Abstract
Antibodies recognizing denatured human leucocyte antigen (HLA) can co-react with epitopes on intact HLA or recognize cryptic epitopes which are normally unaccessible to HLA antibodies. Their specificity cannot be distinguished by single antigen beads (SAB) alone, as they carry a mixture of intact and denatured HLA. In this study, we selected pretransplant sera containing donor-specific HLA class I antibodies (DSA) according to regular SAB analysis from 156 kidney transplant recipients. These sera were analysed using a SAB preparation (iBeads) which is largely devoid of denatured HLA class I, and SAB coated with denatured HLA class I antigens. A total of 241 class I DSA were found by regular SAB analysis, of which 152 (63%) were also found by iBeads, whereas 28 (11%) were caused by reactivity with denatured DNA. Patients with DSA defined either by regular SAB or iBeads showed a significantly lower graft survival rate (P = 0·007) compared to those without HLA class I DSA, whereas reactivity to exclusively denatured HLA was not associated with decreased graft survival. In addition, DSA defined by reactivity to class I SAB or class I iBeads occurred more frequently in female patients and in patients with historic HLA sensitization, whereas reactivity to denatured HLA class I was not associated with any of these parameters. Our data suggest that pretransplant donor-specific antibodies against denatured HLA are clinically irrelevant in patients already sensitized against intact HLA.
Keywords: denatured HLA, donor specific antibodies, kidney transplantation, single-antigen bead array
Introduction
The use of coloured microbeads coated with purified human leucocyte antigen (HLA) antigens has enabled the precise determination of HLA antibody specificities by flow cytometry [1]. The high sensitivity and resolution of this HLA antibody detection by single antigen bead array (SAB) suggests the potential use of this technique for a virtual cross-match in the allocation of renal allografts [2, 3]. However, the significance of antibodies that react with denatured HLA class I molecules is unclear, and it may be clinically relevant to distinguish between reactions to denatured HLA and intact HLA molecules [4–7]. Denatured HLA class I is defined as the HLA α-chain from which β2-microglobulin and peptide within the groove is removed. This causes cryptic epitopes to be exposed, allowing antibody binding to normally unaccessible epitopes [8].
The distinction between antibodies reactive with intact or denatured HLA cannot be made using regular SAB only, as they carry a mixture of both [9, 10]. Also, flow cytometry cannot distinguish reliably between these antibodies as activated cells, including B and T cells, carry both intact and denatured HLA [11] and the technique does not discriminate between HLA and non-HLA antigens binding to the cell surface. Recently, a new preparation of SAB has become available (designated iBeads; formerly known as clean beads), which is largely devoid of denatured HLA and may overcome these concerns [12]. In pretransplant sera from 156 patients who contain class I immunoglobulin (Ig)G donor-specific HLA antibodies (DSA) according to regular SAB analysis, we assessed whether or not the presence of antibodies specific for denatured versus intact HLA is associated with graft survival, kidney graft function and demographic data known to be associated with HLA immunization. In this analysis, patients without class I IgG DSA according to regular SAB analysis served as reference population. In addition, we examined similarities and differences in DSA reacting with classic SAB, iBeads and beads coated with denatured HLA.
Patients and methods
Patients and detection of HLA antibodies
We have analysed sera taken prior to 837 kidney transplants performed after a negative cross-match, using the basic complement-dependent NIH technique on unseparated peripheral blood mononuclear cells. Retransplants with repeated HLA mismatches were not performed. Serology was used for typing of patients and donors from 1990 to 1993, and molecular typing techniques [sequence-specific primers (SSP) or sequence-specific oligonucleotides (SSO)] were used from 1993 to 2008. Patients were primarily Caucasian, aged between 18 and 70 years. Standard immunosuppression before 1997 was cyclosporin/prednisone, and after 1997 patients were treated with tacrolimus/mycophenolate mofetil with prednisone for the first 4 months. Graft loss was defined according to the Eurotransplant graft failure system as irreversible end-stage renal failure, necessitating renal replacement therapy for patient survival. According to this definition, 87 graft failures occurred prior to month 6 after transplantation and 122 thereafter. DSA were rarely defined by solid-phase assays in the period prior to 2009, and results known prior to transplantation did not influence the initial and/or maintenance immunosuppression. DSA was assigned for the HLA-A,-B,-C,-DR and-DQ antigens. Pretransplant sera were stored at −80°C. In 156 cases, HLA class I DSA were found by single antigen beads (One Lambda, Canoga Park, CA, USA) when a threshold of > 1000 mean fluorescence intensity (MFI) signal above background values was used. These patients are included in the current study.
