Abstract
Background and Purpose
Venules within the gut wall may have intrinsic mechanisms for maintaining the circulation even upon the intestinal wall distension. We aimed to explore spontaneous and nerve-mediated contractile activity of colonic venules.
Experimental Approach
Changes in the diameter of submucosal venules of the rat distal colon were measured using video microscopy. The innervation of the microvasculature was investigated using fluorescence immunohistochemistry.
Key Results
Submucosal venules exhibited spontaneous constrictions that were abolished by blockers of L-type Ca2+ channels (1 μM nicardipine), Ca2+-ATPase (10 μM cyclopiazonic acid), IP3 receptor (100 μM 2-APB), Ca2+-activated Cl− channels (100 μM DIDS) or store-operated Ca2+ entry channels (10 μM SKF96365). Transmural nerve stimulation (TNS at 10 Hz) induced a phasic venular constriction that was blocked by phentolamine (1 μM, α-adrenoceptor antagonist) or sympathetic nerve depletion using guanethidine (10 μM). Stimulation of primary afferent nerves with TNS (at 20 Hz) or capsaicin (100 nM) evoked a sustained venular dilatation that was attenuated by calcitonin gene-related peptide (CGRP) 8-37 (2 μM), a CGRP receptor antagonist. Immunohistochemistry revealed sympathetic and primary afferent nerves running along submucosal venules.
Conclusions and Implications
Submucosal venules of the rat distal colon exhibit spontaneous constrictions that appear to primarily rely on Ca2+ release from sarcoplasmic reticulum and subsequent opening of Ca2+-activated Cl– channels that trigger Ca2+ influx through L-type Ca2+ channels. Venular contractility is modulated by sympathetic as well as CGRP-containing primary afferent nerves, suggesting that submucosal venules may play an active role in regulating the microcirculation of the digestive tract.
Keywords: microvasculature, autonomic nerve, afferent nerve, neuropeptide, intestine
Introduction
The mechanisms of arteriole regulation of blood flow through the microcirculation of the intestine have been extensively studied. Sympathetic nerves, extrinsic primary afferent nerves and intrinsic neurons of the enteric nervous system all regulate the contractility of arterioles in the submucosa of the intestines (Hirst, 1977; Vanner, 1994; Kotecha and Neild, 1995; Vanner and Surprenant, 1996). Several hormones and paracrine substances have also been shown to modulate the contractility of intestinal arterioles (Hansen et al., 1998). In contrast, the functional properties of venules in the intestine are less well understood, and venules have been considered to play a passive role in regulating submucosal microcirculation. For example, submucosal venules of the guinea pig small intestine do not respond to neuronal stimulations (Hirst, 1977).
We have recently reported that venules in the mucosa of the rat and mouse bladders display spontaneous rhythmic constrictions (Hashitani et al., 2011; 2012,). Because the bladder wall is distended during filling phases, rhythmic spontaneous constrictions of suburothelial venules may be important for an active drainage. As the wall of the gastrointestinal tract is invariably distended by its luminal contents, it is reasonable to enquire whether submucosal venules in the gastrointestinal tract may also exhibit spontaneous constrictions. The distal colon was chosen in the present study because this region is chronically distended by faecal pellets. A submucosal preparation of the rat distal colon can be obtained quickly by removing the muscle and mucosal layers and is suitable for this initial examination of the properties of venules in the digestive tract.
In the submucosa of the human colon, sympathetic nerves project not only to the arteries, but also to veins with diameters larger than 250 μm (De Fontgalland et al., 2008). Submucosal arterioles in the intestine are constricted upon sympathetic nerve stimulation (Kotecha and Neild, 1995) and dilated upon release of calcitonin gene-related peptide (CGRP) and substance P (SP) from peripheral endings of capsaicin-sensitive primary afferent nerves (Vanner, 1994). However, whether sympathetic and primary afferent nerves innervate and modulate the diameter of the smaller venules of the intestine remains to be explored.
In the present study, we have demonstrated that submucosal venules of the rat distal colon exhibited spontaneous constrictions, and examined the mechanisms underlying their generation using various inhibitors of Ca2+ mobilization. The effects of phentolamine, guanethidine, capsaicin and CGRP8-37, a CGRP receptor antagonist, on contractile responses evoked upon transmural nerve stimulation (TNS) were examined to evaluate the role of sympathetic and primary afferent nerves. Immunohistochemistry was also conducted to identify the projection of sympathetic and CGRP-containing nerves to the venules.
Methods
Tissue preparation
Male Wistar rats 6–8 weeks old (Japan SLC, Shizuoka, Japan) were anaesthetised with sevoflurane and exsanguinated by decapitation. The distal half of the distal colon was cut along the mesenteric border. The experimental protocols of the present study were approved by the Nagoya City University Medical School experimental animal committee. The muscle layers and mucosa of the tissue were removed using sharp tweezers under a dissection microscope to obtain submucosal preparations. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Video imaging with Dimatrak
A submucosal preparation of the rat colon was pined flat in a recording chamber, and physiological salt solution (PSS) bubbled with 95% O2-5% CO2 at 35°C was superfused at a flow rate of 1.2 mL·min−1. Changes in the diameter of submucosal venule were recorded with a video camera and analysed with Diamtrak, edge-tracking software (Neild, 1989).
Neural selectivity of TNS with different parameters (50 μs duration, 10 Hz, 1 s or 100 μs duration, 20 Hz, 5 s) was confirmed by their sensitivity to 1 μM tetrodotoxin (TTX). Phentolamine was superfused 10 min prior to electrical field stimulation (TNS) or the addition of noradorenaline. The perfusion of guanethidine was started 30 min prior to TNS. CGRP8-37 was superfused 5 min prior to TNS or the application of capsaicin or CGRP.
