0·083 between days 0 and 9 for each of CD4+, DP or DN subsets; P < 0·007 for CD8– subset). Error bars, standard error of the mean; #P = 0·001 between PBMC and i.v. groups; †P = 0·03 between PBMC and i.v. groups; †P = 0·004.
We also assessed absolute numbers of blood NKT cells over time after α-GalCer administration by measuring concurrently total lymphocyte counts. All animals in all groups had detectable, albeit variable, baseline NKT cell numbers (mean 5951 NKT cells/ml blood, range 145–21 200 NKT cells/ml blood), consistent with observations on peripheral NKT cell levels in humans, other macaque species and our previous data in pigtail macaques [1, 11, 12, 41, 49, 50]. On the whole, total NKT cell numbers in blood sampled over time mirrored NKT cell frequencies enumerated within the lymphocyte population by flow cytometry (Fig. 1b). Across the whole cohort, average NKT cell percentages were 0·11 at 10 min and 0·03 at 2/3 days after α-GalCer administration.
Effect of dose and mode of α-GalCer administration and impact on NKT cell subsets
The PBMC mode of delivery of α-GalCer was more effective than i.v. delivery for enhancing NKT cell frequencies at days 9 and 20 across all doses (Fig. 2b; P = 0·001 between the PBMC and i.v. groups in a two-way anova with Bonferroni's post-hoc test). Intravenous administration of α-GalCer resulted more commonly in sustained lower frequencies of NKT cells compared to PBMC administration (P = 0·033 at day 20). WB delivery of α-GalCer was not different to the other modes of delivery for NKT cell frequencies at days 9 and 20.
A high dose of α-GalCer was detrimental for recovery of NKT cells. Animals given ≥10 μg α-GalCer had significantly lower NKT cell frequencies across days 9 and 20 (P = 0·004) compared to animals given 1 μg α-GalCer. NKT cell levels across all doses had expanded significantly by day 9 after the initial decline observed at days 2/3 (P < 0·001). α-GalCer administration, however, resulted in no overall expansion of NKT cells from baseline levels, by day 9 or later (P = 0·438) (Fig. 2b).
To determine factors regulating the differences in NKT cell frequencies we studied the impact of delivery of α-GalCer on peripheral NKT cell subsets over time (Fig. 2c). Our previous data from pigtail macaques showed that the major peripheral blood NKT cell subsets were CD4+CD8– and CD4–CD8+ and that the CD4+ NKT cell subset declined after SIV infection [12]. In this study, ex-vivo peripheral blood NKT cell subset frequencies across all 27 animals at day 0 were as follows: CD4+ NKT cells (mean 49%; range 16–74%), CD8+ NKT cells (mean 40%; range 5–77%); CD4+CD8+ double-positive (DP) NKT cells (mean 7·9%; range 0–29%) and CD4–CD8– double-negative (DN) NKT cells (mean 1·8%; range 0–7·5%). The CD4+, DP or DN NKT cell subset frequencies at day 9 after α-GalCer administration were not statistically different from baseline levels (P ≥ 0·083). However, the CD8+ NKT cell subset frequency was significantly lower at day 9 compared to baseline values (P = 0·007).
Activation profile of NKT cells after α-GalCer administration
In addition to numerical levels of NKT cells, the capacity of NKT cells to be activated upon stimulation may be an important measure of NKT cell function. NKT cells from the baseline time-point (day 0) activated in vitro in WB assays with PMA/ionomycin exhibited clear intracellular IFN-γ staining (Fig. 3) [41]. In the absence of mitogenic PMA/ionomycin stimulation, we detected very little IFN-γ production from NKT cells at days 9 and 20 after in-vivo α-GalCer administration (<4% IFN-γ expression, data not shown). This is probably because, at these later time-points, the NKT cells are no longer in an activated state in response to α-GalCer challenge, which is consistent with results from studies in mice [51]. However, the peak of NKT cell expansion at day 9 did not coincide with higher levels of mitogen-induced IFN-γ expression. Furthermore, IFN-γ production at days 9 or 20/21 was lower than baseline levels (P < 0·001).
Figure 3.
