Abstract
Background and Purpose
G-protein coupled receptor (GPR)55 is a novel lipid sensing receptor activated by both cannabinoid endogenous ligands (endocannabinoids) and other non-cannabinoid lipid transmitters. This study assessed the effects of various GPR55 agonists on glucose homeostasis.
Experimental Approach
Insulin secretion and changes in intracellular Ca2+ and cAMP in response to glucose and a range of GPR55 agonists [endogenous ligands (OEA, PEA), chemically synthetic cannabidiol (CBD) analogues (Abn-CBD, 0–1602), an analogue of rimonabant (AM-251) and antagonist (CBD)] were investigated in clonal BRIN-BD11 cells and mouse pancreatic islets. Cytotoxicity was assessed by LDH release, cellular localization by double-staining immunohistochemistry and in vivo effects assessed in mice.
Key Results
The most potent and selective GPR55 agonist was the synthetic CBD analogue, Abn-CBD (pEC50 10.33), maximum stimulation of 67% at 10−4 mol·L−1 (P < 0.001) in BRIN-BD11 cells. AM-251 (pEC50 7.0), OEA (pEC50 7.0), 0–1602 (pEC50 7.3) and PEA (pEC50 6.0) stimulated insulin secretion. Results were corroborated by islet studies, with no cytotoxic effects. Concentration-dependent insulin secretion by GPR55 agonists was glucose-sensitive and accompanied by elevations of [Ca2+]i (P < 0.01–P < 0.001) and cAMP (P < 0.05–P < 0.01). GPR55 agonists exhibited insulinotropic and glucose lowering activity in vivo. GPR55 was expressed on BRIN-BD11 cells and confined to islet beta cells with no distribution on alpha cells.
Conclusion and Implications
These results demonstrate GPR55 is distributed in pancreatic beta cells and is a strong activator of insulin secretion, with glucose-lowering effects in vivo. Development of agents agonizing the GPR55 receptor may have therapeutic potential in the treatment of type 2 diabetes.
Keywords: GPR55, lipid agonists, BRIN-BD11 cells, isolated islets, insulin secretion, glucose tolerance
Introduction
G-protein coupled receptor 55 (GPR55) was recently described as an orphan cannabinoid receptor GPCR but displays limited sequence homology with cannabinoid receptors [(13.5%) and CB2 (14.4%)] (Ross, 2008; Romero-Zerbo et al., 2011). It has the highest degree of sequence homology with platelet-activating factor purinergic P2Y9 (29%) and the two orphan GPCRs, GPR23 (30%) and GPR35 (27%) (Mackie and Stella, 2006). The human GPR55 gene is located on chromosome 2 at q37 and encodes the 319 amino acid protein which is expressed in numerous mammalian tissues (Oka et al., 2007).
GPR55 is a rhodopsin-like GPCR (class A) that is highly expressed in the CNS, ileum, spleen, adipose tissue and endocrine pancreas coupling primarily to Gα12/13 (Kapur et al., 2009). Gαq has also been implicated in the activation of GPR55 through augmenting intracellular calcium from inositol triphosphate stores and activation of PLC (Anavi-Goffer et al., 2013). GPR55 contains phosphorylation sites for PKA and PKC including patterns for glycosylation and cysteines found on extracellular loops 1 and 2 (Henstridge et al., 2011).
Cannabidiol (CBD) is the non-psychoactive component of Cannabis sativa which is an antagonist to GPR55 and to ligands of CB1 and CB2 receptors (Lin et al., 2011). CBD has been described as weak partial antagonist with low affinity for CB1 receptor (Roser et al., 2010) whereas several recent studies have utilized its higher potency antagonizing GPR55 (Whyte et al., 2009; Sylantyev et al., 2013). Other known antagonists of GPR55 and CB1 include CBD analogue O-1918 and SR141716A (rimonabant), respectively (Vilches-Flores et al., 2010). GPR55 is novel lipid sensing receptor activated by both cannabinoid endogenous ligands (endocannabinoids) and other non-cannabinoid lipid transmitters such as L-α-lysophosphatidylinositol (LPI) (Zang et al., 2010). LPI is accepted as an endogenous agonist of GPR55 due to its ability to elevate intracellular Ca2+ in various cell lines (Moreno-Navarrete et al., 2012).
Recently, the endocannabinoid system, a ubiquitous lipid signalling network has been hypothesized to have regulatory effects on glucose homeostasis including insulin secretion, glucose utilization, gastric emptying and lipogenesis (Ross, 2008). Phospholipid-derived endocannabinoids including anandamide, 2-arachidonoylglycerol, palmitoylethanolamine (PEA) and oleoylethanolamide (OEA) regulate various physiological functions throughout the body (Li et al., 2011). In vivo PEA production from adipose tissue occurs independently of other endocannabinoids, initiating anti-inflammatory effects and microglial cell migration (Brown, 2007; Hoareau and Roche, 2010). OEA agonizes the PPAR-α which upon activation reduces food intake and increases lipid oxidation while anadamide increases food ingestion and storage of lipids (Pavón et al., 2008). Levels of these endocannabinoids are regulated in vivo by degradative enzymes namely fatty acid amide hydrolase and monoacylglycerol lipase (Patti, 2010). Interestingly, OEA acts independently of the CBD pathway and is degraded by FFAH to free oleate which has cytoprotective effects on pancreatic beta-cell lines when incubated with lipotoxic long-chain saturated fatty acids (Stone et al., 2012). Type 2 diabetes is associated with increased activation of the endocannabinoid system in adipose tissue and elevated circulating concentrations of endocannabinoids (Moreno-Navarrete et al., 2012).
Atypical cannabinoids are not recognized by the CB1 or CB2 receptors, instead are potent synthetic agonists of GPR55 including O-1602 and abnormal cannabidiol (Abn-CBD) (Godlewski et al., 2009). In a recent study, O-1602 stimulated intracellular Ca2+ and insulin secretion in pancreatic islets while in vivo treatment resulted in decreased glucose and increase in plasma insulin concentrations (Romero-Zerbo et al., 2011). Furthermore O-1602 effects on bone metabolism occur via GPR55 (Whyte et al., 2009), while its effects on glucose metabolism and food intake may occur independently of GPR55 (Díaz-Arteaga et al., 2011). AM-251 is an analogue of rimonabant and a CB1 antagonist that can cross the blood-brain barrier and inhibits both feeding and weight gain in obesity-related diseases (Bermúdez-Silva et al., 2008). Abn-CBD is a synthetic analogue of CBD and in contrast to CBD is a selective agonist of GPR55 which causes vasodilation at endothelial sites (Brown, 2007).