Immunohistochemistry
The protocols of tissue preparation (Mitsui, 2010) and immunohistochemical staining (Mitsui and Hashitani, 2013) are the same as described previously. Briefly, the submucosa with the mucosa attached was flattened, pinned and immersed in Zamboni fixative for 10 min. Tissue was then fixed in the same fixative for 5 h at 4°C. The mucosa was removed, and the remained submucosal whole mounts were immersed in 0.3% Triton X-100 in PBS for 10 min, immersed in Block Ace for 20 min and incubated with primary antibodies for 4 days at 4°C. Tissue was then incubated with biotinylated anti-rabbit antibody for 30 min. Whole mounts were incubated with a fluorescent probe-conjugated streptavidin and/or a secondary antibody for 2 h. Specimens were examined using a confocal laser scanning microscope (LSM 5 PASCAL, Carl Zeiss, Oberkochen, Germany).
Antibodies used in immunohistochemistry were as follows: rabbit anti-tyrosine hydroxylase (TH) antibody (1:1000, Millipore, Billerica, MA, USA), rabbit anti-CGRP antibody (1:50, Progen Biotechnik, Heidelberg, Germany), guinea pig anti-CGRP antibody (1:500, Peninsula Laboratories, San Carlos, CA, USA), rabbit anti-SP antibody (1:1000, Immunostar, Hudson, WI, USA), biotinylated swine anti-rabbit IgG antibody (1:300, Dako, Glostrup, Denmark), Alexa488-conjugated streptavidin (10 μg·mL−1, Molecular Probes, Eugene, OR, USA), Cy-3-conjugated streptavidin (4.5 μg·mL−1, Molecular Probes) and Alexa488-conjugated goat anti-guinea pig IgG antibody (1:200, Molecular Probes).
Solutions
The compositions of PSS were 137.5 mM Na+, 4.7 mM K+, 2.5 mM Ca2+, 1.2 mM Mg2+, 15.5 mM HCO3−, 1.2 mM H2PO4−, 134 mM Cl− and 15 mM glucose. Nicardipine (L-type Ca2+ channel blocker), nifedipine (L-type Ca2+ channel blocker), cyclopiazonic acid (CPA; Ca2+-ATPase inhibitor), 2-aminoethoxydiphenyl borate (2-APB; IP3 receptor inhibitor) caffeine (IP3 receptor inhibitor), tetracaine (Ca2+-indeuced Ca2+ release inhibitor), niflumic acid (Ca2+-activated Cl− channel blocker), 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS; Ca2+-activated Cl− channel blocker), SKF96365 (blocker of store-operated Ca2+ entry channels), noradorenaline, phentolamine (α-adrenoceptor antagonist), guanethidine (sympathetic nerve blocker), capsaicin [transient receptor potential vanilloid 1 (TRPV1) activator], L-nitro arginine (NOS inhibitor) and TTX (voltage-dependent Na+ channel blocker) were purchased from Sigma (St Louis, MO, USA), and human CGRP, human CGRP8-37 (CGRP receptor antagonist) and SP were from the Peptide Institute (Osaka, Japan). YM-244769 (Na+/Ca2+ exchanger type 3 blocker) was synthesized by Professor T Iwamoto (Fukuoka University, Japan). Nicardipine, CPA, 2-APB, tetracaine, niflumic acid, SKF96365 and YM-244769 were dissolved in dimethyl sulphoxide. Capsaicin was dissolved in methanol. Other drugs were dissolved in distilled water. The final concentration of solvents did not exceed 1:1000. The concentration of drugs used was the similar to that used in previous studies of microvasculature (Vanner, 1994; Kotecha and Neild, 1995; Hashitani et al., 2011; 2012,). Drug target nomenclature conforms with the Guide to Receptors and Channels (Alexander et al., 2011).
Data analysis
Data are expressed as mean ± SEM. F-test followed by unpaired Student's t-test was used for experiments using capsaicin. A normality test (Kolmogolov–Sminov test) followed by paired Student's t-test was used in other experiments to examine the effects of drugs. P < 0.05 was considered statistically significant. n-values indicate the numbers of animals used.
Results
General observations
Submucosal venules in the rat distal colon had a diameter of 70.5 ± 2.9 μm (mean ± SEM, n = 68, ranging from 32 to 137 μm) and exhibited rhythmic spontaneous constrictions at a mean frequency of 6.1 ± 0.3·min−1 (Figure 1A). Spontaneous constrictions had a mean amplitude of 17.7 ± 0.7% of the resting diameter and a mean half duration of 2.8 ± 0.1 s. Spontaneous venular constrictions were not affected by TTX (1 μM, n = 3). In contrast, submucosal arterioles running along the venules did not show any spontaneous constrictions.
Figure 1.
