Abstract
Background and Purpose
Mas oncogene-related gene (Mrg) receptors are exclusively distributed in small-sized neurons in trigeminal and dorsal root ganglia (DRG). We investigated the effects of MrgC receptor activation on inflammatory hyperalgesia and its mechanisms.
Experimental Approach
A selective MrgC receptor agonist, bovine adrenal medulla peptide 8-22 (BAM8-22) or melanocyte-stimulating hormone (MSH) or the μ-opioid receptor (MOR) antagonist CTAP was administered intrathecally (i.t.) in rats injected with complete Freund's adjuvant (CFA) in one hindpaw. Thermal and mechanical nociceptive responses were assessed. Neurochemicals were measured by immunocytochemistry, Western blot, ELISA and RT-PCR.
Key Results
CFA injection increased mRNA for MrgC receptors in lumbar DRG. BAM8-22 or MSH, given i.t., generated instant short and delayed long-lasting attenuations of CFA-induced thermal hyperalgesia, but not mechanical allodynia. These effects were associated with decreased up-regulation of neuronal NOS (nNOS), CGRP and c-Fos expression in the spinal dorsal horn and/or DRG. However, i.t. administration of CTAP blocked the induction by BAM8-22 of delayed anti-hyperalgesia and inhibition of nNOS and CGRP expression in DRG. BAM8-22 also increased mRNA for MORs and pro-opiomelanocortin, along with β-endorphin content in the lumbar spinal cord and/or DRG. MrgC receptors and nNOS were co-localized in DRG neurons.
Conclusions and Implications
Activation of MrgC receptors suppressed up-regulation of pronociceptive mediators and consequently inhibited inflammatory pain, because of the activation of up-regulated MrgC receptors and subsequent endogenous activity at MORs. The uniquely distributed MrgC receptors could be a novel target for relieving inflammatory pain.
Keywords: dorsal root ganglia (DRG), inflammatory hyperalgesia, Mas-related gene (Mrg) receptors, μ-opioidergic activity, spinal dorsal horn
Introduction
Mas oncogene-related gene (Mrg) receptors (Dong et al., 2001), also known as sensory neuron-specific receptors (SNSR; Lembo et al., 2002), are a large family of GPCRs (receptor nomenclature follows Alexander et al., 2011). This type of receptor has been identified in mice (Dong et al., 2001), rats (Lembo et al., 2002; Zylka et al., 2003), humans (Dong et al., 2001; Lembo et al., 2002), gerbils (Zylka et al., 2003), rhesus monkeys (Burstein et al., 2006) and macaques (Zhang et al., 2005). The Mrg receptor family comprises many members, divided into four groups (MrgA–MrgD) in rodents (Dong et al., 2001), but seven groups (MrgX1–MrgX7) in humans (Dong et al., 2001; Choi and Lahn, 2003). Importantly, most members of the Mrg receptor family are exclusively distributed in small diameter neurons of trigeminal and dorsal root ganglia (DRG; Dong et al., 2001; Lembo et al., 2002), implying their involvement in nociceptive transmission. This highly restricted distribution of Mrg receptors may allow nociception to be processed or modulated in a highly selective manner and provides a drug target which should have minimal side effects in the CNS (Simonin and Kieffer, 2002).
Surprisingly, the functional roles of Mrg receptors in various types of pain have not yet been fully described. Attention has been drawn to MrgC receptors, partly because of the availability of the specific agonists, bovine adrenal medulla peptide 8-22 (BAM8-22; Lembo et al., 2002; Guan et al., 2010) and (Tyr6)-γ2-MSH-6-12 [melanocyte-stimulating hormone (MSH)] (Han et al., 2002; Lembo et al., 2002). MrgC receptors are not involved in pain processing under normal conditions (physiological pain) as the deletion of MrgC genes (Ndong et al., 2009; Guan et al., 2010) and pharmacological activation of the receptors (Cai et al., 2007b; Guan et al., 2010) did not alter nociceptive thresholds, but these receptors can modulate pathological pain. For example, spinal application of BAM8-22 attenuates mechanical allodynia induced by peripheral nerve injury (Guan et al., 2010). However, there is a disagreement regarding the role of MrgC receptors in inflammatory pain. Rats with SNSR1 (corresponding to MrgC receptors) knocked-down by small interfering RNA (siRNA) show a reduction in thermal hyperalgesia in the complete Freund's adjuvant (CFA) model, suggesting a role of MrgC receptors in the induction of inflammatory pain (Ndong et al., 2009). Data from mice indicate that MrgC receptors display protective actions in inflammatory pain as animals with MrgC cluster knock-out showed enhanced wind-up responding to C fibre inputs and/or increased pain responses following an intraplantar (i.pl.) injection of formalin (Guan et al., 2010), CFA or carrageenan (Liu et al., 2009).