Analysis of sera for reactivity against denatured and intact HLA
The presence of intact HLA on beads was examined using the W6/32 monoclonal antibody (mAb) which reacts with a conformational epitope on intact HLA class I molecules (Sanbio, Uden, the Netherlands). The mAb HC10 (OneLambda) recognizes the β2-microglobulin free heavy chain of HLA class I and was used to assess the presence of denatured HLA on beads. Binding of these mAbs to the beads was assessed by incubation with phycoerythrin-conjugated goat anti-mouse IgG. Pretransplant sera were analysed using iBeads, which are largely devoid of denatured HLA and SAB with denatured HLA. All tests were performed according to the manufacturer's protocol (One Lambda). In order to denature HLA on the surface of regular SAB, beads were treated for 1 h with 0·3 M glycine-HCL with 1% bovine serum albumin (BSA) at pH 2·7, as described previously [11]. For direct comparison of MFI results between the different beads (MFI values obtained by W6/32 and HC10, plus the bead-to-bead comparisons), ‘raw MFI values’, i.e. no normalization, was applied. The presence of DSA defined by > 1000 MFI above background values was assigned individually by reactivity towards regular SAB, iBeads or beads with denatured HLA. For example, a serum could be found to contain antibodies against HLA-B44 by all three bead preparations, and also antibodies against HLA-A3 according to regular SAB and iBead analysis, but not by denatured SAB. DSA binding exclusively to denatured HLA was defined as either DSA binding to denatured HLA but not to regular SAB and iBeads, or binding both to denatured HLA and regular SAB but not to iBeads. Functionally monospecific DSA reactive with public epitopes on different HLA gene products were grouped into cross-reactive groups (CREG) according to schemes published earlier [13]. CREGs were defined as 1C (A1, A3, A23, A24, A25, A26, A34, A11, A29, A30, A31, A32, A33), 2C (A2, A68, A69, A23, A24, B57, B58), 5C (B51, B52, B18, B35, B62, B63, B57, B58, B49, B50), 7C (B7, B8, B13, B54, B55, B27, B60, B61, B41), 8C (B8, B14, B38, B39, B18), 12C (B44, B45, B13, B49, B50, B60, B61, B41) and Bw4 and Bw6. This classification is used in the Supporting information, Table S1.
Statistical analysis
Graft-survival rates were computed using the Kaplan–Meier method and groups were compared by the log-rank test. In all circumstances death-censored graft survival is reported, i.e. the definition of graft failure did not include patient death with a functioning graft. Graft survival was compared between patients with and without class I DSA according to reactivity against different bead preparations (regular SAB, iBeads and beads coated with denatured HLA antigens) using log-rank analysis. Continuous variables were analysed by Student's t-test and categorical data with Fisher's exact test. MFI values between regular SAB, iBeads and beads coated with denatured HLA antigens were compared by linear regression. Statistical analyses were performed using spss version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Detection of intact and denatured HLA on different bead preparations
We first assessed reactivity of the HC10 and W6/32 mAbs with regular SAB, SAB containing denatured HLA and iBeads. This showed that the use of W6/32 yielded comparable MFI values on most regular SAB, whereas MFI values obtained after testing with HC10 ranged from 0 to 17 000 (Fig. 1a). Denaturing of all HLA molecules on the beads completely abolished binding of W6/32 to all beads (Fig. 1b). Reactivity of HC10 with iBeads was much less compared to that on regular SAB, whereas W6/32 yielded different MFI values per bead ranging from 2000 to 17 000 (Fig. 1c).
Figure 1.
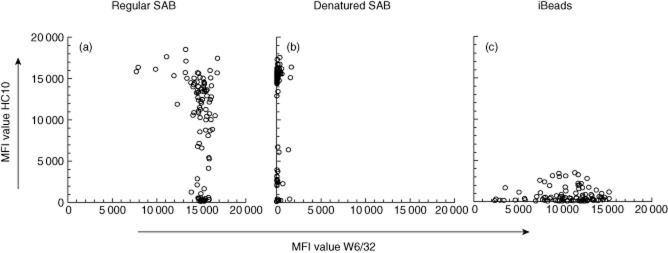
Reactivity of W6/32 and HC10 on different bead preparations. Regular single antigen bead (SAB) (a), denatured SAB coated with denatured human leucocyte antigen (HLA) (b) and iBeads (c) were incubated with W6/32 or HC10. The beads used to assess reactivity against denatured HLA (b) were treated with acid, as described in the Patients and methods section. Shown are the raw mean fluorescence intensity (MFI) values with those of W6/32 on the x-axis and HC10 on the y-axis. Specificities found by HC10 > 1000 MFI on iBeads were B*44:03, B*47:01, B*15:11, B*39:01, A*66:01, B*18:01, B*51:02, A*69:01, A*33:03, B*15:10, A*33:01, B*15:16, A*34:02, B*15:02, A*66:02, A*25:01, B*13:01, B*15:13, B*37:01, A*34:01, A*68:02, A*26:01, B*35:01, B*53:01 and B*38:01.