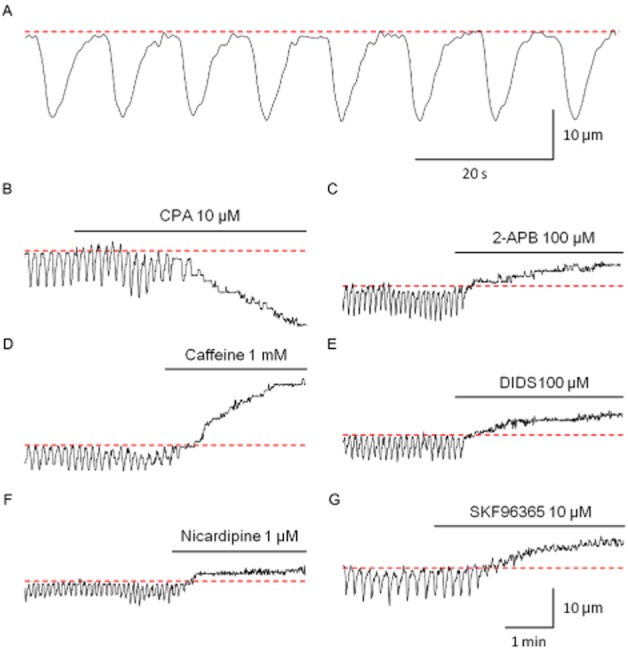
Spontaneous constrictions in submucosal venules of the rat distal colon. A submucosal venule exhibited spontaneous rhythmic constrictions (A). CPA (10 μM) abolished spontaneous venular constrictions and induced a sustained constriction (B). 2-APB (100 μM, C), caffeine (1 mM, D) or DIDS (100 μM, E) abolished spontaneous constrictions with a venular dilation. Nicardipine (1 μM) abolished spontaneous constrictions and dilated the venular wall (F). SKF96365 (10 μM, G) abolished spontaneous constrictions with a venular dilation. All recordings were made from different preparations. Resting diameters indicated by dotted lines were 74 μm (A); 40 μm (B); 32 μm (C); 44 μm (D); 43 μm (E); 49 μm (F); and 52 μm (G). The scale bars in G also apply to B–F.
L-nitro arginine (100 μM), a NOS inhibitor, significantly reduced the resting diameter of the venules by 21.7 ± 5.0% (n = 5, P < 0.05), and virtually abolished rhythmic spontaneous constrictions in three out of these five preparations.
Role of intracellular Ca2+ stores in generating spontaneous venular constrictions
CPA (10 μM, Figure 1B), a sarcoplasmic reticulum (SR) Ca2+-ATPase inhibitor, abolished the spontaneous constrictions and induced a sustained constriction (53.5 ± 6.6% of resting diameter, n = 6). 2-APB (100 μM, Figure 1C) or caffeine (1 mM, Figure 1D), which are known to inhibit InsP3-induced Ca2+ release from SR, abolished the spontaneous constrictions and dilated venules by 25.8 ± 5.6% (2-APB, n = 4) or 40.8 ± 8.7% (caffeine, n = 6) of resting diameter respectively. Tetracaine (100 μM), which inhibits Ca2+-induced Ca2+ release from SR via ryanodine receptors, also caused venule dilation (13.9 ± 2.8% of resting diameter, n = 6). Spontaneous constrictions were abolished in three out of six preparations, and were suppressed in the remaining three preparations. DIDS (100 μM, Figure 1E) or niflumic acid (100 μM), Ca2+-activated Cl− channel blockers, abolished the spontaneous rhythmic activity and dilated the venules by 19.1 ± 8.0% (DIDS, n = 4) or 34.0 ± 11.4% (niflumic acid, n = 3) of the resting diameter respectively.
Role of extracellular Ca2+ influx in generating spontaneous venular constrictions
Nicardipine (1 μM, Figure 1F) or nifedipine (1 μM), L-type Ca2+ channel blockers, dilated the venules by 11.0 ± 1.6% (nicardipine, n = 5) or 8.7 ± 1.2% (nifedipine, n = 5) of the resting diameter, respectively, and abolished all spontaneous constrictions. A similar blockade of spontaneous constrictions with vessel dilatation (32.1 ± 7.8% of resting diameter) was observed with SKF96365 (10 μM, n = 5, Figure 1G), a blocker of store-operated Ca2+ entry, but not with YM-244769 (1 μM, n = 4), a blocker of the Na+/Ca2+ exchanger type 3.
Nerve-evoked constriction of submucosal venules
TNS (50 μs duration, 10 Hz, 1 s) induced a phasic constriction of submucosal venules (32.8 ± 3.9% of the resting diameter, n = 10, Figure 2A,C) that was converted to a small dilation by phentolamine (1 μM), an α-adrenoceptor antagonist (0.8 ± 0.5% of resting diameter, n = 5, P < 0.05, Figure 2B,E). Guanethidine (10 μM) also greatly attenuated these nerve-evoked constrictions (4.1 ± 4.1% of resting diameter, n = 5, P < 0.05, Figure 2D,E).
Figure 2.
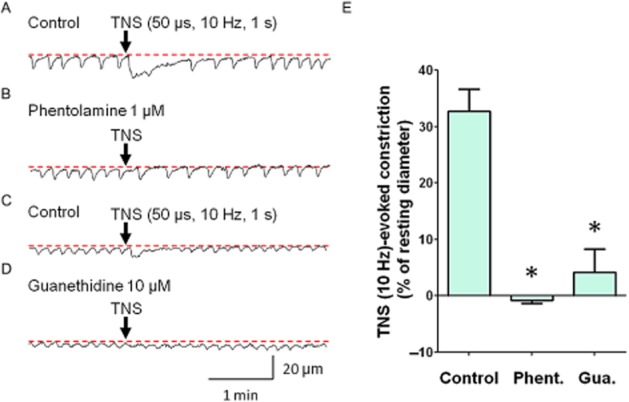
Sympathetic nerve-mediated constriction of the submucosal venules. TNS (50 μs duration, 10 Hz, 1 s) induced a sustained constriction in a submucosal venule (A). In the same venule that had been treated with phentolamine (1 μM), TNS failed to induce a constriction (B). In another submucosal venule, TNS induced a phasic contraction (C). In the same venule that had been treated with guanethidine (10 μM), TNS-induced constriction was largely suppressed (D). These results were summarized in (E). Phentolamine (Phent.; n = 5, *P < 0.05) or guanethidine (Gua.; n = 5, *P < 0.05) significantly attenuated TNS-evoked constriction. Resting diameters were 60 μm (A, B), 37 μm (C) and 41 μm (D). The scale bars in D apply to all traces.