Various classes of GPR55 agonists work through different channels including stimulation of GTPγS binding, intracellular Ca2+ or RhoA activation. However, the present pharmacology of GPR55 and downstream signalling events are controversial depending on the agonist, assay and tissue utilized (Henstridge et al., 2010). Although present research on GPR55 and glucose homeostasis is limited, current studies indicate that GPR55 may be a novel target in the treatment of obesity and type 2 diabetes (Amisten et al., 2013). Currently, few studies have assessed the insulin-releasing potential of various GPR55 agonists in clonal beta BRIN-BD11 cells and pancreatic islets. The agonists utilized in this investigation were endogenous ligands (OEA, PEA), chemically synthetic CBD analogues (Abn-CBD, O-1602) and an analogue of rimonabant (AM-251). This study investigated the cellular localization of GPR55 in clonal BRIN-BD11 cells and pancreatic islets, together with its effects on insulin secretion, beta cell stimulus-secretion coupling pathways and glucose lowering effects in vivo.
Methods
Insulin secretion
Generation and characterization of the insulin secreting BRIN-BD11 cells were outlined previously (McClenaghan et al., 1996). BRIN-BD11 cells were cultured with RPMI-1640 media (11.1 mM glucose) containing antibiotics (100 U·mL−1 penicillin and 0.1 mg·mL−1 streptomycin) and 10% fetal calf serum at 37°C in an atmosphere of 95% air and 5% carbon dioxide. For acute insulin secretion studies, cells were detached using trypsin/EDTA and incubated overnight in 24 well plates with 150 000 cells per well. Cells were then pre-incubated for 40 min at 1.1 mmol·L−1 glucose in Krebs buffer (comprising 4.7 mmol·L−1 KCL, 115 mmol·L−1 NaCl, 1.28 mmol, CaCl22H2O, 10 mmol·L−1 NaHCO3, 5 g·L−1 BSA, 1.2 mmol·L−1 KH2PO4, 1.2 mmol·L−1 MgSO47H2O pH 7.4). Test incubations were then performed at 37°C for 20 min. O-1602, PEA, OEA, AM-251, Abn-CBD and CBD at 10−12–10−4 mol·L−1 were tested at both 5.6 mmol·L−1 and 16.7 mmol·L−1 glucose, as indicated in the Figures (nomenclature follows Alexander et al., 2011). Supernatants were removed, evaluated for LDH release as an indicator of cytotoxicity (as per manufacturer's protocol) or frozen at −20°C until determination of insulin by radioimmunoassay (Flatt and Bailey, 1981).
Pancreatic islets were isolated from normal mice derived from the colony maintained at Aston University, UK (Bailey and Flatt, 1982) by collagenase digestion (Moskalewski, 1969). After overnight culture, groups of 10 islets were incubated for 1 h at 37°C in 1 mL of 1.1 mmol·L−1 glucose Krebs. Test incubations were then carried out for 1 h at 11.1 mmol·L−1 glucose with various GPR55 agonists (10−10–10−4 mol·L−1). Insulin release and insulin content of islets, treated overnight with 1 mL acid ethanol, were determined by radioimmunoassay.
Intracellular Ca2+ and cAMP
For intracellular Ca2+ measurement, monolayers of BRIN-BD11 cells were seeded overnight, at a density of 80 000 cells per well in a 96-well black walled clear bottom plate (Miguel et al., 2004). Cells were washed with 100 μL of Krebs buffer and incubated for 1 h with Flex calcium assay kit reagent at 37°C. O-1602, PEA, OEA, AM-251 and Abn-CBD at 10−4 mol·L−1 were added at 5.6 mmol·L−1 and 16.7 mmol·L−1 glucose. Fluorometric data were obtained using the FLEX Station scanner and test solutions were transferred using fluid transfer workstation at a wavelength of 525 nm (Molecular Devices, Sunnyvale, CA, USA). For cAMP determination, BRIN-BD11 cells were seeded in a 96-well plate at a density of 30 000 cells per well. Cells were washed with 300 μL Krebs buffer for 40 min and 150 μL of GPR55 agonists at 10−10–10−4 mol·L−1 were tested at 11.1 mol·L−1 glucose. After 20 min, test solutions were removed and 0.1 M HCL (150 μL) was added to lyse the cells. Total cAMP production in the cell, supernatants were measured using cAMP enzyme immunoassay kit according to the manufacturer's protocol (Sigma-Aldrich, Poole, UK).
Histology
BRIN-BD11 cells were allowed to attach overnight to polylysine-coated slides and fixed using 4% paraformaldehyde/PBS for 20 min. Antigen retrieval was achieved by incubation in sodium citrate (50 mmol·L−1) at 90°C for 20 min. Pancreatic tissues from normal mice (Bailey and Flatt, 1982) were fixed in 4% PFA/PBS, embedded in paraffin wax and sections cut at 8μm. Sections were mounted onto polylysine-coated slides and dried on a hot plate. Pancreatic sections were dewaxed and antigen retrieval performed as described above. Slides were incubated overnight at 4°C with mouse anti-insulin (1:500), guinea pig anti-glucagon (1:500) and rabbit anti-GPR55 (1:100). After washing in PBS, sections were incubated with Alexa Fluor 488 fluorescein goat anti-rabbit IgG, goat anti-mouse or anti-guinea pig Alexa 594 nm IgG (1:400; Molecular Probes, Life Technologies Ltd, Paisley, UK) for 45 min at 37°C and DAPI nuclear stain for 15 min at 37°C. Finally, slides were washed in PBS, mounted and analysed using a BX51 Olympus microscope equipped with an Olympus XM10 digital camera.
Acute effects of GPR55 agonists in vivo
All animal experiments were carried out in accordance with the UK Animal (Scientific Procedures) Act 1986. Experimental animals were housed individually in an air-conditioned room at 22 ± 2°C with 12 h light: 12 h darkness cycles. Drinking water and standard rodent maintenance diet (Trouw Nutrition, Cheshire, UK) were supplied ad libitum. Non-fasted NIH Swiss mice (Harlan UK Ltd, Harlan, Blackthorn, UK) (14–16 week old, n = 6) received an i.p. injection of glucose alone (18 mmol·L−1 kg−1 body weight) or in combination with GPR55 agonists (0.1 μmol·L−1 kg−1 body weight). Blood samples were obtained, from individual mice at each time point, from the cut tip from tail vein of conscious mice and centrifuged at 13 000 rpm for 3 min at 4°C. Plasma glucose was measured by an automated glucose oxidase procedure using a Beckman glucose analyser and insulin determined by radioimmunoassay. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Statistics
Data are expressed as the mean ± SEM. Results were compared using the Student's t-test or one-way anova on Prism graph pad version 5.0. Differences in data were considered to be statistically significant for P < 0.05.
Materials
O-1602, PEA, OEA, AM-251, Abn-CBD and CBD were purchased from Tocris Bioscience (Bristol, UK). Collagenase (derived from Clostridium histolyticum), cAMP enzyme immunoassay kits, monoclonal guinea-pig anti-glucagon and monoclonal mouse anti-insulin anti-serum were obtained from Sigma-Aldrich. RPMI-1640 media, FBS, streptomycin, Hanks buffer, trypsin and penicillin were sourced from Gibco Life Technologies Ltd (Strathclyde, UK). CytoTox96 non-radioactive cytotoxicity assay kits and FLEX calcium assay reagent were purchased from Promega (Madison, WI, USA) and Molecular Devices respectively. Rabbit anti-GPR55 polyclonal antibody was purchased from Cambridge Bioscience Ltd (Cambridge, UK).