As MrgC receptors possess high therapeutic potential, it is important to clarify the effects of MrgC receptor activation on inflammatory pain. The present study examined the effects of intrathecal (i.t.) administration of BAM8-22 and MSH on inflammatory pain, up-regulation of neuronal NOS (nNOS) and expression of CGRP and c-Fos, which are believed to be ascribed to or indicate hypersensitivity, in the rat model of CFA-induced inflammation. We also investigated the possibility that the stimulation of MrgC receptors increased the activation of μ-opioid receptors (MORs) by endogenous agonists. Some of these results have been presented in abstract form (Hong et al., 2012).
Methods
Animals
All animal care and experimental treatments complied with the guidelines for investigations of experimental pain in conscious animals (Zimmermann, 1983) and were approved by the Animal Care Committee of Fujian Normal University. Efforts were made to minimize animal suffering and the number of animals used in our experiments. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 196 animals were used in the experiments described here. Male Sprague–Dawley rats (250–320 g; Animal Center of Fujian Medical University, Fuzhou, China) were housed individually at 22°C with 50% humidity under a 12 h light/dark cycle and given free access to food and water. Inflammation was induced by s.c. injection of 150 μL of 1 mg·mL−1 CFA into the hindpaw. Control rats received an injection of saline (150 μL).
Intrathecal catheter implantation
Drugs were delivered spinally via i.t. catheter (described below) or percutaneous lumbar puncture (see Supporting Information Appendix S1).
Animals were implanted with chronic indwelling catheters with some modification of the previously described technique (Pogatzki et al., 2000). Briefly, rats were injected with i.p. pentobarbitone (50 mg·kg−1) and shaved along the occiput and neck. An incision was made overlying the atlanto-occipital junction, and the dura mater was exposed by blunt dissection. An incision was made in the dura, and a polyethylene catheter (PE-10; Stoelting, Wood Dale, IL, USA), with a loose knot cemented with dental acrylic 8.0 cm from the end, was threaded caudally to position its tip at the L4-5 segments of the spinal cord. The external end of the catheter was externalized at the back of the neck. The catheter was then flushed with 10 μL of saline and plugged. The rats were housed individually after surgery and allowed to recover for approximately 7 days before being used for behavioural testing. Only the animals with no evidence of neurological deficits after catheter placement were used for subsequent experimentation.
The MrgC receptor agonist, BAM8-22 or MSH, was administered i.t. at 0 and 24 h. To investigate the possible involvement of MORs, some rats also received i.t. injection of the selective MOR antagonist CTAP (Pelton et al., 1985).
Assessment of nociceptive behaviour
A radiant heat stimulus from a Plantar Test Meter (IITC Life Science Inc., Woodland Hills, CA, USA) was applied by aiming a light beam through a hole in the light box through the glass plate to the middle of the plantar surface of the rat's hindpaw. The heat intensity was adjusted to obtain average paw withdrawal latency (PWL) of 7–9 s, and the cut-off time was set at 20 s to prevent tissue damage. PWL at any test time point was measured three times at 1.5 min interval, and the mean value of these measurements was taken (Zeng et al., 2006; Boettger et al., 2007).
Mechanical threshold was measured in the hindpaw using an automated von Frey type system (Dynamic Plantar Anesthesiometer 37400; Ugo Basile, Comerio VA, Italy). The stimulator unit was placed beneath the selected hindpaw with the filament below the plantar surface of the rat. A paw withdrawal response was elicited by applying an increasing force (measured in grams) using a stainless steel filament (0.5 mm diameter). The force was increased at a rate of 2.5 g·s−1 until the rat moved its paw. A force of 50 g for 30 s was used as a cut-off point to preclude possible damage to the paw. The force was measured three times at 1.5 min interval to generate mean values.
Immunohistochemistry
Rats were deeply anaesthetized with sodium pentobarbitone (60 mg·kg−1 i.p.). The animals were perfused intracardially with cold 0.01 M PBS and subsequently with 4% paraformaldehyde in 0.1 M phosphate buffer (PB). The L4-5 segments of the spinal cord and DRG were removed and post-fixed in the same fixative overnight. Tissues were then transferred into 30% sucrose in PB for cryoprotection. Spinal cord sections (40 μm thick) and DRG sections (10 μm thick) were cut on a cryostat (MICROM GmbH, HM550; Walldorf, Germany). Immunocytochemistry was performed at room temperature on free-floating sections (spinal cord) or slides (DRG) using an avidin–biotin complex technique as described previously (Cai et al., 2007a). To permit comparisons across treatment groups, sections from different groups were processed simultaneously. After pretreatment with 0.3% H2O2 and 10% normal goat serum, sections were incubated at 4°C with polyclonal rabbit anti-nNOS (1:2500; Biosciences Pharmingen, San Diego, CA, USA) or anti-CGRP [1:10 000; Santa Cruz Biotechnology (Shanghai) Co., Ltd., Shanghai, China] for 24 h. The tissues were then transferred to biotinylated secondary IgG complex (1:200 in 10% goat serum in PBS) for 2 h followed by exposure to avidin–biotin HRP complex (1:100; Vector Laboratories, Burlingame, CA, USA) for 1 h. The chromogen was developed with 0.01% H2O2 and 0.05% diaminobenzidine. After being thoroughly rinsed with PBS, spinal sections were mounted on gelatin-coated slides, air dried, dehydrated in a series of graded ethanol, cleared in xylene and coverslipped. Using different sections (n = 2), the primary antibody against either nNOS or CGRP was omitted in the immunocytochemical process, which resulted in the absence of staining. The quantification of immunoreactivity (IR) for CGRP and nNOS is described in the Supporting Information Appendix S1.