Bath-applied noradrenaline (1 μM) also induced venular constriction (52.1 ± 3.4% of resting diameter, n = 5), which was inhibited by 1 μM phentolamine (6.2 ± 1.7% of resting diameter, P < 0.05).
Nerve-evoked dilation of submucosal venules
Higher intensity TNS (100 μs duration, 20 Hz, 5 s) evoked a larger, phasic constriction (49.0 ± 5.3% of resting diameter, n = 5, Figure 3A) of submucosal venules. In 5 preparations which had been treated with phentolamine (1 μM), TNS induced a sustained dilation (n = 3, Figure 3B) or an attenuated phasic constriction followed by a sustained dilation (n = 2), and thus the amplitude of TNS-induced dilation was 19.4 ± 5.0% of resting diameter (n = 5). This TNS-evoked dilation was attenuated by CGRP8-37 (2 μM), a CGRP receptor antagonist (6.4 ± 2.0% of resting diameter, P < 0.05, Figure 3C,D). The peak amplitude of the dilation determined during 0–150 s after TNS (100 μs duration, 20 Hz, 5 s) was attenuated, but not abolished by TTX, a voltage-gated Na+ channel blocker (from 17.1 ± 4.2% to 10.2 ± 1.8% of resting diameter, n = 5). This is consistent with previous reports that demonstrate the presence of TTX-resistant voltage-gated Na+ channels on primary afferent nerves innervating the gut submucosal microvasculature (Miranda-Morales et al., 2010). The peak amplitude of dilation determined during 150–300 s after TNS was largely suppressed by TTX (from 16.5 ± 3.9% to 4.8 ± 3.0% of resting diameter in TTX, P < 0.05).
Figure 3.
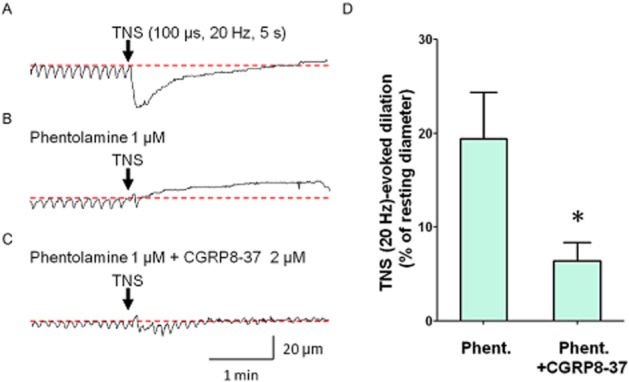
Involvement of CGRP in nerve-evoked dilation of submucosal venules. TNS (100 μs duration, 20 Hz, 5 s) induced a phasic venular constriction (A). In the same preparation treated with phentolamine (1 μM),---TNS failed to cause contraction---, but induced a dilation (B). In the presence of phentolamine and CGRP8-37 (2 μM), a CGRP receptor antagonist, TNS-induced dilation was greatly attenuated in the same venule (C). These results were summarized in (D). TNS-evoked dilation in the presence of phentolamine (Phent.) was significantly attenuated by CGRP8-37 (n = 5, *P < 0.05). Resting diameters were 66 μm (A), 64 μm (B) and 63 μm (C). The scale bars in C apply to all traces.
Capsaicin-evoked dilation of submucosal venules
Capsaicin (100 nM), which stimulates primary afferent nerves through TRPV1 activation, induced a dilation of submucosal venules and abolished spontaneous constrictions (Figure 4A). After washout of capsaicin, the venular diameter returned to the basal level, and the spontaneous constrictions were recovered.
Figure 4.
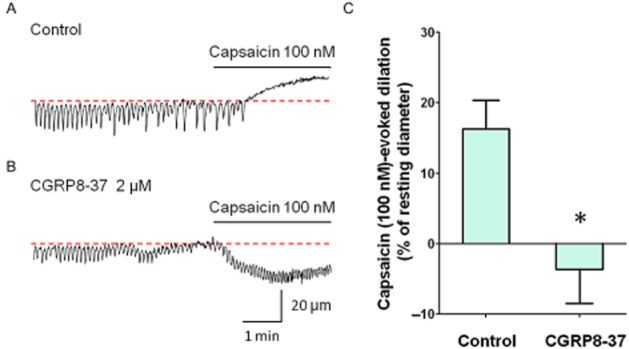
Submucosal venular dilations in response to capsaicin. Capsaicin (100 nM) dilated the venule and prevented the generation of spontaneous constrictions in control venule (A). Capsaicin evoked a constriction in a different venule that had been pretreated with CGRP8-37 (2 μM, B). These results were summarized in (C). The capsaicin-induced dilatory response of submucosal venules was significantly inhibited by CGRP8-37 (n = 6, *P < 0.05). Resting diameters wee 72 μm (A) and 62 μm (B). The scale bars in B also apply to A.
Because the second application of capsaicin even 1 h after the washout induced a smaller vasodilation compared with the first response, capsaicin-induced depletion of primary afferent neurotransmitters appears not to be readily reversible in this preparation. Thus, to examine effects of CGRP receptor antagonist CGRP8-37 on capsaicin-induced dilations of the venule, two submucosal preparations from the same rats were prepared; the first one was used as a control treated with vehicle (distilled water), while the second preparation was treated with CGRP8-37. In the control tissues, capsaicin (100 nM) evoked a 16.3 ± 4.1% dilation of the resting venule diameter (n = 7, Figure 4A). In CGRP8-37 (2 μM) treated preparations, capsaicin (100 nM) failed to cause a dilatation in five out of seven preparations, and even constricted in the remaining two venules (Figure 4B). Thus the capsaicin-evoked dilatory response was greatly inhibited by CGRP8-37 (−3.7 ± 4.8% of resting diameter, P < 0.05, Figure 4C).