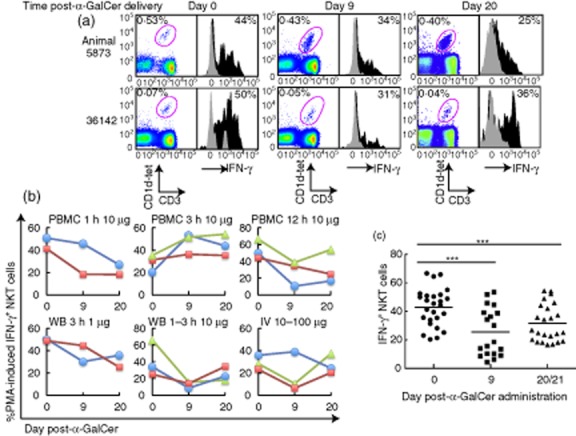
Activation status of peripheral NKT cells. Peripheral blood of macaques was activated ex vivo with phorbol myristate acetate (PMA)/ionomycin at days 0, 9 and 20 following α-galactosylceramide (α-GalCer) administration. (a) Representative dot-plots of NKT cell levels within the lymphocyte population following stimulation and histograms of intracellular interferon (IFN)-γ production from NKT cells. NKT cells were stained with CD1d tetramers loaded with the α-GalCer analogue, PBS-44. Cytokine frequencies shown are background-subtracted values. Grey shaded histograms are unstimulated controls containing 0·41% dimethylsulphoxide (DMSO); black-shaded histograms are mitogen-activated. (b,c) Expression levels of IFN-γ from NKT cells of macaques. Only animals that expressed IFN-γ levels three times above background (unstimulated) at days 0, 9 and 20 are shown (b) and included in statistical analysis (c). Animals across all doses and modes of delivery were included in a two-way analysis of variance (anova) with Bonferroni post-hoc test (day 0, n = 26; day 9, n = 19; day 20/21, n = 24); ***P < 0·001. The mean of each group is represented by a horizontal bar.
Effect of α-GalCer-pulsed blood as a vaccine adjuvant
α-GalCer has been shown to improve vaccine efficacy in murine studies of both influenza and herpes simplex virus vaccinations [29–31, 36]. No studies have analysed the effects of α-GalCer as a vaccine adjuvant in macaques. We evaluated the effectiveness of α-GalCer as an adjuvant in a pilot trial of two macaques given 5 μg α-GalCer delivered via peripheral blood at the same time as the first dose of live attenuated influenza virus A (flu) carrying three SIV-specific CD8 T cell epitopes [42, 43]. Macaques were reinoculated with three further doses of flu over a 19-week period, but without any further administration of α-GalCer (Fig. 4a shows the vaccination schedule). A second cohort of eight animals were given the above regimen but without α-GalCer, and a third cohort of five animals received live attenuated flu without SIV epitopes inserted.
As observed with the administration of α-GalCer onto WB in the absence of vaccination, detection of peripheral blood NKT cell levels in the two immunized macaques declined to approximately 50% within a half hour of α-GalCer delivery, and these cells were essentially undetectable at 2 days, after which NKT cell levels begin to rise (Fig. 4a). However, one animal never recovered its baseline frequency of NKT cells. In contrast, no apparent depletion of NKT cells occurred in animals immunized with flu-SIV or flu-only but not given α-GalCer.
To assess whether α-GalCer had an impact on influenza-specific immunity we studied both antibody response by HI antibody titration and influenza T cell response by MHC class I tetramer (Fig. 4b,c). HI antibodies were observed rarely prior to 14 days after the first influenza inoculation, but thereafter HI antibodies to influenza were present in all animals and, although variable across the small numbers of animals, were of a similar magnitude in animals administered α-GalCer compared to those not administered α-GalCer.
We also studied an influenza-specific T cell response late into the flu infection, at 133 days after the first flu inoculation. We previously mapped an influenza nucleoprotein-specific CD8 T cell response (RA9) restricted by the MHC class I allele Mane-A1*08401 [42]. As all animals expressed this MHC allele, we compared the RA9-specific CD8 T cell response in animals given α-GalCer to those not given α-GalCer. The levels of RA9-specific CD8 T cells produced by animals given a single dose of α-GalCer were relatively low (0·060–0·080% of CD8 T cells) and were within the range of animals that received either flu-SIV or flu-only infections.