For double immunostaining of nNOS with MrgC receptors, DRG sections were first incubated in 10% normal donkey serum and next in a mixture of goat polyclonal antibody against nNOS (1:100; Abcam, Cambridge, MA, USA) with rabbit antisera against MrgC receptors (1:100; Phoenix Pharmaceuticals, Burlingame, CA, USA) for 24 h at 4°C. Sections were then incubated in a mixture of donkey anti-goat IgG conjugated with FITC (1:200; Abcam) and donkey anti-goat IgG conjugated with rhodamine (1:100) for 2 h at room temperature. nNOS-IR appeared green, whereas MrgC receptor-IR appeared red. Images were captured using a confocal microscopy system (C1-Si; Nikon, Tokyo, Japan). For control, omission of the primary antibody resulted in negative staining in all tested sections. Sections of DRG were incubated with MrgC receptor antiserum that was preabsorbed with MrgC receptor protein [1 μM, Phoenix Biotech (Beijing) Co., Ltd., Beijing, China]. These procedures resulted in the complete loss of staining.
Western blot, quantitative real-time PCR and elisa
Methods for these procedures are described fully in Supporting Information Appendix S1.
Data analysis
CFA-induced responses, such as thermal supersensitivity (Malcangio and Bowery, 1994), chemical release (Cabot et al., 2001) in the hindpaw and expression of nociceptive or anti-nociceptive molecules in DRG (Galeazza et al., 1995; Puehler et al., 2004) and spinal dorsal horn (Nahin et al., 1989), are elicited only on the ipsilateral side. Therefore, the expression of MrgC mRNA and opioid molecules on the side contralateral to CFA injection were used as control values.
Data are expressed as mean ± SEM. Statistical significance between groups was examined using anova followed by Tukey's test for multiple comparisons. To detect changes over the time between two groups (treatment group × time), data were analysed using a two-way anova. A P-value of less than 0.05 was considered statistically significant.
Materials
Sodium pentobarbitone was obtained from Shengong, Shanghai, China. CFA and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). BAM8-22, MSH and BAM8-18 were synthesized in Huadatianyuan Co. (Shanghai, China; see Supporting Information Appendix S1).
Results
Activation of MrgC receptors produces instant and delayed attenuations of CFA-induced heat hyperalgesia but does not change CFA-induced mechanical allodynia
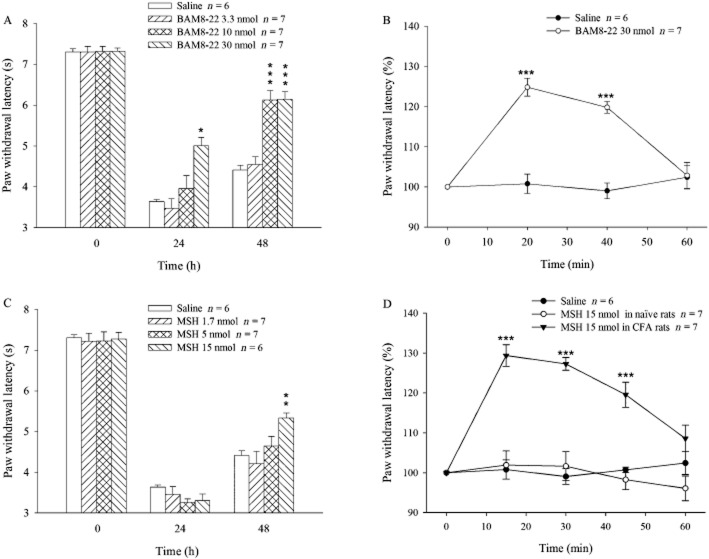
As a pilot study showed that i.t. administration of BAM8-22 inhibited, rather than enhanced, CFA-evoked hyperalgesia in rats (as observed in mice by Guan et al., 2010), the anti-nociceptive effects of activating MrgC receptors were measured. CFA was injected in one hindpaw on day 0 and BAM8-22 (3.3, 10 and 30 nmol), or saline was administered i.t. on days 0 and 1. The CFA injection combined with saline i.t., produced hyperalgesia indicated by a marked decrease in PWL on days 1 and 2 (Figure 1A). However, the hyperalgesic response was attenuated in the group given BAM8-22 at 30 nmol on day 1 and was reduced even further on day 2. These changes were significantly different from corresponding control (P < 0.5 or 0.001). I.t. BAM8-22 at a dose of 10 nmol also attenuated hyperalgesia on day 2 (P < 0.001). Moreover, the highest dose of BAM8-22 administered produced a rapidly developing (by 20 min) but short lasting (<60min) increase in PWL, compared with the saline-treated group. This rapid response is illustrated in Figure 1B, where the BAM8-22-induced changes have been expressed as a percentage of the pre-BAM8-22 value and shows that 30 nmol BAM8-22 induced 120–125% PWL (P < 0.001) for 40 min.