Effects of CGRP and SP on spontaneous venular constrictions
CGRP (10 nM), an inhibitory neurotransmitter of primary afferents, induced a dilatation of six submucosal venules (13.5 ± 1.9% of resting diameter, n = 6, Figure 5A) and abolished spontaneous constrictions. This dilatory response of CGRP was attenuated by CGRP8-37 (2 μM) (4.4 ± 0.6% of resting diameter, P < 0.05, Figure 5B,D). After 1 h washout of CGRP8-37, CGRP-evoked dilation was restored (12.8 ± 2.5% of resting diameter).
Figure 5.
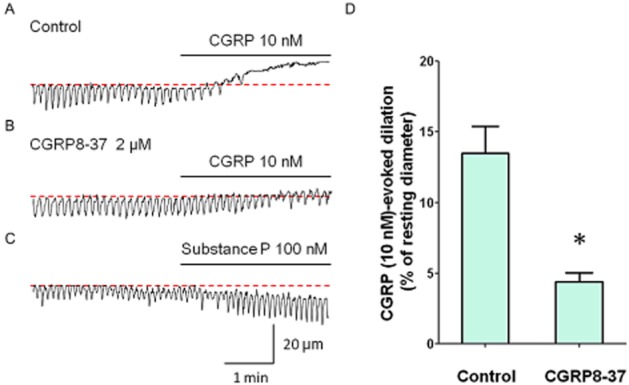
Effects of exogenously applied primary afferent neuropeptides on submucosal venules. CGRP (10 nM) prevented the generation of spontaneous constriction with a dilation of venule (A). In the same venule that had been pretreated with CGRP8-37 (2 μM), CGRP-induced dilatation was largely attenuated. These results were summarized in (D). CGRP (10 nM)-evoked dilation of the venule was significantly attenuated by CGRP8-37 (n = 3, *P < 0.05). In a different venule, SP (100 nM) evoked a constriction of the venule (C). Resting diameters were 76 μm (A), 81 μm (B) and 71 μm (C). The scale bars in C apply to all traces.
In contrast, SP (100 nM), another neurotransmitter of primary afferent nerves, constricted the venular wall (n = 7, 12.4 ± 3.5% of resting diameter, Figure 5C). Effects of SP on venular spontaneous constrictions were variable; SP increased the amplitude or had no effect or increased the frequency or abolished the spontaneous activity.
Immunohistochemical observations
Venules and arterioles were observed to run parallel in whole mounts of the submucosa of rat distal colon (Figure 6A). Sympathetic nerves innervating the submucosal microvasculature were detected by immunohistochemistry for TH (Figure 6B). Although bundles of sympathetic fibres ran along the submucosal arterioles, single varicose sympathetic nerve fibres were observed to project to the submucosal venule (Figure 6B).
Figure 6.
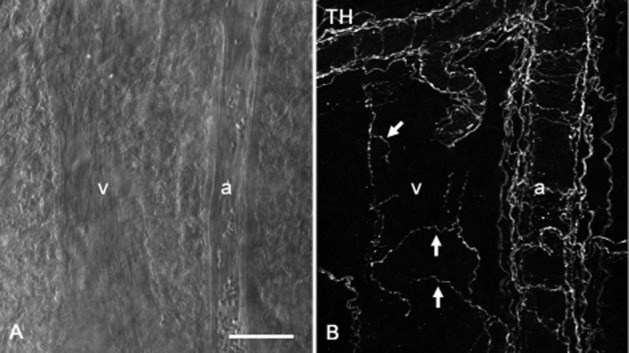
Sympathetic innervations of submucosal microvasculatures of the rat distal colon. A venule (v) and an arteriole (a) running parallel were observed in the submucosal specimen using a differential interference contrast microscope (A). In the same specimen, TH-immunoreactive sympathetic varicose nerve fibres (arrows) were sparsely distributed around a submucosal venule (v), while bundles of sympathetic fibres ran along a submucosal arteriole (a) (B). Scale bar: 50 μm.
CGRP-immunoreactive varicose nerve fibres were observed to run along both arterioles and venules (Figure 7A,B). CGRP and SP also co-localized in single varicose nerve fibres projecting to submucosal venules (Figure 7C–E).
Figure 7.
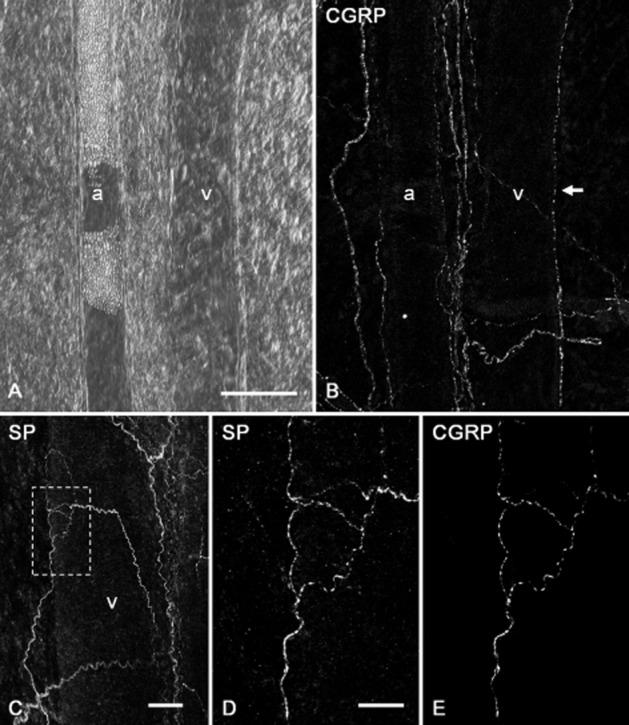
Projections of CGRP-immunoreactive nerve fibres to submucosal microvasculatures of the rat distal colon. A differential interference contrast microscopy showed an arteriole (a) and a venule (v) ran pararell in a submucosal specimen (A). In the same specimen, CGRP-immunoreactive varicose nerve fibres were distributed along the submucosal arteriole (a), and one varicose nerve fibre positive for CGRP (arrow) ran along the submucosal venule (v) (B). Nerve fibres immunoreactive for SP were also detected around the submucosal venule (v) (C). An enlarged image of the area surrounded by doted box in C shows single varicose nerve fibres positive for SP (D). In the same specimen, the SP-positive varicose fibres were also immunoreactive for CGRP (E). Scale bars: 100 μm (A), 50 μm (C) and 20 μm (D).