Pretreatment with α-GalCer does not alter SIV infection
NKT cells activated following α-GalCer delivery in mice remain in an activated state, with production of IFN-γ for the first 48 h [52, 53]. Our studies showed that NKT cells are no longer detected in the peripheral blood 2 days post α-GalCer delivery, and we reasoned that at this time-point, NKT cells may be in a more activated state and primed to respond to infection. We therefore evaluated whether SIV infection or disease progression was influenced by stimulation of NKT cells with α-GalCer prior to SIV infection. We administered 5 μg α-GalCer pulsed onto peripheral blood 2 days prior to intravenous SIVmac251 infection in two naive macaques, and followed markers of SIV infection systemically in peripheral blood (Fig. 5). Peripheral blood NKT cells were depleted in both animals as observed in the earlier studies, although not completely in one animal (Fig. 5). NKT cell proportions returned subsequently to near baseline levels 1–2 weeks after SIV infection. SIV infection resulted subsequently in near complete depletion of NKT cells in one of the two animals, consistent with our previous analyses of total NKT cell levels following SIV infection [12]. Depletion occurred primarily in the CD4+ subset of NKT cells (Fig. 5b). The animal that showed near complete depletion of NKT cells (red-filled square) had a significantly lower pretreatment baseline CD8+ NKT cell frequency of 0·61% compared to 29% in the other animal administered α-GalCer (blue-filled square), and a higher CD4+ NKT cell frequency of 77% (cf. 51%) (data not shown). As in our previous study [12], the proportion of CD8+ NKT cells rose concomitantly (Fig. 5c), while variable depletion was observed in the CD4+CD8+ (DP) subset (Fig. 5d). Despite NKT cell modulation after α-GalCer administration, peripheral SIV viraemia and CD4 T cell levels were very similar between the animals administered α-GalCer to those not administered α-GalCer (Fig. 5e,f). In addition, preliminary data carried out on these two animals found no changes in levels of serum cytokines such as IL-2, IL-4, IFN-γ or TNF at 2 weeks post-α-GalCer administration compared to pre-administration levels (data not shown).
Discussion
NKT cells can be harnessed to modulate infections to microbes by exploiting their ability to enhance innate and adaptive immune responses [37]. NKT cell numbers vary widely in humans, and this may influence the ability of these cells to influence immunity (reviewed in [1, 7]), and similar variability in NKT cell numbers is observed in macaques [12, 49, 50]. Macaques represent an important model for the study of influenza and HIV infection and immunity [42, 43, 54–57] and may be a valuable means to study the impact of NKT cell activation on these diseases, and also whether NKT cell numbers can be enhanced by in-vivo activation. Therefore, we undertook a study to determine the response of macaque NKT cell activation in vivo, as well as a pilot study into the ability of NKT cell activation to enhance immune responses and/or ameliorate infection. We observed a transient reduction in detectable NKT cells within 2 days of α-GalCer administration, followed by restoration of NKT cell numbers, the extent of which appeared to vary in an α-GalCer dose-dependent manner. Thus, a low dose of 1 μg resulted in normal to increased NKT cell numbers, particularly when loaded onto PBMC, while higher doses (10–100 μg) tended towards lower NKT cell numbers compared to the starting population, regardless of the mode of delivery. Our preliminary findings failed to demonstrate an enhanced immune response to influenza or SIV infection following α-GalCer co-administration.
Presentation of α-GalCer upon APC generated in vitro with IL-2 and GM-CSF from cultured autologous PBMC was successful in elevating baseline NKT cell levels in some cancer patients [14–16, 46–48]. We determined the efficacy of introducing α-GalCer-pulsed PBMC or peripheral blood into healthy macaques without the use of long-term cultures or cytokines during the pulsing stage. Similar to previous studies in humans, expansion of NKT cells above baseline levels was observed in some but not all animals after delivery of α-GalCer either i.v., pulsed onto PBMC or pulsed onto peripheral blood. At best, a 2·5–3·5-fold expansion of NKT cells was achieved. Interestingly the two animals that had the most expansion had one of the highest baseline NKT cell frequencies.