Results
Effects of GPR55 agonists and antagonist on insulin secretion from BRIN-BD11 cells
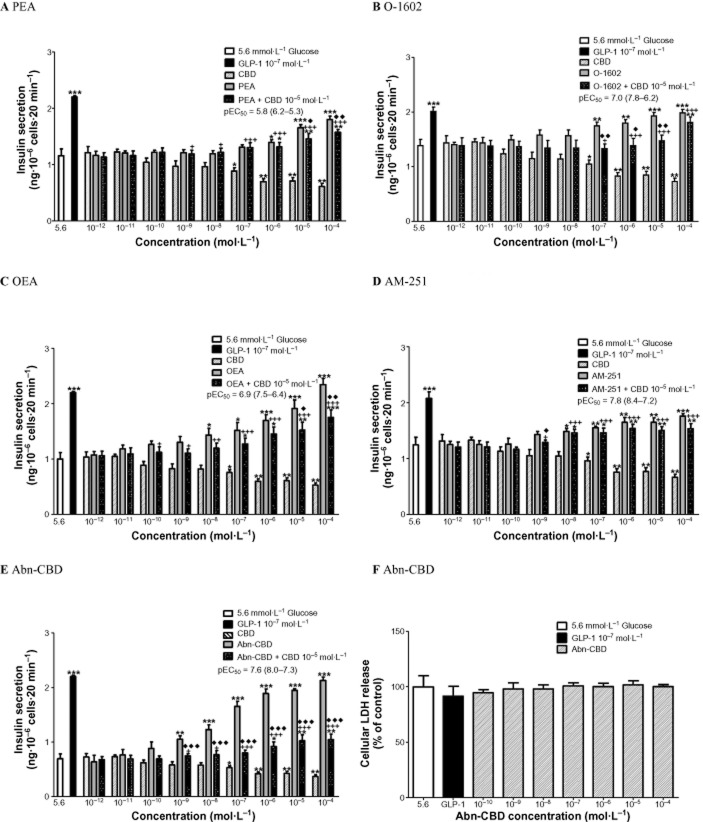
Insulinotropic activity of endogenous GPR55 ligands (PEA, OEA), synthetic CBD analogues (Abn-CBD, O-1602), an analogue of rimonabant (AM-251) and a GPR55 antagonist (CBD) at 10−12–10−4 mol·L−1 were investigated at 5.6 mM and 16.7 mM glucose in clonal BRIN-BD11 cells (Table 1). At 5.6 mM glucose, endogenous agonists PEA (10−6–10−4 mol·L−1) increased insulin secretion by 1.2-to 1.5-fold (P < 0.01–P < 0.001) (Figure 1A) while OEA was more potent [pEC50 6.9 (7.5–6.4, 95% confidence limits)] at 10−8–10−4 mol·L−1 with a 1.4-to 2.4-fold increase in insulin release (P < 0.01–P < 0.001), representing a 55% increase in insulin secretion at 10−4 mol·L−1 at 5.6 mM glucose (Figure 1C). At 5.6 mM glucose, antagonist CBD (10−7–10−4 mol·L−1) inhibited insulin secretion by 1.3-to 1.9-fold (P < 0.05–P < 0.01) (Figure 1A). The antagonist CBD (10−5 mol·L−1) decreased PEA-induced insulin secretion at 10−5–10−4 mol·L−1 by 11–13% (P < 0.05–P < 0.01) and OEA-induced insulin release at 10−5–10−4 mol·L−1 by 21–25% (P < 0.05–P < 0.01) (Figure 1A & C).
Table 1.
Effects of various GPR55 agonists on insulin secretion in clonal BRIN-BD11 cells and isolated islets at normoglycaemic and hyperglycaemic conditions pEC50 = (95% confidence limits)
| BRIN-BD11 cells (5.6 mM glucose) | BRIN-BD11 cells (16.7 mM glucose) | Isolated islets (11.1 mM glucose) | |
|---|---|---|---|
| pEC50 | |||
| Abn-CBD | 7.6 (8.0–7.3) | 10.3 (10.7–10.0) | 6.1 (6.6-5.7) |
| PEA | 5.8 (6.2–5.3) | 6.0 (6.5–5.6) | 5.3 (6.2–4.4) |
| OEA | 6.9 (7.5–6.4) | 7.0 (7.5–6.5) | 5.5 (6.0–5.1) |
| O-1602 | 7.0 (7.8–6.2) | 7.3 (8.0–6.6) | 5.8 (6.6–4.9) |
| AM-251 | 7.8 (8.4–7.2) | 7.0 (7.7–6.4) | 6.9 (8.7–5.1) |
Figure 1.
Effects of (A) PEA (B) O-1602 (C) OEA (D) AM-251 and (E) Abn-CBD, in the absence and presence of CBD, on insulin secretion from BRIN-BD11 cells at 5.6 mmol·L−1 glucose. GLP-1 (10−7 mol·L−1) was used as positive control. (F) Effect of Abn-CBD on LDH release. Results are the mean ± SEM (n = 8) for insulin secretion and (n = 3) for LDH release. *P < 0.05, **P < 0.01 and ***P < 0.001 compared to glucose alone. +P < 0.05 ++P < 0.01 and +++P < 0.001 compared to CBD. ♦P < 0.05 ♦♦P < 0.01 and ♦♦♦P < 0.001 compared to agonist alone.
Synthetic CBD analogues, 0–1602 (10−7–10−4 mol·L−1) augmented insulin release by 1.2-to 1.4-fold (P < 0.01–P < 0.001) (Figure 1B) with a pEC50 of 7.0 (7.8–6.2, 95% confidence limits). The most potent GPR55 agonist tested was Abn-CBD [pEC50 = 7.6 (8.0–7.3, 95% confidence limits)]. Abn-CBD (10−9–10−4 mol·L−1) stimulated insulin release by 1.5-to 3.1-fold (P < 0.01–P < 0.001), with a 67% increase in insulin secretion at the highest concentration tested (10−4 mol·L−1) (Figure 1E). GPR55 antagonist CBD (10−5 mol·L−1) reduced O-1602-induced insulin secretion at 10−7–10−5 mol·L−1 by 22–24% (P < 0.05–P < 0.01) and Abn-CBD-induced insulin secretion at 10−9–10−4 mol·L−1 by 29–52% (P < 0.001) (Figure 1B & E). Insulin secretion was increased 1.2-to 1.4-fold (P < 0.05–P < 0.001) by the rimonabant analogue, AM-251 (10−8–10−4 mol·L−1) with a maximum stimulation of 26% at 10−4 mol·L−1 (P < 0.001) (Figure 1D). AM-251 exhibited a pEC50 of 7.8 (8.4–7.2, 95% confidence limits). CBD at 10−5 mol·L−1 decreased AM-251-induced insulin release at 10−9 mol·L−1 by 10% (P < 0.05) (Figure 1D). pEC50 values ranged from 7.6 (Abn-CBN) to 5.8 (PEA) at 5.6 mM glucose.