Discussion and conclusions
This study for the first time demonstrated that submucosal venules of the rat distal colon exhibited spontaneous rhythmic constrictions. Upon electrical stimulation submucosal venules were contracted by noradrenaline released from sympathetic nerves acting on α-adrenoceptor, and dilated by CGRP released from capsaicin-sensitive primary afferent nerves. This functional innervation was confirmed by immunohistochemistry which showed sympathetic and CGRP-containing afferent nerves running along the venules. Thus, functional properties of submucosal venules in the rat distal colon appear to be very different from those of the guinea pig small intestine that did not exhibit any spontaneous activity or response to nerve stimulation (Hirst, 1977).
Spontaneous venular constrictions and corresponding electrical and Ca2+ activity have been reported in the rat and mouse bladder (Hashitani et al., 2011; 2012,). Spontaneous rhythmic changes in membrane potential of venular smooth muscles have been also described in the submucosa of cat stomach (Morgan, 1983). Spontaneous constrictions can be also detected using video imaging in the venules of the rat stomach and rectum (Mitsui R and Hashitani H, unpublished data). Thus, spontaneous rhythmic activity appears to be a common property of venules in the wall of several hollow organs distended by their luminal contents (e.g. faecal pellets, urine and ingested food). Such distension of hollow organs would result in compression and/or stretching of venular walls that may lead to a reduction in tissue blood flow. We propose that rhythmic spontaneous constrictions of venules in these organs may well establish an active drainage. Such an enhancement of venular blood flow by spontaneous constrictions has been described previously in vivo using venules in the bat wing, an old experimental model of venomotion (Dongaonkar et al., 2012).
Spontaneous venular constrictions in the rat distal colon were suppressed by CPA, 2-APB, low concentration of caffeine (1 mM) or tetracaine, suggesting that Ca2+ release from SR through IP3 or ryanodine receptors is a primary step of their generation. However, molecular mechanisms underlying the cycle of spontaneous SR Ca2+ release and its termination have not been well explored in venular smooth muscle cells. In cardiac myocytes, ryanodine receptor interacts with Ca2+ sensor molecules that detect SR luminal Ca2+ contents to regulate Ca2+-induced Ca2+ release (Györke and Terentyev, 2008). IP3 receptor may also have a similar luminal regulatory mechanism.
Spontaneous constrictions were also prevented by DIDS, niflumic acid, nicardipine or nifedipine, suggesting that Ca2+ released from SR trigger the opening of Ca2+-activated Cl− channels, and the resultant depolarization induces Ca2+ entry through voltage-gated ‘L-type’ Ca2+ channels to contract venular smooth muscle. Since spontaneous constrictions were blocked by SKF96365, Ca2+ influx through store-operated channels may also contribute to refill SR Ca2+ stores to maintain the operation of this cytosolic Ca2+ oscillator.
The results arising from the pharmacological experiments were not contradictory to the assumption that the main target of drugs used was venular smooth muscles rather than venular endothelial cells. In general, drugs known to increase cytosolic Ca2+ concentration ([Ca2+]i) of venular smooth muscle caused a constriction, while those known to decrease [Ca2+]i of venular smooth muscle caused a dilation. If CPA which increases [Ca2+]i predominantly acted on endothelial cells, it would be expected to cause NO release resulting in the dilation of venules as reported in the bladder venule (Hashitani et al., 2012). However, CPA caused a venular constriction without such a prominent dilation in the submucosal venule of rat distal colon.
L-nitro arginine, a NOS inhibitor, reduced the resting diameter of venules and virtually abolished spontaneous constrictions, suggesting that endogenous NO presumably released from endothelium may contribute to maintain the basal venular diameter which allows the generation of rhythmic spontaneous constrictions. It has been reported that rhythmic vasoconstrictions (vasomotion) induced by 0.1 μM–0.5 μM noradrenaline in the rat mesenteric artery are maintained via endothelium-derived NO (Peng et al., 2001). Although precise ionic mechanism has not been determined, cGMP-dependent depolarization subsequent to Ca2+ release from SR in arteriolar smooth muscles appears to be required to initiate this vasomotion.
It might be possible that some circular muscle cells and interstitial cells of Cajal remained attached to the ‘submucosal’ preparations. However, any synchrony between venular constrictions and circular muscle contractions was not evident. Moreover, the frequency of spontaneous contractions of circular muscle layer in the rat colon (12.8 ± 0.8·min−1; Plujà et al., 2001) has been reported to be different from that of the venular spontaneous constrictions (6.1 ± 0.3·min−1). Thus, it is highly unlikely that the rhythmicity of venules was affected by the circular smooth muscles.
For Diamtrak experiments, submucosal preparations were pinned flat and thus microvasculature was stretched. Stretching in longitudinal as well as circular directions by perivascular connective tissue might affect the spontaneous rhythmicity of submucosal venule. Thus, our experimental conditions may well be more relevant to the distal colon in vivo where faecal pellets in the lumen distend the colonic wall.