Previous studies in humans have shown a decrease in circulating numbers of NKT cells upon i.v. injection of α-GalCer, particularly at 2 days post-administration, and a lack of rebound to baseline levels [17, 58]. Our data are partially consistent with this finding, although with the caveat that the extent of rebound appears to be α-GalCer dose-dependent. The early transient depletion of macaque peripheral NKT cells due to α-GalCer stimulation, also noted in mice studies, most probably reflects TCR down-regulation causing NKT cells to be undetectable by CD1d tetramers [52, 53, 59, 60]. However, we cannot exclude the possibility that some of the NKT cell disappearance was due to migration from blood to other tissues. We also observed gradual long-term depletion of blood NKT cells (<75% of baseline levels) in 13 of 19 animals administered 10–100 μg α-GalCer, and followed to 10 weeks after α-GalCer administration. Again, we were unable to determine if this was due to NKT cell death or migration to other sites.
Given that, in some studies, in-vivo activation of NKT cells by α-GalCer results in an anergic response to rechallenge [51, 61], it was also important to determine whether in-vivo activated NKT cells were still capable of producing cytokines at different time-points after α-GalCer administration. While these previously stimulated NKT cells were clearly still capable of robust IFN-γ production, the percentage of cells responding was slightly but significantly lower. At the time this study was conducted, optimal methods of ex-vivo glycolipid antigen-specific activation of macaque NKT cells [41] had not been developed, so we were limited to mitogenic NKT cell activation to induce clear cytokine production. At this stage we cannot exclude the possibility that antigen-specific NKT cell activation following in-vivo α-GalCer stimulation may be different to mitogenic activation, or that expression of cytokines other than IFN-γ may have correlated with expansion.
In mice, NKT cells have preformed IL-4 and IFN-γ mRNA transcripts poised for immediate secretion upon ligand stimulation [62, 63]. Similarly, in macaques, the peak IFN-γ expression may have occurred much earlier after in-vivo α-GalCer stimulation which would have been missed in this study, because NKT cells were essentially undetectable at days 2/3. Furthermore, in mice, in-vivo α-GalCer stimulation results in peak secretion of various cytokines such as IL-4 and IFN-γ at different times after priming, which can precede peak NKT cell expansion [51, 63]. Future studies could assess additional time-points for NKT cell activation after in-vivo α-GalCer administration using IFN-γ expression, expression of other cytokines and other NKT cell activation markers such as CD69, as well as the use of an exhaustion marker such as programmed death (PD)-1 [64, 65], which may be useful in elucidating any apparent NKT cell dysfunction.
While it was encouraging that we detected a clear NKT cell response to in-vivo-administered α-GalCer, our pilot study of the impact of α-GalCer on immunity to influenza or SIV did not reveal an enhanced immune response to these viruses. It is possible that the timing of administration of α-GalCer, together with influenza vaccination (Fig. 4) or prior to SIV exposure (Fig. 5) in macaques, was suboptimal. However, studies of α-GalCer therapy in mouse models of infections were successful in clearing infection and/or augmenting T cell responses using an α-GalCer delivery timing strategy similar to that performed in our studies [29, 31, 32, 66–68]. For example, when α-GalCer was administered at the same time as irradiated sporozoites, enhanced T cell immunity to the recombinant adenovirus vector was detected [67]. Another study by the same group found that α-GalCer delivery 2 days before live sporozoite challenge cleared hepatic parasites completely [66]. In our study, we found no enhancement in CD8 T cell or antibody responses to live attenuated influenza virus after inoculation. α-GalCer may play a more useful role in augmenting CD8 T cell responses to inactivated influenza virus vaccination as noted in a mouse model [29], compared to the live influenza infection model we studied.
In our SIV infection study of two macaques, we reasoned that NKT cells and downstream activation of effector cells may be at their peak of activation 2 days after α-GalCer administration, as evidenced by a loss of circulating NKT cells. Using this approach, however, we found no significant impact of α-GalCer delivery on the course of SIV infection. We cannot exclude the possibility that cytokine production, particularly IFN-γ secretion, or bystander activation of other cell types such as APCs, necessary to combat SIV replication, may have subsided within the 48 h before infection.