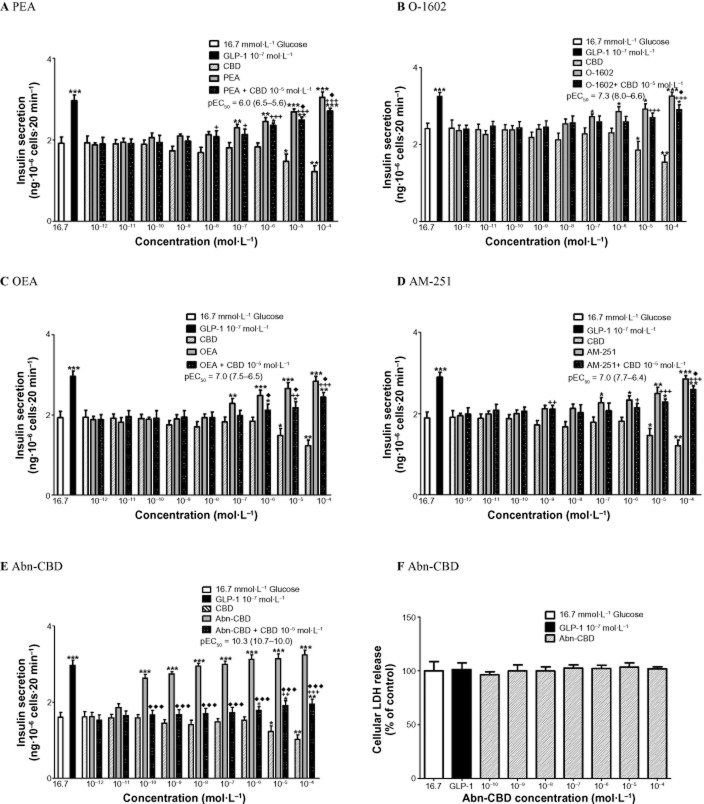
At 16.7 mM glucose, endogenous agonist PEA (10−7–10−4 mol·L−1) enhanced insulin secretion by 1.3-to 1.7-fold (P < 0.01–P < 0.001) (Figure 2A) but was the least potent insulin secreting agonist tested [pEC50 of 6.0 (6.5–5.6, 95% confidence limits)]. OEA (10−7–10−4 mol·L−1) augmented insulin release by 1.2-to 1.5-fold (P < 0.01–P < 0.001) (Figure 2C). At 16.7 mM glucose, antagonist CBD (10−5–10−4 mol·L−1) inhibited insulin secretion by 1.3-to 1.6-fold (P < 0.05–P < 0.01) (Figure 2A). CBD at 10−5 mol·L−1 inhibited PEA-induced insulin release at 10−5–10−4 mol·L−1 by 8–11% (P < 0.05) and OEA-induced insulin secretion at 10−6–10−4 mol·L−1 by 14–18% (P < 0.05) (Figure 2A & C). Synthetic analogues O-1602 (10−7–10−4 mol·L−1) increased insulin secretion by 1.3-to 1.7-fold (P < 0.05–P < 0.001) (Figure 2B) and AM-251 (10−10–10−4 mol·L−1) stimulated the release of insulin by 1.2-to 1.5-fold (P < 0.05–P < 0.001) (Figure 2D). CBD at 10−5 mol·L−1 decreased O-1602-induced insulin release at 10−4 mol·L−1 by 11% (P < 0.05) and AM-251-induced insulin secretion at 10−4 mol·L−1 by 10% (P < 0.05) (Figure 2B & D). Abn-CBD (10−10–10−4 mol·L−1) stimulated the release of insulin by 1.7-to 2.0-fold (P < 0.001) (Figure 2E), with pEC50 of 10.3 (10.7–10.0, 95% confidence limits) and a maximum 51% stimulation of insulin release at 10−4 mol·L−1. CBD at 10−5 mol·L−1 blunted Abn-CBD-induced insulin release from 10−10 to 10−4 mol·L−1 by 37–56% (P < 0.001) (Figure 2E). The pEC50 values ranged from 10.3 (Abn-CBD) to 6.0 (PEA) at 16.7 mM glucose. Comparison of pEC50 values at 5.6 and 16.7 mM glucose showed a more potent insulin secretory response for all agents at 16.7 mM glucose, except AM-251 (Table 1). None of the agonists affected LDH release indicating a lack of cytotoxicity (Figures 1F & 2F).
Figure 2.
Effects of (A) PEA (B) O-1602 (C) OEA (D) AM-251 and (E) Abn-CBD, in the absence and presence of CBD, on insulin secretion from BRIN-BD11 cells at 16.7 mmol·L−1 glucose. GLP-1 (10−7 mol·L−1) used as positive control. (F) Effect of Abn-CBD on LDH release. Results are the mean ± SEM (n = 8) for insulin secretion and (n = 3) for LDH release. *P < 0.05, **P < 0.01 and ***P < 0.001, compared to glucose alone. +P < 0.05 ++P < 0.01 and +++P < 0.001 compared to CBD. ♦P < 0.05 ♦♦P < 0.01 and ♦♦♦P < 0.001 compared to agonist alone.
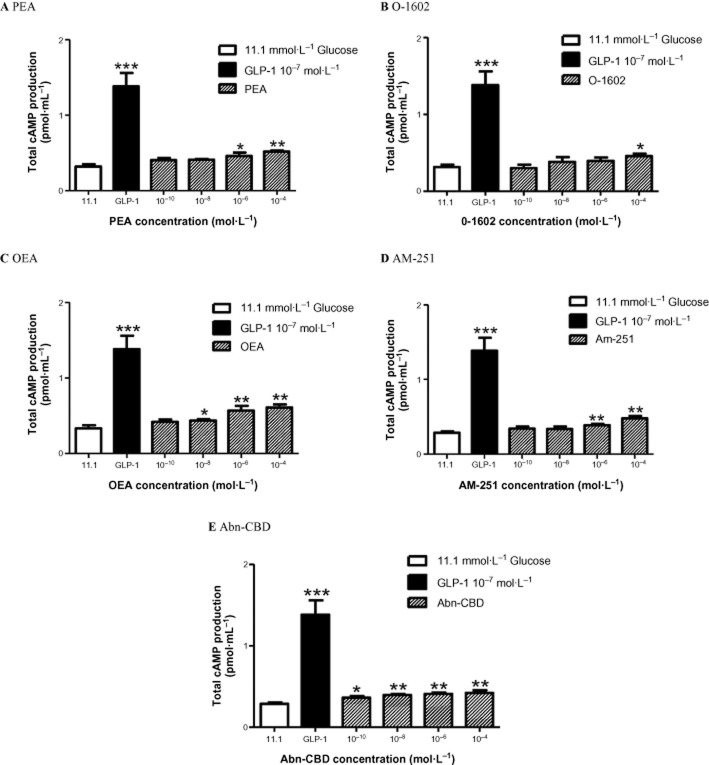
Effect of GPR55 agonists on intracellular Ca2+ and cAMP in BRIN-BD11 cells
Exposure of BRIN-BD11 cells to O-1602, PEA, OEA, AM-251 and Abn-CBD at 10−4 mol·L−1 resulted in prominent stimulatory effects on intracellular Ca2+ concentrations at both 5.6 mmol·L−1 and 16.7 mM glucose (P < 0.05–P < 0.001) (Figure 3). Concentration-dependent stimulation of cAMP production in BRIN-BD11 cells was observed with each GPR55 agonist. Endogenous ligands OEA enhanced cAMP production from 10−8 to 10−4 mol·L−1 (P < 0.05–P < 0.01) (Figure 4C), while PEA augmented cAMP production at 10−6–10−4 mol·L−1 (P < 0.05–P < 0.01) (Figure 4A). O-1602 weakly stimulated cAMP production at the highest concentration tested 10−4 mol·L−1 (P < 0.05) (Figure 4B). Total cAMP production was increased at all concentrations tested (10−10–10−4 mol·L−1) by the synthetic agonist Abn-CBD (P < 0.05–P < 0.01) (Figure 4E). AM-251 moderately enhanced cAMP production at 10−6–10−4 mol·L−1 (P < 0.01) (Figure 4D).