The blood volume in intestine is decreased upon sympathetic nerve stimulation (Folkow et al., 1964). Constrictions of the resistance arteries/arerioles decrease intestinal blood flow, while constrictions of capacitance venules/veins in the gut wall squeeze out blood (Andersson, 1984). In the present study, we have presented functional and morphological evidence of a sympathetic innervation to submucosal venules. These data are consistent with the notion that venules contribute to a redistribution of blood from the colon to other tissues during sympathetic nerve activation with exercise or haemorrhage (Andersson, 1984).
Previous studies have revealed an efferent function of primary afferent nerves containing CGRP in the intestine; CGRP released from capsaicin-sensitive primary afferents dilate arterioles (Vanner, 1994) and relax the smooth muscle layer (Takaki et al., 1989). A dense distribution of nerve fibres immunoreactive for both CGRP and TRPV1, a capsaicin receptor, has also been reported in the mouse colon (Christianson et al., 2006). In the present study, activation of primary afferent nerves by TNS or capsaicin evoked a venular dilation that was mimicked by exogenously applied CGRP, and both of these dilatory responses were attenuated by a CGRP receptor antagonist. The identification of CGRP/SP-positive nerve fibres adjacent to the submucosal venules is consistent with our recent immunohistochemical study that demonstrated sympathetic and primary afferent fibres innervating fine venules (about 20 μm–50 μm diameter) in the mucosa of mouse bladder (Mitsui and Hashitani, 2013). These CGRP/SP-containing nerve fibres originate from extrinsic primary afferents because these neuropeptides do not co-localize in intrinsic neurons (i.e. enteric neurons) in the submucosa of rat distal colon (Mitsui, 2010). In contrast to the SP-induced relaxations of submucosal arterioles reported in the small intestine (Vanner, 1994), exogenous application of SP induced a venular constriction. These results indicate that CGRP released from perivascular primary afferent nerves plays a major role in nerve-mediated dilation of the submucosal venule in the rat distal colon.
In phentolamine-treated preparations, TNS-evoked venular dilation was greatly attenuated by a CGRP receptor antagonist. This dilatory response was sensitive to TTX, but was not completely abolished by TTX. This is consistent with previous reports showing that TTX-resistant CGRP-containing primary afferent nerves are involved in vasodilation (Thengchaisri and Rivers, 2005), and that action potential conduction of capsaicin-sensitive primary afferents innervating the submucosal arteriole of the intestines was reported to be TTX-resistant (Miranda-Morales et al., 2010). TTX-resistant voltage-gated Na+ current has been reported in capsaicin-sensitive primary afferents innervating the rat distal colon (Su et al., 1999).
The distension of the colonic wall activates capsaicin-sensitive afferent nerves to evoke electrolyte and water secretion into the colonic lumen (Eutamene et al., 1997; Weber et al., 2001). Activation of capsaicin-sensitive primary afferents may also induce a dilation of submucosal arterioles (Vanner, 1994) and venules (the present study) to keep sufficient fluid within the colonic wall to maintain this water secretion into the lumen. Faecal pellets may distend the distal colonic wall to initiate such a secretion/vasodilation reflex, so that the secreted water lubricates the lumen to facilitate the evacuation of faecal pellets from the distal colon to the anus. In addition to such a physiological condition, the dilation of microvasculature seems to be also important in a pathological condition. Exposure of acid in the mucosa of rat colon results in hyperaemia in the adjacent unexposed and undamaged mucosa partly via capsaicin-sensitive primary afferent nerves, and this reaction may contribute to prevent mucosal damage (Leung, 1992). In the rat stomach, mucosal damage caused by hypertonic saline and acid is restored by mucosal hyperaemia mediated via capsaicin-sensitive primary afferents (Grønbech and Lacy, 1996).
We consider that spontaneous rhythmic constrictions of the venules actively drain blood even when the distal colon wall is chronically distended by faecal pellets in physiological conditions. On the other hand, acute activation of sympathetic or primary afferent nerves may dramatically change the diameter of venules. The rhythmic spontaneous activity is usually important for drainage while the neural regulation mechanisms may be mainly important for responding to acute changes of environment in physiological and pathophysiological conditions.
In conclusion, submucosal venules of the rat distal colon exhibit rhythmic spontaneous constrictions and receive sympathetic and primary afferent neuronal inputs. Thus, fine venules may play more active role in the regulation of submucosal microcirculation of intestine than previously thought.
Acknowledgments
The authors would like to thank Dr. R. J. Lang (Monash University) for his critical reading of the paper. This study was supported by a Medical Research Grant of Nagoya City University Hospital to R.M., Grant-in-Aid for Research in Nagoya City University to R.M., Grant-in-Aid for Scientific Research (B) (No. 22390304) from the Japan Society for Promotion of the Science (JSPS) to H.H., and Grant-in-Aid for challenging Exploratory Research (No. 21659377) from JSPS to H.H.