Figure 1.
PWL in response to radiant heat. Animals were treated with i.pl. CFA (150 μL) on day 0, and i.t. BAM8-22 (3.3, 10.0 and 30.0 nmol) or MSH (1.7, 5.0 and 15.0 nmol) on days 0 and 1. PWL was measured on days 0, 1 and 2 prior to any injections in (A) and (C). PWL was also measured on day 1 after the injection of BAM8-22 (B) or MSH (D). In (B) and (D), PWL was calculated as percentage of pretreatment baseline PWL (100%). *P < 0.05, **P < 0.01, ***P < 0.001: compared with saline group (A and C) or pretreatment baseline (B and D).
To confirm the effect of MrgC receptor activation on CFA-induced hyperalgesia, we used another MrgC receptor agonist, MSH, with a structure different from that of BAM8-22. As shown in Figure 1C, i.t. administration of MSH on day 0 did not change CFA-induced hyperalgesia on day 1. However, PWL was significantly increased on day 2 in the group treated with 15 nmol MSH compared with the CFA/saline group (P < 0.01). MSH (15 nmol) administered on day 1 also produced the rapid increase in PWL compared with the pre-treated level, an increase that lasted for 45 min (P < 0.001; Figure 1D). In contrast, MSH (15 nmol) given i.t. to naïve animals failed to alter thermal pain threshold (Figure 1D).
The effect of BAM8-22 on CFA-induced mechanical sensitivity was also assessed. CFA was injected at 0 h and saline or BAM8-22 (30 nmol) was given i.t. at 0, 24, 48 and 72 h. Baseline mechanical threshold in the hindpaw was 34.2 ± 0.4 g in CFA + saline group. The threshold was decreased to 16.2 ± 0.6 g on day 1 and maintained throughout the experiment (20–23 g on days 2–4; n = 8, data not shown). However, the daily administration of BAM8-22 for 4 days did not change CFA-induced hypersensitivity compared with CFA + saline group (n = 9, data not shown).
Activation of MrgC receptors attenuates CFA-induced expression of CGRP, nNOS and c-Fos in DRG or spinal dorsal horn
Because CGRP plays a pivotal role in pathogenesis of inflammatory hyperalgesia (Seybold et al., 1995; Fehrenbacher et al., 2003), we examined the effect of i.t. BAM8-22 on CFA-induced CGRP expression. A number of small to medium sized neurons exhibited staining for CGRP in DRG. On the side contralateral to CFA injection, the proportion of CGRP-IR positive small or medium neurons (Figure 2A and E) was lower than the corresponding proportions on the ipsilateral side (P < 0.01; Figure 2B and E), illustrating the unilateral induction of CGRP by CFA. Following i.t. administration of BAM8-22, the proportion of CGRP-positive neurons in small and medium subpopulations on the ipsilateral side was reduced, to values significantly different from the CFA/saline group (P < 0.05–0.01; Figure 2C and E).
Figure 2.

Effect of i.t. administration of BAM8-22 on CFA-evoked expression of CGRP in DRG. CFA was injected in the right hindpaw on day 0, and BAM8-22 (30 nmol) was administered i.t. on days 0 and 1. The L4-6 DRG were collected on day 2. Photomicrographs of transverse sections of DRG show CGRP-IR in small (small arrows), medium (medium arrows) and large (large arrows) neurons on the contralateral (A and C) and ipsilateral (B and D) sides in CFA/saline (A and B) and CFA/BAM8-22 (C and D) groups. Histograms show means ± SEM of CGRP-IR-positive neurons in DRG (E). **P < 0.01 compared with the contralateral side. #P < 0.05, ##P < 0.01; compared with CFA/saline group. n = 4 each. Scale bar = 50 μm.
Because no antagonist of MrgC receptors is available, another MrgC receptor agonist, MSH, was used to confirm the receptor activation. As illustrated in Figure 3A, CFA injection increased the expression of nNOS in the spinal dorsal horn compared with saline treatment (P < 0.05). The i.t. administration of the non-active MrgC homologue BAM8-18 (30 nmol) did not change CFA-induced increase in nNOS expression (P > 0.05). However, after i.t. MSH (15 nmol), CFA injection induced a level of nNOS that was significantly lower than that in the saline group (P < 0.01; Figure 3A). Similarly, i.t. MSH, but not BAM8-18, inhibited the CFA-induced c-Fos protein expression in dorsal horn, compared with saline group (P < 0.01; Figure 3B).
Figure 3.