Figure 3.
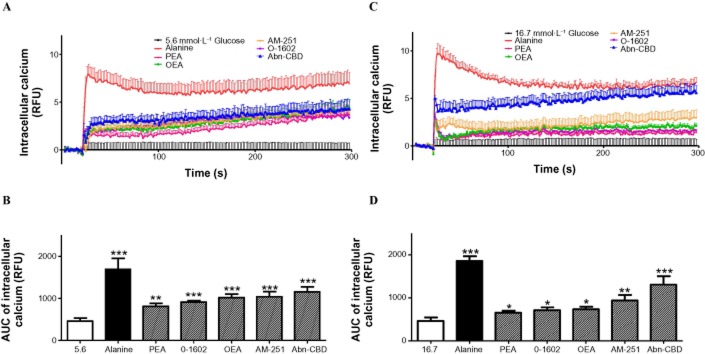
Effects of various GPR55 agonists (10−4 mol·L−1) and alanine (10 mmol·L−1) on intracellular Ca2+ in BRIN-BD11 cells at 5.6 mmol·L−1 or 16.7 mmol·L−1 glucose expressed as (A,C) RFU (B,D) Area under the curve. Results are the mean ± SEM (n = 8). *P < 0.05, **P < 0.01 and ***P < 0.001 compared to control.
Figure 4.
Effects of (A) PEA (B) O-1602 (C) OEA (D) AM-251 and (E) Abn-CBD on cAMP production in BRIN-BD11 cells at 11.1 mmol·L−1 glucose. GLP-1 (10−7 mol·L−1) used as positive control. Values are the mean ± SEM (n = 3). *P < 0.05, **P < 0.01 and ***P < 0.001 compared to 11.1 mmol·L−1 glucose control.
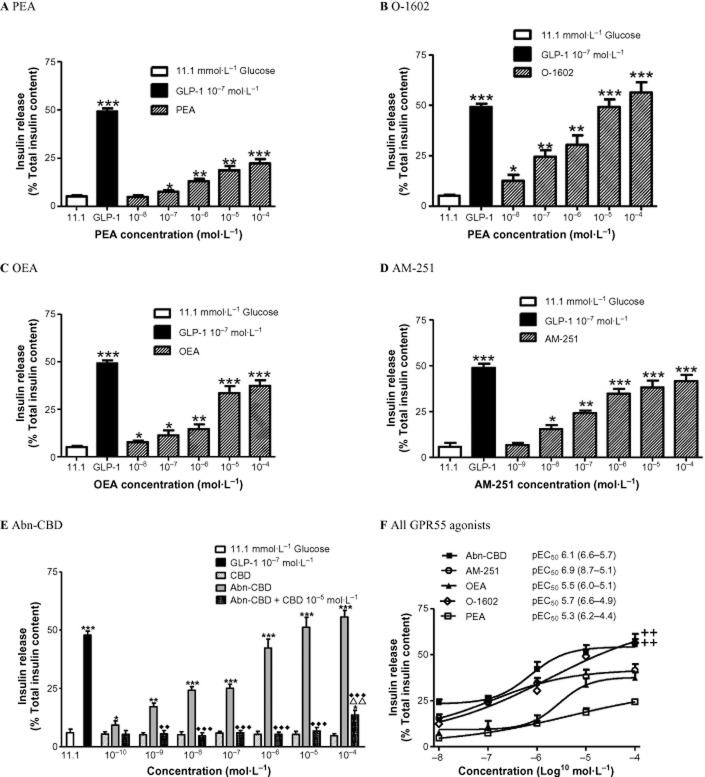
Effect of GPR55 agonists and antagonist on insulin secretion from isolated islets
As shown in Figure 5, the most potent GPR55 agonist tested was AM-251 with a pEC50 of 6.9 (8.7–5.1, 95% confidence limits), with maximum 42% stimulation of insulin secretion (%total insulin content) at 10−4 mol·L−1 (P < 0.001). Abn-CBN stimulated insulin release from isolated islets by 56% at 10−4 mol·L−1 (P < 0.001) displaying a pEC50 value of 6.1 (6.6–5.7, 95% confidence limits). The antagonist CBD had no effect on insulin secretion at 10−10–10−4 mol·L−1 while CBD at 10−5 mol·L−1 inhibited Abn-CBD-induced insulin release from pancreatic islets by 12–45% at 10−9–10−4 mol·L−1 (P < 0.01–P < 0.001) (Figure 5E). Both Abn-CBD and AM-251 were more potent than the naturally occurring agonist PEA (P < 0.01). Endogenous agonist, OEA, augmented insulin release by 37% (P < 0.001; 10−4 mol·L−1) with a pEC50 of 5.5 (6.0–5.1, 95% confidence limits). O-1602 stimulated insulin secretion from isolated islets by 56% [P < 0.001; pEC50 = 5.8 (6.6–4.9, confidence limits)]. Contrastingly, PEA was the least potent insulin secreting agonist with a pEC50 value of 5.3 (6.2–4.4, 95% confidence limits), enhancing insulin release by 22% at 10−4 mol·L−1 (P < 0.001).
Figure 5.
Effects of (A) PEA (B) O-1602 (C) OEA (D) AM-251 (E) Abn-CBD in the absence and presence of CBD (F) all GPR55 agonists tested, with pEC50 values on insulin release (% of insulin content) from isolated mouse islets at 11.1 mmol·L−1 glucose. GLP-1 (10−7 mol·L−1) used as the positive control. Values are the mean ± SEM (n = 3). *P < 0.05, **P < 0.01 and ***P < 0.001, compared to 11.1 mmol·L−1 glucose control. ++P < 0.01, compared to PEA. ΔΔP < 0.01, compared to CBD. ♦♦P < 0.01, ♦♦♦P < 0.001, compared to Abn-CBN.