HLA antibodies recognize mainly intact HLA
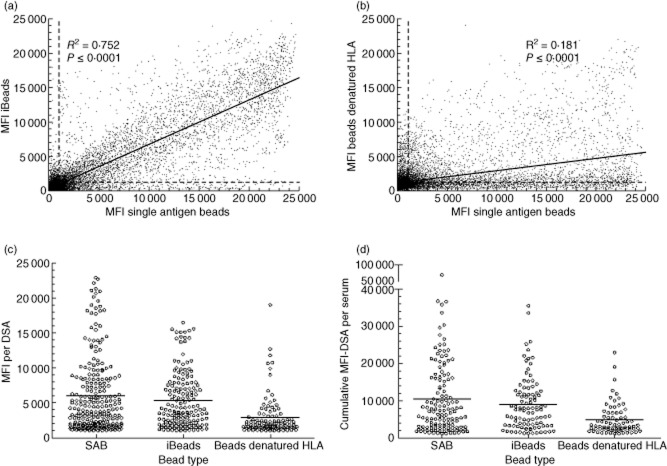
To compare HLA antibody reactivity against regular SAB, SAB with denatured HLA and iBeads, we selected pretransplant sera from 156 kidney recipients containing class I DSA according to regular SAB analysis. A comparison of all raw MFI data between the three different types of beads showed that MFI values from individual beads present in the regular SAB test correlated with those obtained using iBead values (Fig. 2a, r2 = 0·752). On average, iBeads yielded 25% lower MFI values than regular SAB. Of note is that iBeads with high MFI values were present while the MFI values of corresponding beads in the regular SAB test were low, but also vice versa. Further comparison between reactivity towards SAB versus beads with denatured HLA (Fig. 2b) showed that most of the reactivity to HLA antigens present on regular SAB is reduced severely after denaturation of HLA antigens. Reactivity towards most beads was completely absent after denaturation of HLA, although beads were present retaining their HLA-antibody binding capacity despite denaturation of HLA. The data displayed in Fig. 2 indicate that the main reactivity of HLA antibodies is directed against intact HLA antigens present on regular SAB and iBeads.
Figure 2.
Comparison of mean fluorescence intensity (MFI)-values and donor-specific human leucocyte antigen (HLA) antibodies (DSA) between different bead preparations. Raw MFI-values of single antigen bead (SAB) analysis, iBeads and beads coated with denatured HLA from 156 sera were obtained by testing on the same lot number of single antigen beads. (a) Shows > 15·000 luminex data points comparing SAB with iBeads and (b) contains the same number of comparisons between SAB and beads with denatured HLA. Cut-off reference lines discriminating positive from negative reactions (as indicated in the Patients and methods section) are indicated in (a) and (b) by striped lines. The solid lines in (a) and (b) show linear regressions including the R2 and P-values. MFI values of DSA assigned by the different bead preparations are depicted in (c), whereas the cumulative MFI values of all DSA found in individual sera are shown in (d). The lines in panels (c) and (d) represent average values found.
The number and specificity of DSA assigned depends largely upon the bead type used
We next defined the number and specificity of DSA defined by reactivity towards the three different types of beads (Table 2 and Supporting information, Table S1). In 156 sera, 241 DSA were identified by regular SAB whereas, respectively, 187 and 154 DSA were identified by iBeads and beads coated with denatured HLA. From the DSA defined by regular SAB, 152 of 241 (63%) were also found by iBeads, 28 (11%) were caused by reactivity with denatured HLA (pattern 3 Table 2), 53 DSA were defined identically by all three types of beads (pattern 1) and 61 by SAB only (pattern 5). Combined positivity on regular beads and iBeads was observed most frequently (n = 99). Remarkably, many DSA were found on one bead type only (61 with regular SAB, 25 with iBeads and 63 beads coated with denatured HLA).