Glossary
- 2-APB
2-aminoethoxydiphenyl borate
- CGRP
calcitonin gene-related peptide
- CPA
cyclopiazonic acid
- DIDS
4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid
- SP
substance P
- TH
tyrosine hydroxylase
- TNS
transmural nerve stimulation
Conflict of interest
All authors of the paper entitled ‘Properties of submucosal venules in the rat distal colon’ have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson PO. Vascular control in the colon and rectum. Scand J Gastroenterol Suppl. 1984;93:65–78. [PubMed] [Google Scholar]
- Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- De Fontgalland D, Wattchow DA, Costa M, Brookes SJ. Immunohistochemical characterization of the innervation of human colonic mesenteric and submucosal blood vessels. Neurogastroenterol Motil. 2008;20:1212–1226. doi: 10.1111/j.1365-2982.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- Dongaonkar RM, Quick CM, Vo JC, Meisner JK, Laine GA, Davis MJ, et al. Blood flow augmentation by intrinsic venular contraction in vivo. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1436–R1442. doi: 10.1152/ajpregu.00635.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eutamene H, Theodorou V, Fioramonti J, Bueno L. Rectal distention-induced colonic net water secretion in rats involves tachykinins, capsaicin sensory, and vagus nerves. Gastroenterology. 1997;112:1595–1602. doi: 10.1016/s0016-5085(97)70041-6. [DOI] [PubMed] [Google Scholar]
- Folkow B, Lewis DH, Lundgren O, Mellander S, Wallentin I. The effect of graded vasoconstrictor fibre stimulation on the intestinal resistance and capacitance vessels. Acta Physiol Scand. 1964;61:445–457. [PubMed] [Google Scholar]
- Grønbech JE, Lacy ER. Role of sensory afferent neurons in hypertonic damage and restitution of the rat gastric mucosa. Gastroenterology. 1996;111:1474–1483. doi: 10.1016/s0016-5085(96)70008-2. [DOI] [PubMed] [Google Scholar]
- Györke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- Hansen MB, Dresner LS, Wait RB. Profile of neurohumoral agents on mesenteric and intestinal blood flow in health and disease. Physiol Res. 1998;47:307–327. [PubMed] [Google Scholar]
- Hashitani H, Takano H, Fujita K, Mitsui R, Suzuki H. Functional properties of suburothelial microvessels in the rat bladder. J Urol. 2011;185:2382–2391. doi: 10.1016/j.juro.2011.02.046. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Mitsui R, Shimizu Y, Higashi R, Nakamura K. Functional and morphological properties of pericytes in suburothelial venules of the mouse bladder. Br J Pharmacol. 2012;167:1723–1736. doi: 10.1111/j.1476-5381.2012.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977;273:263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha N, Neild TO. Actions of vasodilator nerves on arteriolar smooth muscle and neurotransmitter release from sympathetic nerves in the guinea-pig small intestine. J Physiol. 1995;489:849–855. doi: 10.1113/jphysiol.1995.sp021097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung FW. Role of capsaicin-sensitive afferent nerves in mucosal injury and injury-induced hyperemia in rat colon. Am J Physiol. 1992;262:G332–G337. doi: 10.1152/ajpgi.1992.262.2.G332. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Morales M, Ochoa-Cortes F, Stern E, Lomax AE, Vanner S. Axon reflexes evoked by transient receptor potential vanilloid 1 activation are mediated by tetrodotoxin-resistant voltage-gated Na+ channels in intestinal afferent nerves. J Pharmacol Exp Ther. 2010;334:566–575. doi: 10.1124/jpet.110.165969. [DOI] [PubMed] [Google Scholar]
- Mitsui R. Immunohistochemical characteristics of submucosal Dogiel type II neurons in rat colon. Cell Tissue Res. 2010;340:257–265. doi: 10.1007/s00441-010-0954-z. [DOI] [PubMed] [Google Scholar]
- Mitsui R, Hashitani H. Immunohistochemical characteristics of suburothelial microvasculature in the mouse bladder. Histochem Cell Biol. 2013;140:189–200. doi: 10.1007/s00418-012-1074-5. [DOI] [PubMed] [Google Scholar]
- Morgan KG. Comparison of membrane electrical activity of cat gastric submucosal arterioles and venules. J Physiol. 1983;345:135–147. doi: 10.1113/jphysiol.1983.sp014970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild TO. Measurement of arteriole diameter changes by analysis of television images. Blood Vessels. 1989;26:48–52. [PubMed] [Google Scholar]
- Peng H, Matchkov V, Ivarsen A, Aalkjaer C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res. 2001;88:810–815. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- Plujà L, Albertí E, Fernández E, Mikkelsen HB, Thuneberg L, Jiménez M. Evidence supporting presence of two pacemakers in rat colon. Am J Physiol Gastrointest Liver Physiol. 2001;281:G255–G266. doi: 10.1152/ajpgi.2001.281.1.G255. [DOI] [PubMed] [Google Scholar]
- Su X, Wachtel RE, Gebhart GF. Capsaicin sensitivity and voltage-gated sodium currents in colon sensory neurons from rat dorsal root ganglia. Am J Physiol. 1999;277:G1180–G1188. doi: 10.1152/ajpgi.1999.277.6.G1180. [DOI] [PubMed] [Google Scholar]
- Takaki M, Jin JG, Nakayama S. Possible involvement of calcitonin gene-related peptide (CGRP) in non-cholinergic non-adrenergic relaxation induced by mesenteric nerve stimulation in guinea pig ileum. Brain Res. 1989;478:199–203. doi: 10.1016/0006-8993(89)91499-6. [DOI] [PubMed] [Google Scholar]
- Thengchaisri N, Rivers RJ. Remote arteriolar dilations caused by methacholine: a role for CGRP sensory nerves? Am J Physiol Heart Circ Physiol. 2005;289:H608–H613. doi: 10.1152/ajpheart.01290.2004. [DOI] [PubMed] [Google Scholar]
- Vanner S. Corelease of neuropeptides from capsaicin-sensitive afferents dilates submucosal arterioles in guinea pig ileum. Am J Physiol. 1994;267:G650–G655. doi: 10.1152/ajpgi.1994.267.4.G650. [DOI] [PubMed] [Google Scholar]
- Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. Am J Physiol. 1996;271:G223–G230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- Weber E, Neunlist M, Schemann M, Frieling T. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J Physiol. 2001;536:741–751. doi: 10.1111/j.1469-7793.2001.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