Expression of GPR55 in BRIN-BD11 cells and mouse islets
Figure 6 displays the distribution of GPR55, insulin and nuclear stain (DAPI) in BRIN-BD11 cells. DAPI (blue) displayed the nuclei of BRIN-BD11 cells (Figure 6A), insulin (red) was evenly distributed (Figure 6B) similar to GPR55 (green) (Figure 6C). Double immunofluorescence of insulin and GPR55 indicated areas of colocalization (yellow) (Figure 6D). The distribution of GPR55, DAPI, insulin and glucagon in mouse pancreatic islets is shown in Figure 7. GPR55 (green) was expressed across the islet (Figure 7C, D) with a similar staining pattern to insulin (red) (Figure 7E). Double immunofluorescence of insulin and GPR55 indicated that insulin secreting beta cells express GPR55 (Figure 7G) while there was no evidence of the receptor in glucagon secreting alpha cells (no yellow staining) (Figure 7H).
Figure 6.
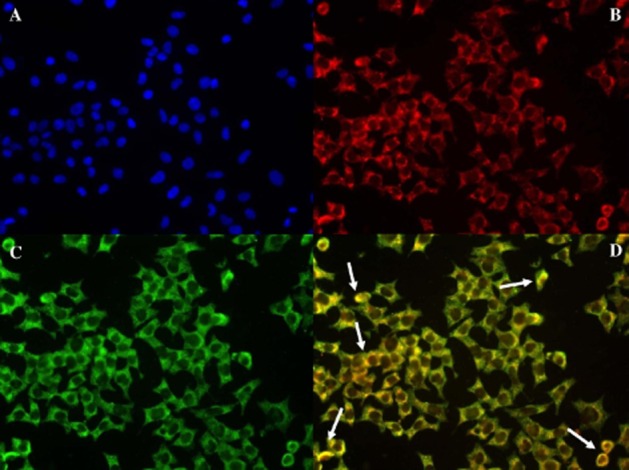
Distribution in BRIN-BD11 cells of (A) DAPI nuclear stain, (B) insulin (C) GPR55 (D) merge of GPR55 colocalized with insulin at ×40 magnification. Examples of colocalization indicated by arrows.
Figure 7.
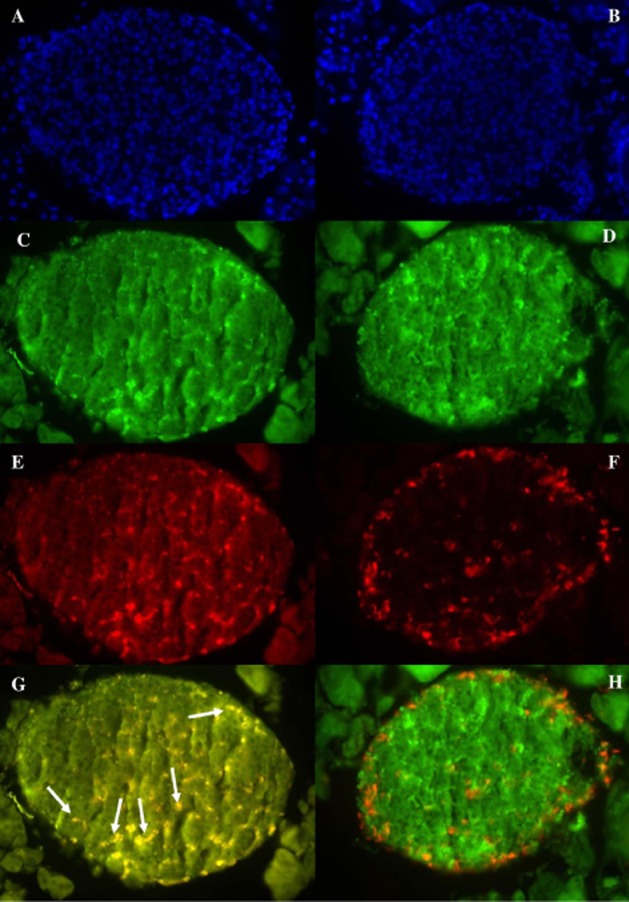
Distribution in mouse pancreatic islets of (A, B) DAPI nuclear stain, (C, D) GPR55 (E) insulin (F) glucagon (G) merge of GPR55 colocalized with insulin. Example of colocalization indicated by arrows. (H) merge of GPR55 and glucagon in mouse pancreatic tissue at ×40 magnification.
Acute effects of GPR55 agonists on glucose tolerance and insulin release in vivo
Administration of 0.1 μmol·kg−1 of OEA, AM-251 and O-1602 to non-fasted NIH mice resulted in reduced plasma glucose after 105 min by 18–25% (P < 0.05) while the GPR55 agonist Abn-CBD decreased plasma glucose by 28% at 60 min (P < 0.05) and by 37% at 105 min (P < 0.01) compared to glucose alone (Figure 8A). Both AM-251 (P < 0.05) and Abn-CBD (P < 0.01) enhanced insulin release by 32–40% after 15 min while OEA, AM-251 and Abn-CBD (P < 0.01) stimulated plasma insulin by 30–37% after 30 min (Figure 8B). All agonists tested with exception of PEA augmented glucose-induced insulin release and decreased the glycaemic excursion (P < 0.05–P < 0.01) when compared to glucose alone (Figure 8C, D).
Figure 8.
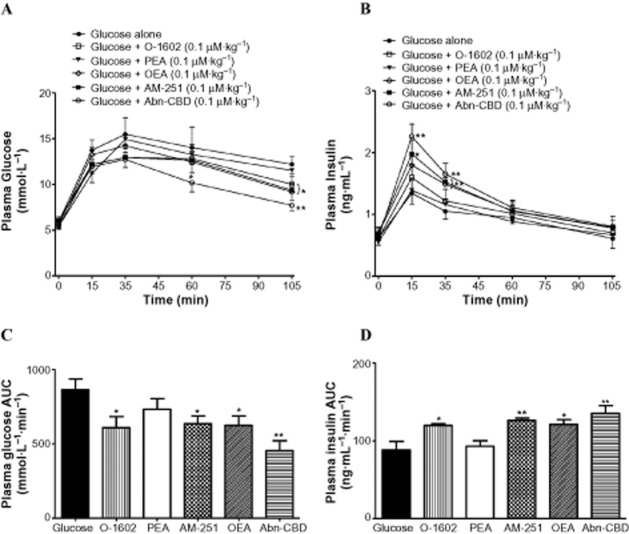
Acute effects of GPR55 agonists on (A,C) plasma glucose and (B,D) insulin responses following an i.p. glucose load. Glucose (18 mmol·kg−1 body weight) was administered alone or in combination with agonists (0.1 μmol·kg−1 body weight) to non-fasted NIH Swiss mice. Plasma glucose and insulin AUC values for 0–105 min post-injection are also shown. Values are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared to 18 mmol·L−1 glucose.