Table 2.
Patterns of DSA found in pretransplant sera (n = 156).
| Bead type | DSA pattern of reactivity |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| SAB | + | + | + | − | + | − | − |
| iBead | + | + | − | + | − | + | − |
| Denatured | + | − | + | + | − | − | + |
| Total no. found | 53 | 99 | 28 | 10 | 61 | 25 | 63 |
The presence of donor-specific human leucocyte antigen (HLA) antibodies (DSA) was defined according to reactivity against single antigen bead (SAB), iBeads or beads with denatured HLA. The presence or absence of reactivity against a specific type of bead is indicated by a + or − sign leading to seven patterns of reactivity against the three bead preparations. Each DSA from each serum was counted. Pattern 1 occurs when antibodies react with both intact and denatured HLA, patterns 2 and 6 represent antibodies recognizing only intact HLA, patterns 3 and 7 are considered as reactivity towards denatured HLA only, the specificity of antibodies yielding pattern 4 is unclear and discussed separately, whereas pattern 5 probably arises from low levels of antibodies against intact HLA. In the table are the actual DSA numbers found per pattern of reactivity in 156 sera.
No frequent DSA specificities or CREGs could be found by regular beads or iBeads, scoring negative on beads with denatured HLA. In addition, DSA or CREGs found by beads with denatured HLA were also found by regular beads or iBeads. Most individual sera contain multiple DSA showing different patterns of reactivity against the three different bead preparations. A separate analysis showed that the observed frequency of DSA reactivity defined by the three different types of beads was not skewed by a limited number of sera (data not shown).
MFI values of DSA found by regular beads, iBeads and beads with denatured HLA ranged up to 23 000, 16 500 and 19 000, with average values of 6000, 5400 and 2900, respectively (Fig. 2c). Cumulative MFI values of all DSA present in individual sera ranged up to 70 000, 35 500 and 22 800 using regular beads, iBeads and beads with denatured HLA, respectively, with average values of 10 500, 8900 and 4800 (Fig. 2d).
Reactivity against intact but not denatured HLA is related to kidney graft function and graft loss
Comparison of demographic parameters with DSA defined by iBeads and SAB with denatured HLA showed that class I DSA were found more frequently according to iBead analysis in patients with historic HLA sensitization (P = 0·007), and trends for immunization were found for female recipients (P = 0·07) and patients awaiting retransplantation (P = 0·09) (Table 1), These differences were not found when beads were used containing denatured HLA. No other relation could be found between any of other the parameters shown in Table 1 and results of reactivity towards iBeads or beads containing denatured HLA antigens.
Table 1.
Characteristics of the study population according to reactivity with different bead preparations (n = 837).
| Class I DSA (no. patients) | Bead type |
||
|---|---|---|---|
| Regular SAB | iBeads | Denatured SAB | |
| Pos | Pos/Neg | Pos/Neg | |
| 156 | 120/36 | 64/92 | |
| Recipient sex (no. male) | 68 | 48/20 | 25/43 |
| (no. female) | 88 | 72/16* | 39/49 |
| Recipient age (years ± s.d.) | 46 ± 13 | 47 ± 14/45 ± 13 | 46 ± 14/47 ± 13 |
| Donor sex (no. male) | 77 | 58/19 | 36/41 |
| (no. female) | 79 | 62/17 | 28/51 |
| Donor age (years ± s.d.) | 45 ± 15 | 45 ± 14/44 ± 16 | 46 ± 14/44 ± 15 |
| Transplant type | |||
| no. living | 31 | 23/8 | 13/18 |
| no. deceased | 125 | 97/28 | 51/74 |
| Transplant (no. = 1) | 130 | 97/33 | 55/75 |
| (no. > 1) | 26 | 23/3 | 9/17 |
| Cold ischaemia time (h ± s.d.) | 19 ± 12 | 19 ± 12/20 ± 12 | 20 ± 13/18 ± 12 |
| HLA-A,-B-DR mismatches (no.) | |||
| 0 | 6 | 3/3 | 2/4 |
| 1–4 | 144 | 112/32 | 57/87 |
| 5–6 | 6 | 5/1 | 5/1 |
| Induction therapy applied | 21 | 15/6 | 7/14 |
| not applied | 135 | 105/30 | 57/78 |
| hPRA < 5 | 74 | 50/24** | 29/45 |
| ≥ 5 | 82 | 70/12 | 35/47 |
Patients were defined as single antigen bead-donor-specific human leucocyte antigen (HLA) antibodies (SAB-DSA)-positive when mean fluorescence intensity (MFI)-values of HLA antibodies against kidney grafts were 1000 above background values. The sera from 156 patients with HLA class I DSA defined by regular SAB were analysed further using iBeads and beads with denatured HLA. Maintenance immunosuppression was applied as indicated in the Patients and methods section, with a change in 1997. Interleukin (IL)-2 blockade as induction therapy has been applied since 2005 in patients with an established high immunological risk (i.e. second or more kidney transplantation or panel reactive antigen > 40%). hPRA, highest historical pretransplant value of panel-reactive antibodies; s.d.: standard deviation. Asterisks indicate the following P-values
P = 0·07
P = 0·007.