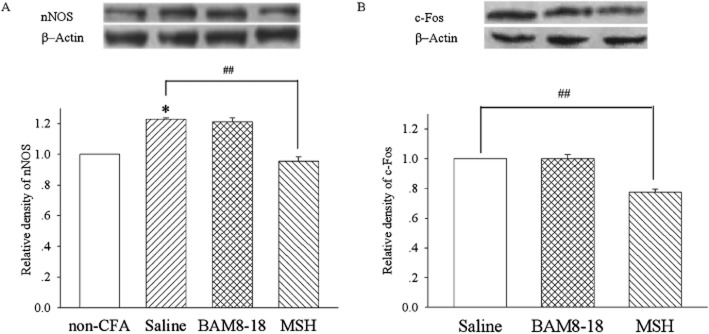
Effect of i.t. administration of MSH on CFA-evoked expression of nNOS and c-Fos proteins in the spinal dorsal horn. CFA was injected i.pl. in the right hindpaw on day 0, and MSH (15 nmol), saline or BAM8-18 (30 nmol) was administered i.t. on days 0 and 1. The lumbar spinal cord was collected on day 2. The expression of nNOS (A) and c-Fos (B) proteins in the spinal dorsal half was assayed by Western blot on the side ipsilateral to CFA injection. *P < 0.05 compared with non-CFA group. ##P < 0.01 compared with CFA/saline group. n = 4 each.
CFA injection up-regulates the expression of MrgC receptor mRNA
We reported earlier that CFA-induced inflammation increased the expression of the endogenous MrgC receptor ligand BAM22 in the spinal dorsal horn and DRG (Cai et al., 2007a). We therefore evaluated the effects of CFA injection on the expression of mRNA for MrgC receptors. As demonstrated in Figure 4, there was a basal level of MrgC mRNA expression in lumbar DRG in animals injected with saline in the hindpaw. Following CFA injection, MrgC receptor mRNA was increased over basal values on days 1 and 2 respectively (P < 0.05).
Figure 4.

Levels of MrgC receptor mRNA in DRG. CFA (150 μL) or saline was injected i.pl. in the right hindpaw on day 0, and L4-6 DRG were collected on days 1 (24 h) and 2 (48 h). The levels are expressed relative to the level of MrgC receptor mRNA in saline group. Data are shown as mean ± SEM. *P < 0.05. n = 5.
Activation of MrgC receptors leads to endogenous activation of MORs
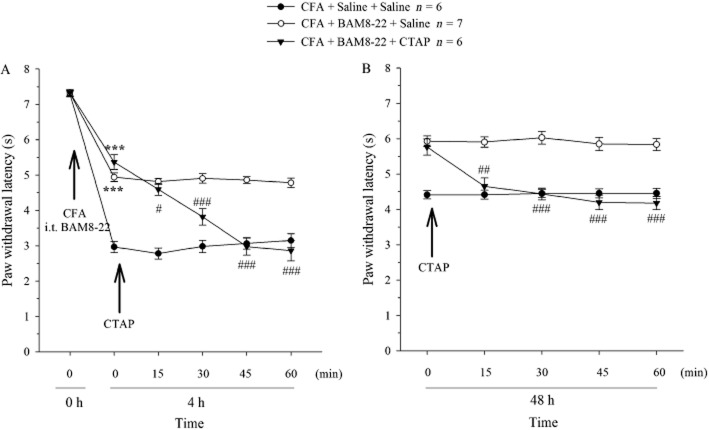
The results showing that the short-acting compounds BAM8-22 and MSH (Grazzini et al., 2004) produced long-lasting inhibition of CFA-induced hyperalgesia suggested a non-MrgC receptor-mediated mechanism. Therefore, the possible involvement of endogenous agonists of MORs was assessed. PWL was decreased at 4 h (on day 0) following CFA plus i.t. saline injections (P < 0.001). After CFA plus i.t. BAM8-22 (30 nmol) administered at 0 h, PWL was less decreased at 4 h (Figure 5A; P < 0.001 vs. CFA/saline group), showing that BAM8-22 attenuated CFA-induced hyperalgesia. In a separate group treated with CFA/BAM8-22, CTAP (10 nmol) given i.t. at 4 h reduced PWL in the next 60 min to a level that was significantly different from pre-CTAP level (P < 0.05–0.001), reversing the anti-nociceptive effects of the BAM8-22 treatment. However, saline administered i.t. at 4 h did not change the CFA/BAM8-22-induced response. Moreover, i.t. CTAP (10 nmol) did not change thermal nociceptive threshold in naïve rats (n = 6, data not shown). Similarly, when CTAP (10 nmol) was given i.t. at 48 h, the BAM8-22-induced increase in PWL was also abolished, compared with the pretreatment level or saline group (P < 0.01–0.001; Figure 5B).
Figure 5.