Discussion
New drugs acting to enhance beta cell function are needed for the treatment of type 2 diabetes and much interest has focused on GPCRs and their activation by ligands/agonists. GPCRs comprise a large family of membrane proteins that are attracting pharmaceutical interest as therapeutic agents. Currently, more than 1000 GPCRs have been identified, some of which have no known ligand (orphan GPCRs) (Ahern, 2009). Free fatty acids are signalling molecules that augment insulin and glucagon secretion in pancreatic islets and were proposed to exert their actions through GPR40 (Winzell and Ahren, 2007). One well studied GPCR agonist is exenatide, and in recent phase II clinical trials the orally available GPR40 agonist TAK-875 displayed glucose lowering abilities in patients with type 2 diabetes (Araki et al., 2011).
The present study explored the secretion of insulin in response to pharmacological GPR55 agonists using the clonal BRIN-BD11 cells. The GPR55 agonists, O-1602, OEA, PEA, AM-251 and Abn-CBD, enhanced insulin secretion in a concentration-dependent manner. At basal glucose, Abn-CBD was the most potent GPR55 agonist, followed by AM-251, OEA, O-1602 and endogenous agonist PEA based on their pEC50 values. Similarly at 16.7 mM glucose, GPR55 agonists in order of decreasing pEC50 values are Abn-CBD, AM-251, OEA, O-1602 and PEA. The combination of GPR55 agonists with antagonist CBD determined the selectivity of the agonists for GPR55. At both basal and stimulatory glucose, Abn-CBD displayed a high selectivity for GPR55 with significant reduction in insulinotropic abilities. OEA and O-1602 were moderately selective for GPR55 while AM-251 and PEA were less selective for the receptor. Cytotoxicity testing revealed that none of the GPR55 agonists had adverse effects. Interestingly, previous research has shown that the GPR55 agonist OEA has cytoprotective effects on pancreatic beta cell lines when incubated with lipotoxic long chain saturated fatty acids (Stone et al., 2012).
To complement the above findings, insulin secretion studies were carried out using isolated mouse islets. Similar to the above findings Abn-CBD and AM-251 were found to be potent agonists, based on pEC50 values. Abn-CBD selectivity for GPR55 was confirmed in pancreatic islets by the addition of antagonist CBD which eliminated the potent stimulatory ability of Abn-CBD alone. OEA exhibited intermediate potency whereas the endogenous agonist PEA and CBD analogue O-1602 were found to be less effective. These findings suggest that some agonists have a low affinity for GPR55 and may activate other receptors including CBD and non-CBD receptors. A study evaluating O-1602 in pancreatic islets found that insulin secretion was stimulated in wild-type mice while in GPR55 knock-out mice insulin secretion was blunted indicating 0–1602 is indeed working through GPR55 (Romero-Zerbo et al., 2011). In the present study, all the GPR55 agonists had greater potency in the clonal BRIN-BD11 cells than in isolated mouse islets and the results demonstrate that glucose sensitized insulin-secreting cells to the actions of GPR55 agonists.
The mechanism of agonist-induced insulin secretion was investigated by exploring intracellular Ca2+ and cAMP production using BRIN-BD11 cells. All GPR55 agonists (0–1602, OEA, PEA, AM-251 and Abn-CBD at 10−4 mol·L−1) caused a prompt increase in intracellular Ca2+ suggesting modulation of insulin release is mediated partly through Ca2+ activated pathways. A recent study using 0–1602, by Romero-Zerbo et al., 2011 using isolated islets also implicated changes in intracellular Ca2+. In the present study, GPR55 agonists also caused a moderate increase in total cAMP production, with greatest effects being observed with Abn-CBD. These results indicate that the GPR55 agonists predominately work through the Ca2+ dependent pathway and to a lesser extent the adenylyl cyclase pathway. It is well recognized that GPCRs may activate pathways in islets such as cAMP, intracellular Ca2+, diacylglycerol and inositol 1.4,5-trisphosphate (Amisten et al., 2013). Few studies have reported the effects of PEA, AM-251 and Abn-CBD on insulin secretion in pancreatic islets. However, Ning et al. found that OEA-induced insulin secretion was associated with increased cAMP production and intracellular Ca2+ (Ning et al., 2008).
The naturally occurring agonists evaluated in this study are not GPR55 specific and have the ability to activate several different receptors. Endogenous agonist OEA activates non-CB1/CB2 receptors including GPR119 and has a high affinity for the PPAR-α receptor (Overton et al., 2006). PEA is reported to have a high potency for GPR55 with low affinity for CB1/CB2 receptors, PPAR-α receptor and GPR119 (Ryberg et al., 2007). The CBD analogue O-1602 is a selective potent agonist of GPR55 and lesser extent CB1/CB2 receptors (Whyte et al., 2009). AM-251 is a potent CB1 antagonist and activates GPR55 augmenting intracellular calcium in endothelial cells expressing GPR55 (Waldeck-Weiermair et al., 2008). The ligand Abn-CBD is a selective agonist of GPR55 and mediates endothelial vasodilation which was previously found to be independent of the CB1/CB2 receptors (Járai et al., 1999).
In order to investigate the cellular localization of GPR55 in BRIN-BD11 cells and pancreatic islets, double immunofluorescence was carried out and demonstrated that GPR55 was specifically colocalized with insulin on BRIN-BD11 cells and pancreatic beta cells. A recent study by Romero-Zerbo et al. (2011) demonstrated GPR55 mRNA in pancreatic islets and considerable protein distribution in insulin secreting beta cells. While no distribution was observed on glucagon secreting alpha cells in this study, the effect of GPR55 on the beta cells may have a paracrine effect on neighbouring cells. To further validate the in vitro findings, administration of GPR55 agonists were examined in vivo. All GPR55 agonists, except PEA, demonstrated glucose lowering and insulinotropic abilities in mice. The effects of Abn-CBD were particularly notable; however, further studies are necessary to determine the long-term effects and agonist-GPR55 binding profile and pharmacokinetics of these GPR55 agonists in vivo. One recent study demonstrated that O-1602 when injected acutely in mice, results in a decrease in plasma glucose and increase in plasma insulin (Romero-Zerbo et al., 2011).
In conclusion, a broad range of GPR55 agonists stimulated insulin-secretion with elevation of intracellular Ca2+ and production of cAMP. This highlights a role for GPR55 in insulin secretion and suggests that agonizing GPR55 may have therapeutic potential for type 2 diabetes.
Acknowledgments
These studies were supported by the Department of Education and Learning, Northern Ireland.
Glossary
- Abn-CBD
abnormal cannabidiol
- CB1
Cannabinoid receptor 1
- CB2
Cannabinoid receptor 2
- CBD
Cannabidiol
- GPR55
G-protein coupled receptor 55
- OEA
oleoylethanolamide
- PEA
palmitoylethanolamine
Conflict of interest
The authors have no conflict of interest to report.