The 156 patients with class I DSA according to SAB at year 1 had a serum creatinine comparable to patients without them [161 (87) μmol/l versus 157 (88) μmol/l; P = 0·99]. However, at year 5 kidney function in the group with class I DSA was worse [158 (79) μmol/l versus 170 (109) μmol/l; P = 0·02]. No statistically significant difference was seen at either years 1 or 5 with regard to creatinine between iBeads or beads containing denatured HLA antigens [year 1: 158 (101) μmol/l; versus 154 (20) μmol/l; P = 0·49; year 5: 177 (110) μmol/l; versus 144 (53) μmol/l; P = 0·28]. For rejection episodes a distinction was made between clinical rejection and biopsy-proven episodes. In total, 221 patients underwent a clinical rejection episode, from which 179 were biopsy-proven. Of the class I DSA SAB-positive patients, 47 had a clinical rejection and in 36 cases this was biopsy-proven. However, there was no statistically significant difference in either type of rejection episodes when compared to the patients who did not have class I DSA SAB positivity (P = 0·34 and P = 0·11, respectively). There was also no statistical difference between rejection episodes in patients who were positive for iBeads (clinical rejection 40/biopsy-proven rejection 30) or beads containing denatured HLA antigens (clinical rejection seven/biopsy-proven rejection six; P = 0·47/0·35).
We next examined the relation between graft loss and the pretransplant presence of DSA against HLA class I, defined by reactivity against the different bead preparations. Patients with class I DSA according to regular SAB analysis showed a significantly lower graft survival rate compared to those without (Fig. 3a, P = 0·007). Analysis by iBeads showed class I DSA to be present in 120 of 156 sera. Graft survival rates were also lower in these patients compared to those without DSA, whereas the remaining 36 patients showed graft survival rates not statistically significantly different to patients without DSA (Fig. 3a). Further analysis indicated that 44 of 120 patients with iBead-defined class I DSA also had class II DSA, and their graft survival rates were significantly lower compared to 76 of 120 patients without class II DSA (P = 0·013). Sera from 20 patients contained only DSA binding exclusively to denatured HLA class I (patterns 3 + 7 in Table 2). Graft survival rates of these patients were similar (Fig. 3a) to those without DSA, irrespective of the presence of class II DSA (data not shown). In a separate analysis we examined the impact of luminex-defined HLA antibodies on short-term (within 1 year) and long-term graft survival. At year 1 after transplantation no significant difference was found in graft survival between patients with or without DSA detected either by regular SAB or iBeads (data not shown). However, resetting the graft survival analysis at 100% 1 year after transplantation showed a long-term effect of DSA defined either by regular SAB or iBeads (Fig. 3b).
Figure 3.
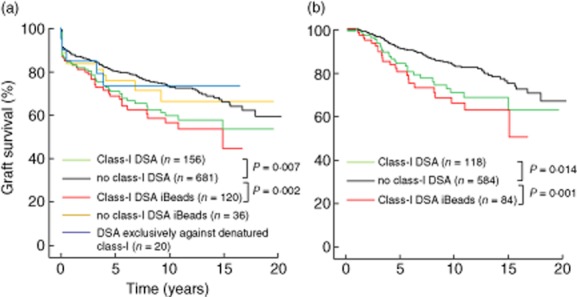
Comparison of graft survival in patients stratified according to reactivity with different bead preparations. All graft survival analysis were death censored. (a) Graft survival is shown of patients with (n = 156) or without (n = 681) human leucocyte antigen (HLA) class I donor-specific HLA antibodies (DSA) according to regular single antigen bead (SAB) analysis, performed as described in the Patients and methods section. All 156 sera with class I DSA were retested with iBeads and graft survival results from those patients are indicated as ‘class I DSA iBeads’ or ‘no class I DSA iBeads’. The same sera were also retested with beads containing denatured HLA class I. Graft survival from all 14 patients with pretransplant sera reacting exclusively against denatured HLA are indicated as ‘DSA exclusively against denatured class I’. (b) Analysis of long-term effect of luminex-defined class I DSA. In this analysis graft survival was reset at 100% at year 1 after transplantation.