Effect of CTAP on BAM8-22-induced anti-nociceptive responses. CFA was injected i.pl. on day 0 and i.t. BAM8-22 (30 nmol), or saline was administered i.t. on days 0 and 1. CTAP (10 nmol) or saline was given i.t. at 4 h (A) or 48 h (B). #P < 0.05, ##P < 0.01 and ###P < 0.001 compared with pre-CTAP value. ***P < 0.001 compared with CFA + BAM8-22 + CTAP group.
The involvement of MORs was further evaluated by measuring CGRP mRNA levels. To match the same protocol performed in the behavioural study (see Figure 1), CFA (i.pl.) was given at 0 h and BAM8-22 (60 nmol) was administered i.t. via lumbar puncture at 0 and 24 h. In a separate group, the same protocol was repeated except that CTAP (20 nmol) was injected i.t. at 24 and 48 h. I.pl. injection of saline or i.pl. CFA plus i.t. saline was used as control. The L4-6 DRG were harvested at 48.5 h. As illustrated in Figure 6, treatment with CFA + i.t. saline increased CGRP mRNA levels by twofold compared with i.pl. saline (P < 0.001), and this increase was abolished by i.t. BAM8-22 (P < 0.001 vs. CFA + i.t. saline). However, in the presence of CTAP, the treatment with CFA + BAM8-22 still increased CGRP mRNA levels by twofold (P < 0.001 vs. CFA + i.t. BAM8-22).
Figure 6.
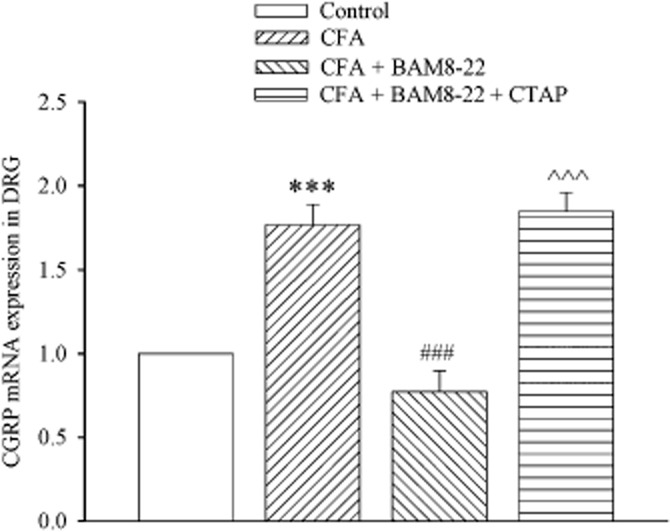
Effect of i.t. administration of BAM8-22 on CFA-evoked CGRP mRNA levels in DRG in the presence or absence of CTAP. CFA was injected i.pl. at 0 h and i.t. BAM8-22 (60 nmol) or saline was administered by lumbar puncture at 0 and 24 h. CTAP (20 nmol) or saline was given by lumbar puncture at 24 and 48 h. Saline was injected i.pl. at 0 h as control. The L4-6 DRG were harvested at 48.5 h. ***P < 0.001 compared with saline group. ###P < 0.001 compared with CFA + i.t. saline group. ∧∧∧P < 0.001 compared with CFA + i.t. BAM8-22 group. n = 4–5.
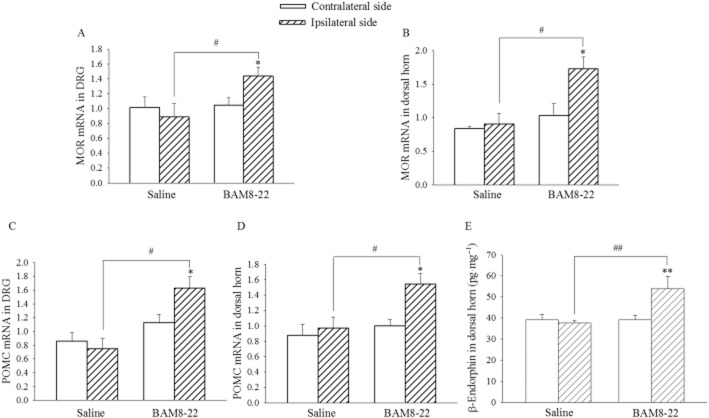
To strengthen the evidence for the activation of MORs initiated by MrgC receptor agonists, mRNA expression of MOR as well as pro-opiomelanocortin (POMC) and β-endorphin content in the lumbar spinal cord and DRG were assayed. CFA was injected i.pl. on day 0, and BAM8-22 (30 nmol) or saline was administered i.t. on days 0 and 1. Tissue samples were harvested on day 2. As illustrated in Figure 7A–D, the expression of MOR mRNA and POMC mRNA in the spinal cord and DRG on the ipsilateral side was not different from the contralateral side following treatment with CFA. However, i.t. BAM8-22 significantly increased the expression of MOR mRNA and POMC mRNA in the spinal cord and DRG on the ipsilateral side (P < 0.05). The β-endorphin content of spinal dorsal horn on the ipsilateral side was similar to that on the contralateral side, in the CFA/saline group. Following treatments with CFA and i.t. BAM8-22, the β-endorphin content on the ipsilateral side was increased significantly above values from the contralateral side or in the CFA/saline group (P < 0.01; Figure 7E).