References
- Ahern B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther. 139:359–391. doi: 10.1016/j.pharmthera.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Anavi-Goffer S, Baillie G, Irving AJ, Gertsch J, Greig IR, Pertwee RG, et al. Modulation of L-α-Lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signalling by cannabinoids. J Biol Chem. 2012;287:91–104. doi: 10.1074/jbc.M111.296020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Hirayama M, Hiroi S, Kaku K. GPR40-induced insulin secretion by the novel agonist TAK-875: first clinical findings in patients with type 2 diabetes. Diabetes Obes Metab. 2011;14:271–278. doi: 10.1111/j.1463-1326.2011.01525.x. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Flatt PR. Influence of genetic background and age on the expression of the obese hyperglycaemic syndrome in Aston ob/ob mice. Int J Obes. 1982;6:11–21. [PubMed] [Google Scholar]
- Bermúdez-Silva FJ, Suárez J, Baixeras E, Cobo N, Bautista D, Cuesta-Muñoz AL, et al. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia. 2008;51:476–487. doi: 10.1007/s00125-007-0890-y. [DOI] [PubMed] [Google Scholar]
- Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 2007;152:567–575. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Arteaga A, Vázquez MJ, Vazquez-Martínez R, Pulido MR, Suarez J, Velásquez DA, et al. The atypical cannabinoid O-1602 stimulates food intake adiposity in rats. Diabetes Obes Metab. 2011;14:234–243. doi: 10.1111/j.1463-1326.2011.01515.x. [DOI] [PubMed] [Google Scholar]
- Flatt PR, Bailey CJ. Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia. 1981;20:573–577. doi: 10.1007/BF00252768. [DOI] [PubMed] [Google Scholar]
- Godlewski G, Offertaler L, Wagner JA, Kunos G. Receptors for acylethanolamines-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009;89:105–111. doi: 10.1016/j.prostaglandins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Schröder R, Kargl JK, Platzer W, Martini L, et al. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br J Pharmacol. 2010;160:604–614. doi: 10.1111/j.1476-5381.2009.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Kargl J, Andradas C, Brown AJ, Irving A, et al. Minireview: recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol Endocrinol. 2011;25:1835–1848. doi: 10.1210/me.2011-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoareau L, Roche R. Palmitoylethanolamide, adipocytes and obesity-related inflammatory states. Drug Discov Today Dis Mech. 2010;7:205–212. [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Zhao P, Sharir H, Bai Y, Caron MG, Barak LS, et al. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J Biol Chem. 2009;284:29817–29827. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Bowe JE, Huang GC, Amiel SA, Jones PM, Persaud SJ. Cannabinoid receptor agonists and antagonists stimulate insulin secretion from isolated human islets of Langerhans. Diabetes Obes Metab. 2011;13:903–910. doi: 10.1111/j.1463-1326.2011.01422.x. [DOI] [PubMed] [Google Scholar]
- Lin XH, Yuece B, Li YY, Feng YJ, Feng JY, Yu LY, et al. A novel CB receptor GPR55 and its ligands are involved in regulation of gut movements in rodents. Neurogastroenterol Motil. 2011;23:862–871. doi: 10.1111/j.1365-2982.2011.01742.x. [DOI] [PubMed] [Google Scholar]
- McClenaghan NH, Barnett CR, Ah-Sing E, Abdel-Wahab YH, O'Harte FP, Yoon TW, et al. Characterisation of a novel glucose-responsive insulin-secreting cell line, BRIN BD11, produced by electrofusion. Diabetes. 1996;45:1132–1140. doi: 10.2337/diab.45.8.1132. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. AAPS J. 2006;8:298–306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel JC, Patterson S, Abdel-Wahab YHA, Mathias PC, Flatt PR. Time-correlation between membrane depolarization and intracellular calcium in insulin secretion BRIN-BD11 cells: studies using FLIPR. Cell Calcium. 2004;36:43–50. doi: 10.1016/j.ceca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Catalán V, Whyte L, Díaz-Arteaga A, Vázquez-Martínez R, Rotellar F, et al. The L-α-Lysophosphatidylinositol/ GPR55 system and its potential role in human obesity. Diabetes. 2012;61:281–291. doi: 10.2337/db11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalewski S. Studies on the culture and transplantation of isolated islets of Langerhans of the guinea pig. Proc K Ned Akad Wet C. 1969;72:157–171. [PubMed] [Google Scholar]
- Ning Y, O'Neill K, Lan H, Pang L, Shan LX, Hawes BE, et al. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br J Pharmacol. 2008;155:1056–1065. doi: 10.1038/bjp.2008.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, et al. Deorphanisation of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agent. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Patti M. Rehashing endocannabinoid antagonists: can we selectively target the periphery to safely treat obesity and type 2 diabetes? J Clin Invest. 2010;120:2646–2648. doi: 10.1172/JCI44099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavón FJ, Serrano A, Pérez-Valero V, Jagerovic N, Hernández-Folgado L, Bermúdez-Silva FJ, et al. Central versus peripheral antagonism of cannabinoid CB1 receptor in obesity: effects of LH-21, a peripherally acting neutral cannabinoid receptor antagonism, in Zucker rats. J Neuroendocrinol. 2008;20:116–123. doi: 10.1111/j.1365-2826.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- Romero-Zerbo SY, Rafacho A, Diaz-Arteaga A, Suárez J, Quesada I, Imbernon M, et al. A role for the putative cannabinoid receptor GPR55 in the islets of Langerhans. J Endocrinol. 2011;211:177–185. doi: 10.1530/JOE-11-0166. [DOI] [PubMed] [Google Scholar]
- Roser P, Vollenweider FX, Kawohl W. Potential antipsychotic properties of central cannabinoid (CB1) receptor antagonists. World J Biol Psychiatry. 2010;11:208–219. doi: 10.3109/15622970801908047. [DOI] [PubMed] [Google Scholar]
- Ross RA. The enigmatic pharmacology of GPR55. Trends Pharmacol Sci. 2008;30:156–163. doi: 10.1016/j.tips.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–10101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone VA, Dhayal S, Smith DM, Lenaghan C, Brocklehurst KJ, Morgan NG. The cytoprotective effects of oleoylethanolamide in insulin-secreting cells do not require activation of GPR119. Br J Pharmacol. 2012;165:2758–2770. doi: 10.1111/j.1476-5381.2011.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylantyev S, Jensen TP, Ross RA, Rusakov DA. Cannabinoid-and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci U S A. 2013;110:5193–5198. doi: 10.1073/pnas.1211204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilches-Flores A, Delgado-Buenrostro NL, Navarrete-Vazquez G, Villalobos-Molina R. CB1 cannabinoid receptor expression is regulated by glucose and feeding in rat pancreatic islets. Regul Pept. 2010;163:81–87. doi: 10.1016/j.regpep.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Waldeck-Weiermair M, Zoratti C, Osibow K, Balenga N, Goessnitzer E, Waldhoer M, et al. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci. 2008;121:1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte LS, Ryberg E, Sims NA, Ridge SA, Mackie K, Greasley PJ, et al. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci U S A. 2009;106:16511–16516. doi: 10.1073/pnas.0902743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzell MS, Ahren B. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol Ther. 2007;116:437–448. doi: 10.1016/j.pharmthera.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Zang X, Maor Y, Wang JF, Kunos G, Groopman JE. Endocannabinoid-like N-arachidonoyl serine is a novel pro-angiogenic mediator. Br J Pharmacol. 2010;160:1583–1594. doi: 10.1111/j.1476-5381.2010.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]