Discussion
In pretransplant sera from 156 kidney transplant recipients with donor-specific HLA class I antibodies assessed by regular SAB, we show that only antibodies against intact HLA identify patients at risk for graft loss and are related to kidney graft function, whereas additional DSA against denatured HLA do not. In addition, we find that a substantial number of DSA can co-recognize intact and denatured HLA, indicating that discrimination between reactivities against intact versus denatured HLA is an oversimplification of the actual specificities present.
In a previous analysis on post-transplant sera, no significant difference in graft survival rate could be found between patients without HLA antibodies versus patients with antibodies against denatured HLA [7]. In that study patients with antibodies reacting only against intact HLA showed a significant decrease in graft survival rate. A subsequent analysis indicated that DSA against intact class I was associated with a poor graft prognosis, whereas the clinical relevance of DSA against denatured HLA was not analysed further. Two recent case reports showed that the pretransplant presence of DSA against exclusively denatured HLA were not associated with humoral rejection after kidney or heart transplantation [9, 10]. Furthermore, in the recently published consensus guidelines on HLA antibody detection it was recommended that awareness must be present with regard to the presence of denatured DNA on beads which can influence the result of antibody testing. The clinical relevance of pretransplant natural HLA antibodies reacting with denatured HLA is unknown at present, as it has not been addressed in large studies. It has been speculated that these antibodies arise after exposure to microbial antigens cross-reactive with cryptic epitopes on HLA [4]. In that study specificities towards HLA antigens were found that are rare (such as A*3101, B76, etc.) in the general population [4]. We found antibodies against denatured HLA-B8 to be the most frequently occurring antigen recognized in pattern 4 (Supporting information, Table S1), with numbers too low to determine whether or not they are clinically relevant. This difference in specificities of natural antibodies reported earlier in 2008 versus those in our study may be caused by a difference in the manufacturing date of single antigen beads. Intact HLA class I molecules are recognized by the W6/32 mAb, whereas denatured HLA can be identified using the HC10 mAb, recognizing the β2-microglobulin free heavy chain of HLA class I [11, 14]. With these mAbs it has been shown that denatured HLA is not present on resting B or T cells. However, activated lymphocytes, malignant cells and also normal arterial endothelial cells are able to express denatured HLA [15, 16]. Potentially, antibodies recognizing denatured HLA can bind to these cells, resulting in effector mechanisms such as transduction of intracellular activation signals or sublytic complement activation, causing the release of tissue factor [17, 18]. However, the results from our study indicate that these antibodies are not detrimental after kidney transplantation. It could be speculated that the density of denatured HLA is too low on the cell surface to yield sufficient antibody binding for activation of endothelial cells.
Data on the reactivity of the W6/32 and HC-10 mAbs with the different bead preparations showed that reactivity of W6/32 with regular SAB yielded comparable MFI values, suggesting a comparable density of HLA per bead. Average MFI values (14 700; data not shown) of W6/32 on regular SAB were higher than those found using iBeads (10 600), indicating a lower density of HLA on iBead preparations. This difference was also found after analysing patients' sera for DSA using these beads. Although reactivity of HC10 against iBeads was largely absent compared to that on regular SAB, several HLA molecules induced MFI values above 1000 on iBeads, indicating that some positive results by patient sera may not be clinically relevant for the specificities involved. Reactivity of W6/32 against iBeads was much more variable per bead compared to that observed on regular beads. These data indicate that iBeads are largely devoid of denatured HLA and that the density of HLA differs between beads, unlike that on regular SAB. Early batches of regular SAB were also found to contain different quantities of HLA, which was corrected later, and it can be expected that future batches of iBeads will address this issue accordingly. In this study we did not normalize for these differences using W6/32. Multiple regular beads yielded high MFI values with HC10 as well as W6/32, as shown in Fig. 1a, indicating the co-presence of intact and denatured HLA on their surface. As the relation between MFI values and the precise number of HLA molecules coated on the beads is unknown, no estimate can be provided with regard to the percentage of denatured HLA on these beads. In addition, in previous studies HC10 was found to bind strongly to HLA molecules carrying the motif 57PxxWDR62, but weakly or not at all to molecules where the R62 amino acid is replaced by glycine, such as is present in most HLA-A2 molecules [19]. The three beads carrying HLA-A2 examined in this study all containing G62 were recognized, although weakly (average MFI = 2800), which may be explained by the general ability of solid-phase assays to detect low-affinity antibody binding. Beads with HLA molecules completely lacking recognition by HC10 were carrying A23, A24, A30, A31, A80 and B57. These beads are displayed at the bottom left corner in Fig. 1b, and are also present at the bottom of Fig. 1a.