Figure 7.
Influence of i.t. BAM8-22 on mRNA levels of MOR as well as POMC and β-endorphin content in the spinal dorsal horn and/or DRG. CFA was injected i.pl. in the right hindpaw on day 0, and BAM8-22 (30 nmol) or saline was administered i.t. on days 0 and 1. The lumbar spinal dorsal horn and L4-6 DRG were collected on day 2. mRNA levels of MOR (A and B) and POMC (C and D) and β-endorphin content (E) in the spinal cord and/or DRG were assayed. The data represent normalized averages derived from the threshold cycles in quantitative PCR. *P < 0.05 compared with the contralateral side. #P < 0.05, ##P < 0.0; compared with saline group. n = 5.
MrgC receptors attenuate CFA-induced nNOS expression and co-localize with nNOS in DRG neurons
To explore the mechanisms underlying the activity of MrgC receptors, the effect of i.t. BAM8-22 on CFA-induced nNOS expression in DRG was examined. To match the behavioural changes, the experimental protocol was similar to the behavioural study. CFA (i.pl.) was given at 0 h, and BAM8-22 (30 nmol) was administered i.t. at 0 and 24 h. Saline or CTAP (10 nmol) was injected i.t. at 24 and 48 h. Animals treated with i.pl. saline or i.pl. CFA at 0 h were used as controls. DRG at L4-6 were harvested at 48.5 h. In accordance with previous studies, a number of small-to medium-sized neurons exhibited staining for nNOS in DRG. On the side ipsilateral to i.pl. saline injection, relatively few (15–20%) small or medium neurons were nNOS-IR positive (Figure 8A and E), but i.pl. CFA markedly increased the expression of nNOS-IR in both populations of neurons (P < 0.001 compared with saline group; Figure 8B and E). Following i.t. administration of BAM8-22, the proportion of CFA-induced nNOS-IR neurons in small and medium subpopulations was clearly reduced, and the reductions were significant compared with CFA group (P < 0.01–0.001; Figure 8C and E). However, in the presence of CTAP, this inhibitory effect of BAM8-22 on CFA-induced nNOS expression was completely reversed in both small and medium neurons (P < 0.01–0.001, compared with CFA + BAM8-22 (no CTAP) group; Figure 8D and E).
Figure 8.
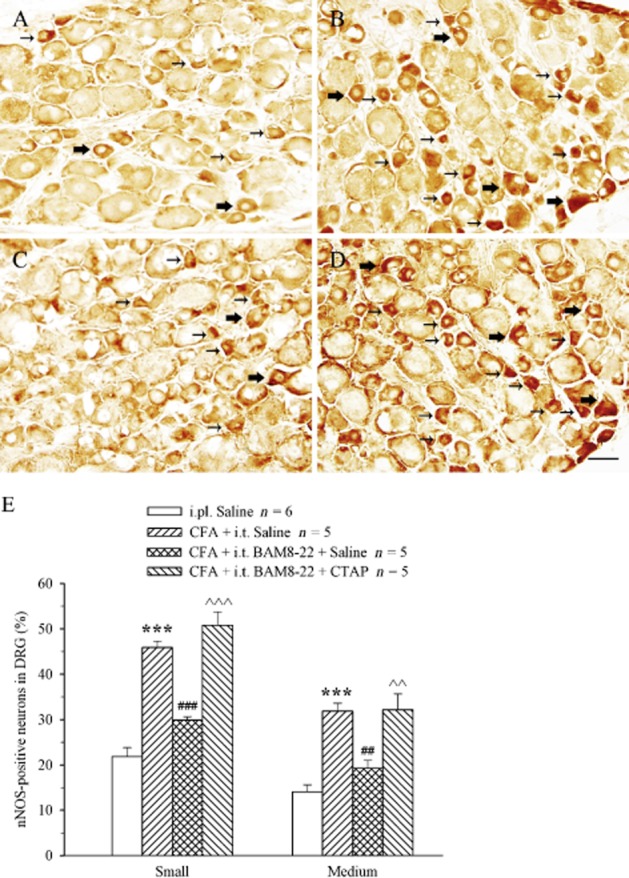
Effect of i.t. administration of BAM8-22 on CFA-evoked expression of nNOS in DRG in the presence or absence of CTAP. Saline (A) or CFA (B, C and D) was i.pl. at 0 h. BAM8-22 (30 nmol; B, C and D) and saline (C) or CTAP (10 nmol; D) were administered i.t. at 0/24 and 24/48 h respectively. The L4-6 DRG were collected at 48.5 h. Photomicrographs of transverse sections of DRG show nNOS-IR expressions in small (small arrows) and medium (large arrows) neurons. Histograms show means ± SEM of nNOS-IR-positive neurons (E). ***P < 0.001 compared with saline group. ###P < 0.001 compared with CFA + i.t. saline group. ∧∧∧P < 0.001 compared with CFA + i.t. BAM8-22 group. Scale bar = 50 μm.