There are several limitations to our study that warrant further attempts to modulate NKT cells in macaque models. First, we have not studied α-GalCer delivery via autologous DC. Although this method has been highly successful in both mice and small human cancer trials [14–16, 20, 46–48], such methods will not be practical in diverse clinical settings. Secondly, the timing of α-GalCer delivery may not have been optimal to influence influenza or SIV-specific immunity. For example, it remains possible that NKT cells stimulated by α-GalCer would be more effective at controlling SIV if timed to coincide more closely with peak viraemia levels 2 weeks after infection. Thirdly, we did not study tissue NKT cell levels or their activation status. We observed a modest expansion of peripheral NKT cells, and we know that substantial expansion of NKT cells can occur in the spleen and liver of mice following α-GalCer administration [51, 60]. Further studies are needed to determine if trafficking and expansion may have occurred in such sites. Taken together, a more extensive study comparing the kinetics of α-GalCer administration and a broader analysis of various cytokines, bystander cell types and different tissue sources is required to determine correctly the influence of α-GalCer on influenza-or SIV-specific immunity.
In summary, we show that macaque peripheral NKT cells can be modulated safely with α-GalCer by pulsing onto PBMC or WB. Lower α-GalCer doses (<10 μg) appeared to result in better NKT cell expansion in peripheral blood than higher α-GalCer doses (10 or 100 μg). However, in preliminary experiments, we did not observe enhanced immunity to influenza or SIV infection associated with α-GalCer co-administration. Further studies are warranted to explore and optimize the impact of NKT cells on infectious diseases in macaque models.
Acknowledgments
We thank Angela Chan, Thakshila Amarasena, Marcin Chiula, Sheilajen Alcantara, Kon Kyparissoudis and Wendy Winnall, and Drs Karen Laurie and Aeron Hurt of the World Health Organization (WHO) for expert assistance and advice. This research was supported by the Australian National Health and Medical Research (NHMRC) awards 510448, 629000 and 454569. D.I.G. is supported by an NHMRC senior principal research fellowship 454309; S.J.K. is supported by an NHMRC research fellowship 508937.
Disclosure
The authors declare that they have no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Equivalent staining of pigtail macaque peripheral blood mononuclear cells (PBMC) with CD1d tetramers loaded with either PBS-44 or KRN7000. Frozen PBMC from three macaques were stained singly with CD3 in combination with either CD1d tetramers loaded with PBS-44 conjugated to phycoerythrin (PE) fluorochrome or CD1d tetramers loaded with KRN7000 conjugated to allophycocyanin (APC) fluorochrome.
References
- 1.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11:131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 3.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4–CD8– T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 5.Eger KA, Sundrud MS, Motsinger AA, Tseng M, Van Kaer L, Unutmaz D. Human natural killer T cells are heterogeneous in their capacity to reprogram their effector functions. PLoS ONE. 2006;1:e50. doi: 10.1371/journal.pone.0000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 8.Montoya CJ, Catano JC, Ramirez Z, Rugeles MT, Wilson SB, Landay AL. Invariant NKT cells from HIV-1 or Mycobacterium tuberculosis-infected patients express an activated phenotype. Clin Immunol. 2008;127:1–6. doi: 10.1016/j.clim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med. 2002;195:869–879. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Vliet HJ, von Blomberg BM, Hazenberg MD, et al. Selective decrease in circulating V alpha 24+V beta 11+ NKT cells during HIV type 1 infection. J Immunol. 2002;168:1490–1495. doi: 10.4049/jimmunol.168.3.1490. [DOI] [PubMed] [Google Scholar]
- 11.Motsinger A, Azimzadeh A, Stanic AK, et al. Identification and simian immunodeficiency virus infection of CD1d-restricted macaque natural killer T cells. J Virol. 2003;77:8153–8158. doi: 10.1128/JVI.77.14.8153-8158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez CS, Chan AC, Kyparissoudis K, De Rose R, Godfrey DI, Kent SJ. Peripheral NKT cells in simian immunodeficiency virus-infected macaques. J Virol. 2009;83:1617–1624. doi: 10.1128/JVI.02138-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandberg JK, Fast NM, Palacios EH, et al. Selective loss of innate CD4(+) V alpha 24 natural killer T cells in human immunodeficiency virus infection. J Virol. 