Comparison of HLA antibodies present in patients' sera binding to intact, denatured HLA or a mixture showed that all possible patterns of reactivity can be found in sera, indicating that a strict separation of antibodies binding to either intact or denatured HLA is not possible. This may be caused by the presence of linear epitopes present on the α-chain of HLA, which probably retain their antibody-binding capacity after denaturation, whereas tertiary HLA epitopes are lost after denaturation [20]. Indeed, in some cases antibody binding is completely unchanged after denaturing of HLA molecules by acid, as shown in Fig. 2b (upper right corner). In our study we found that 28 of the 241 DSA (11%) found by regular SAB were caused by reactivity with denatured HLA and in the clinical routine diagnostics these antibodies may be considered as DSA when testing by regular SAB only. HLA antibodies binding specifically to one type of bead can be explained by the density of HLA per bead in an intact, denatured or mixed configuration. An unexpected finding was the reactivity with iBeads and beads with denatured HLA (pattern 4), but negative with regular SAB containing a mixture of intact and denatured HLA. Although this comprises a small percentage, in theory such reactivity cannot occur unless the enzymatic treatment protocol yielding iBeads caused some cryptic epitopes to be exposed. This option is supported by the results shown in the upper left corner of Fig. 2a, where beads can be seen yielding MFI levels < 2000 MFI on regular SAB and > 10 000 MFI on iBeads. These beads have an MFI in a range between 600 and 4700 MFI on beads with denatured HLA. Finally, a number of specificities were found by iBeads only (pattern 6) but their number was too low to define their clinical relevance. These data indicate that multi-centre and multivariate evaluation needs to be performed upon introduction of iBeads into routine HLA antibody monitoring.
In this study we did not retest sera which were negative according to regular SAB, as they would also not have been analysed further in a regular diagnostic setting for the presence of antibodies against denatured HLA. Furthermore, we have not evaluated the clinical relevance of post-transplant DSA as a major confounder for graft survival. Important differences were observed when comparing HLA antibody binding to intact versus denatured HLA molecules with regard to clinical characteristics and outcome. First, HLA antibodies in current sera are well known to occur more frequently in females, patients awaiting retransplantation and those with historic immunization, but we show that this association is lost when denatured HLA is used as target. Secondly, and most importantly, our data show that the relation between pretransplant presence of HLA class I DSA and graft survival – including long-term graft survival – is lost when DSA are defined by binding to denatured HLA. Taken together, our data indicate that antibodies against denatured HLA are not associated with HLA immunization and are probably irrelevant for outcome of graft survival in patients sensitized against intact HLA. As a consequence, detection of these antibodies on regular SAB can be regarded as false positive results which may lead to the incorrect assignment of sensitization grade.
Disclosure
The authors of this paper have no conflicts of interest to disclose.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1. Details on donor-specific human leucocyte antigen (HLA) antibodies (DSA) and cross-reactive groups (CREGS) found in pretransplant sera (n = 156). The result of the actual DSA and grouping into CREGs is shown as a supplement to Table . Sera can contain multiple DSA and CREGs with different reactivity patterns.