2002;76:7528–7534. doi: 10.1128/JVI.76.15.7528-7534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida T, Horiguchi S, Tanaka Y, et al. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57:337–345. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunii N, Horiguchi S, Motohashi S, et al. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–1098. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamasaki K, Horiguchi S, Kurosaki M, et al. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol. 2011;138:255–265. doi: 10.1016/j.clim.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Giaccone G, Punt CJ, Ando Y, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 18.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 21.Schneiders FL, Scheper RJ, von Blomberg BM, et al. Clinical experience with alpha-galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol. 2011;140:130–141. doi: 10.1016/j.clim.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Emoto M, Emoto Y, Yoshizawa I, et al. Alpha-GalCer ameliorates listeriosis by accelerating infiltration of Gr-1+ cells into the liver. Eur J Immunol. 2010;40:1328–1341. doi: 10.1002/eji.200939594. [DOI] [PubMed] [Google Scholar]
- 23.Juno JA, Keynan Y, Fowke KR. Invariant NKT cells: regulation and function during viral infection. PLoS Pathog. 2012;8:e1002838. doi: 10.1371/journal.ppat.1002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novak J, Lehuen A. Mechanism of regulation of autoimmunity by iNKT cells. Cytokine. 2011;53:263–270. doi: 10.1016/j.cyto.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Hong S, Wilson MT, Serizawa I, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 26.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 27.De Santo C, Salio M, Masri SH, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Exley MA. Editorial: NKT get the 'flu: NKT cells as (mostly) good guys in influenza; monocytic cells as double agents. J Leukoc Biol. 2012;91:349–352. doi: 10.1189/jlb.0911468. [DOI] [PubMed] [Google Scholar]
- 29.Guillonneau C, Mintern JD, Hubert FX, et al. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc Natl Acad Sci USA. 2009;106:3330–3335. doi: 10.1073/pnas.0813309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galli G, Pittoni P, Tonti E, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youn HJ, Ko SY, Lee KA, et al. A single intranasal immunization with inactivated influenza virus and alpha-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine. 2007;25:5189–5198. doi: 10.1016/j.vaccine.2007.04.081. [DOI] [PubMed] [Google Scholar]
- 32.Reilly EC, Thompson EA, Aspeslagh S, Wands JR, Elewaut D, Brossay L. Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection. PLoS ONE. 2012;7:e37991. doi: 10.1371/journal.pone.0037991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Chen A, Li X, et al. DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine. 2008;26:1807–1816. doi: 10.1016/j.vaccine.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Dondji B, Deak E, Goldsmith-Pestana K, et al. Intradermal NKT cell activation during DNA priming in heterologous prime-boost vaccination enhances T cell responses and protection against Leishmania. Eur J Immunol. 2008;38:706–719. doi: 10.1002/eji.200737660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindqvist M, Persson J, Thorn K, Harandi AM. The mucosal adjuvant effect of alpha-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J Immunol. 2009;182:6435–6443. doi: 10.4049/jimmunol.0900136. [DOI] [PubMed] [Google Scholar]
- 37.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 38.Chea S, Dale CJ, De Rose R, Ramshaw IA, Kent SJ. Enhanced cellular immunity in macaques following a novel peptide immunotherapy. J Virol. 2005;79:3748–3757. doi: 10.1128/JVI.79.6.3748-3757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Rose R, Fernandez CS, Smith MZ, et al. Control of viremia and prevention of AIDS following immunotherapy of SIV-infected macaques with peptide-pulsed blood. PLoS Pathog. 2008;4:e1000055. doi: 10.1371/journal.ppat.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez CS, Reece JC, Saepuloh U, et al. Screening and confirmatory testing of MHC class I alleles in pig-tailed macaques. Immunogenetics. 2011;63:511–521. doi: 10.1007/s00251-011-0529-5. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez CS, Cameron G, Godfrey DI, Kent SJ. Ex-vivo alpha-galactosylceramide activation of NKT cells in humans and macaques. J Immunol Methods. 2012;382:150–159. doi: 10.1016/j.jim.