References
- 1.El-Awar N, Lee J, Terasaki PI. HLA antibody identification with single antigen beads compared to conventional methods. Hum Immunol. 2005;66:989–997. doi: 10.1016/j.humimm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Bielmann D, Hönger G, Lutz D, Mihatsch MJ, Steiger J, Schaub S. Pretransplant risk assessment in renal allograft recipients using virtual crossmatching. Am J Transplant. 2007;7:626–632. doi: 10.1111/j.1600-6143.2007.01667.x. [DOI] [PubMed] [Google Scholar]
- 3.Cecka JM, Kucheryavaya AY, Reinsmoen NL, Leffell MS. Calculated PRA: initial results show benefits for sensitized patients and a reduction in positive crossmatches. Am J Transplant. 2011;11:719–724. doi: 10.1111/j.1600-6143.2010.03340.x. [DOI] [PubMed] [Google Scholar]
- 4.Morales-Buenrostro LE, Terasaki PI, Marino-Vázquez LA, Lee JH, El-Awar N, Alberú J. ‘Natural’ human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation. 2008;86:1111–1115. doi: 10.1097/TP.0b013e318186d87b. [DOI] [PubMed] [Google Scholar]
- 5.Jacob EK, De Goey SR, Gandhi MJ. Positive virtual crossmatch with negative flow crossmatch results in two cases. Transpl Immunol. 2011;25:77–81. doi: 10.1016/j.trim.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–49. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 7.Cai J, Terasaki PI, Anderson N, Lachmann N, Schönemann C. Intact HLA not beta2m-free heavy chain-specific HLA class I antibodies are predictive of graft failure. Transplantation. 2009;88:226–230. doi: 10.1097/TP.0b013e3181ac6198. [DOI] [PubMed] [Google Scholar]
- 8.El-Awar N, Terasaki PI, Nguyen A, et al. Epitopes of human leukocyte antigen class I antibodies found in sera of normal healthy males and cord blood. Hum Immunol. 2009;70:844–853. doi: 10.1016/j.humimm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Poli F, Benazzi E, Innocente A, et al. Heart transplantation with donor-specific antibodies directed toward denatured HLA-A*02:01: a case report. Hum Immunol. 2011;72:1045–1048. doi: 10.1016/j.humimm.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Pereira S, Perkins S, Lee JH, et al. Donor-specific antibody against denatured HLA-A1: clinically nonsignificant? Hum Immunol. 2011;72:492–498. doi: 10.1016/j.humimm.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Matko J, Bushkin Y, Wei T, Edidin M. Clustering of class I HLA molecules on the surfaces of activated and transformed human cells. J Immunol. 1994;152:3353–3360. [PubMed] [Google Scholar]
- 12.El-Awar NR, Terasaki PI, Hajeer A, et al. A novel HLA class I single antigen bead preparation eliminates false positive reactions attributed to natural antibodies in the sera of normal males and pre-transplant patients. Hum Immunol. 2010;71(Suppl. 1):S26. [Google Scholar]
- 13.Rodey GE, Neylan JF, Whelchel JD, Revels KW, Bray RA. Epitope specificity of HLA class I alloantibodies. I. Frequency analysis of antibodies to private versus public specificities in potential transplant recipients. Hum Immunol. 1994;39:272–280. doi: 10.1016/0198-8859(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 14.Barnstable CJ, Bodmer WF, Brown G, et al. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens – new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 15.Bijen CB, Bantema-Joppe EJ, de Jong RA, et al. The prognostic role of classical and nonclassical MHC class I expression in endometrial cancer. Int J Cancer. 2010;126:1417–1427. doi: 10.1002/ijc.24852. [DOI] [PubMed] [Google Scholar]
- 16.Pasquinelli G, Pistillo MP, Ricci F, et al. The ‘in situ’ expression of human leukocyte antigen class I antigens is not altered by cryopreservation in human arterial allografts. Cell Tissue Bank. 2007;8:195–203. doi: 10.1007/s10561-006-9025-9. [DOI] [PubMed] [Google Scholar]
- 17.Jin YP, Singh RP, Du ZY, Rajasekaran AK, Rozengurt E, Reed EF. Ligation of HLA class I molecules on endothelial cells induces phosphorylation of Src, paxillin, and focal adhesion kinase in an actin-dependent manner. J Immunol. 2002;168:5415–5423. doi: 10.4049/jimmunol.168.11.5415. [DOI] [PubMed] [Google Scholar]
- 18.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918–1926. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 19.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nat Rev Immunol. 2012;12:431–442. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- 20.Duquesnoy RJ, Takemoto S, de Lange P, et al. HLA matchmaker: a molecularly based algorithm for histocompatibility determination. III. Effect of matching at the HLA-A, B amino acid triplet level on kidney transplant survival. Transplantation. 2003;75:884–889. doi: 10.1097/01.TP.0000055101.20821.AC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Details on donor-specific human leucocyte antigen (HLA) antibodies (DSA) and cross-reactive groups (CREGS) found in pretransplant sera (n = 156). The result of the actual DSA and grouping into CREGs is shown as a supplement to Table . Sera can contain multiple DSA and CREGs with different reactivity patterns.