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Jegaskanda S, Reece JC, De Rose R, et al. Comparison of influenza and SIV specific CD8 T cell responses in macaques. PLoS ONE. 2012;7:e32431. doi: 10.1371/journal.pone.0032431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sexton A, De Rose R, Reece JC, et al. Evaluation of recombinant influenza virus–simian immunodeficiency virus vaccines in macaques. J Virol. 2009;83:7619–7628. doi: 10.1128/JVI.00470-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motohashi S, Ishikawa A, Ishikawa E, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 45.Motohashi S, Nagato K, Kunii N, et al. A phase I–II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–2501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 46.Nieda M, Okai M, Tazbirkova A, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa A, Motohashi S, Ishikawa E, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 48.Nicol AJ, Tazbirkova A, Nieda M. Comparison of clinical and immunological effects of intravenous and intradermal administration of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells. Clin Cancer Res. 2011;17:5140–5151. doi: 10.1158/1078-0432.CCR-10-3105. [DOI] [PubMed] [Google Scholar]
- 49.Gansuvd B, Goodwin J, Asiedu CK, et al. Invariant natural killer T cells from rhesus macaque spleen and peripheral blood are phenotypically and functionally distinct populations. J Med Primatol. 2008;37:1–11. doi: 10.1111/j.1600-0684.2007.00222.x. [DOI] [PubMed] [Google Scholar]
- 50.Rout N, Else JG, Yue S, Connole M, Exley MA, Kaur A. Paucity of CD4+ natural killer T (NKT) lymphocytes in sooty mangabeys is associated with lack of NKT cell depletion after SIV infection. PLoS ONE. 2010;5:e9787. doi: 10.1371/journal.pone.0009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uldrich AP, Crowe NY, Kyparissoudis K, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crowe NY, Uldrich AP, Kyparissoudis K, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;171:4020–4027. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 53.Wilson MT, Johansson C, Olivares-Villagomez D, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T. HIV/AIDS: in search of an animal model. Trends Biotechnol. 2007;25:333–337. doi: 10.1016/j.tibtech.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Hatziioannou T, Ambrose Z, Chung NP, et al. A macaque model of HIV-1 infection. Proc Natl Acad Sci USA. 2009;106:4425–4429. doi: 10.1073/pnas.0812587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lackner AA, Veazey RS. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu Rev Med. 2007;58:461–476. doi: 10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- 57.Richt JA, Rockx B, Ma W, et al. Recently emerged swine influenza A virus (H2N3) causes severe pneumonia in Cynomolgus macaques. PLoS ONE. 2012;7:e39990. doi: 10.1371/journal.pone.0039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woltman AM, Ter Borg MJ, Binda RS, et al. Alpha-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir Ther. 2009;14:809–818. doi: 10.3851/IMP1295. [DOI] [PubMed] [Google Scholar]
- 59.Harada M, Seino K, Wakao H, et al. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int Immunol. 2004;16:241–247. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- 60.Subleski JJ, Hall VL, Wolfe TB, et al. TCR-dependent and-independent activation underlie liver-specific regulation of NKT cells. J Immunol. 2011;186:838–847. doi: 10.4049/jimmunol.1001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parekh VV, Wilson MT, Olivares-Villagomez D, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stetson DB, Mohrs M, Reinhardt RL, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuda JL, Gapin L, Baron JL, et al. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci USA. 2003;100:8395–8400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parekh VV, Lalani S, Kim S, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182:2816–2826. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang WS, Kim JY, Kim YJ, et al. Cutting edge: programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J Immunol. 2008;181:6707–6710. doi: 10.4049/jimmunol.181.10.6707. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, et al. alpha-galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–8466. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. Alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175:3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Equivalent staining of pigtail macaque peripheral blood mononuclear cells (PBMC) with CD1d tetramers loaded with either PBS-44 or KRN7000. Frozen PBMC from three macaques were stained singly with CD3 in combination with either CD1d tetramers loaded with PBS-44 conjugated to phycoerythrin (PE) fluorochrome or CD1d tetramers loaded with KRN7000 conjugated to allophycocyanin (APC) fluorochrome.