Our findings that i.t. administration of BAM8-22 inhibited CFA-induced up-regulation of nNOS suggested a modulating effect of MrgC receptors on nNOS expression. Accordingly, the possible co-localization of MrgC receptors with nNOS was examined. As illustrated in Figure 9A, nNOS was localized in the soma of small-and medium-sized DRG neurons. Staining for MrgC receptors was diffusely distributed throughout the cytoplasm but was excluded from the nucleus (Figure 9B). Figure 9C shows the co-localization of MrgC receptors with nNOS. The specificity of the MrgC staining was assessed by rabbit normal serum (not shown) and preabsorption controls (Figure 9D); all these conditions showed no MrgC immunoreactivity.
Figure 9.

Confocal images showing the localization of nNOS-IR and MrgC receptor IR in DRG neurons. nNOS-IR neurons are identified by FITC fluorescence (A, arrows, green) whereas MrgC receptor-IR-positive neurons by rhodamine (B, arrows, red). nNOS-IR is extensively co-localized with MrgC receptor-IR in DRG neurons (C, arrows, yellow). Incubation of DRG section with anti-MrgC receptor antiserum preabsorbed with 1 μM MrgC receptor protein resulted in the complete absence of staining (D). Scale bar = 50 μm.
Discussion
The present study demonstrated an increase in the expression of MrgC receptor mRNA in L4-6 DRG following the injection of CFA in a hindpaw. The i.t. administration of two MrgC receptor agonists with different structures produced both immediate and delayed attenuations of CFA-evoked thermal hyperalgesia. These effects were associated with the inhibition of up-regulation of nNOS and CGRP as well as c-Fos expression in the spinal dorsal horn or DRG. Furthermore, the delayed anti-hyperalgesia and inhibition of nNOS and CGRP expressions induced by BAM8-22 were abolished by the MOR antagonist CTAP. Treatment with i.t. BAM8-22 also increased the expression of the mRNAs coding for MOR and POMC as well as β-endorphin content in the spinal cord and/or DRG. In addition, MrgC receptors were co-localized with nNOS in DRG neurons. These results suggest that the activation of MrgC receptors can modulate inflammatory pain by suppressing the up-regulation of pronociceptive mediators and dorsal horn neuronal activation, which was partially attributed to the activation of MORs by endogenous agonists, following MrgC receptor activation.
The intriguing feature of MrgC receptors is their unique distribution in primary nociceptive neurons (Dong et al., 2001; Lembo et al., 2002). The study showing that, of the CNS tissues, the spinal cord is the only one displaying specific binding for MSH (Grazzini et al., 2004) implies the localization of MrgC receptors in the central terminal of primary afferents. These receptors do not play a role in pain processing under normal conditions because neither the deletion of MrgC gene (Ndong et al., 2009; Guan et al., 2010) nor the activation of MrgC receptors by i.t. BAM8-22 (Cai et al., 2007b; Guan et al., 2010) or MSH (present study) altered basal nociceptive thresholds. However, MrgC receptors do reduce pain intensity under inflammatory conditions (Liu et al., 2009; Guan et al., 2010). In accordance with previous reports (Galeazza et al., 1995; Safieh-Garabedian et al., 1995), the present study showed that thermal hyperalgesia developed at 4 h and was maintained throughout the experiment following CFA injection. The activation of MrgC receptors by i.t. administration of the highly specific MrgC receptor agonists BAM8-22 (Lembo et al., 2002; Guan et al., 2010) and MSH (Han et al., 2002; Lembo et al., 2002) both produced a rapid (20-40 min) inhibition of CFA-evoked hyperalgesia. These results are in contrast to those from a recent study reporting that MrgC receptor knock-down using siRNA, reduced CFA-induced heat hyperalgesia (Ndong et al., 2009). However, our results are consistent with other earlier studies showing that i.t. administration of BAM8-22 or MSH inhibits the wind-up response (Guan et al., 2010), formalin-induced pain and dorsal horn neuronal activation (Chen et al., 2006; Guan et al., 2010), and NMDA-(Chen et al., 2008) as well as CFA-evoked hypersensitivity (Guan et al., 2010). Moreover, our behavioural observations were supported by the biochemical results (see below). However, the present study showed that MrgC receptor activation did not alter CFA-induced mechanical allodynia. This result may indicate that the interactions of MrgC receptors with inflammation-associated hypersensitivity to mechanical stimuli differs from their interactions with thermal stimuli. Such differences between the inhibition of thermal and mechanical hypersensitivity have been reported earlier (Boettger et al., 2007; Schepers et al., 2008).
The inhibitory effect of MrgC receptors is not seen under normal conditions. Our study showed that peripheral inflammation up-regulated the expression of mRNA coding for MrgC receptors in DRG. This finding is in accordance with the enhanced bioactivity of MrgC receptors in inflammation, as shown by the stronger wind-up or enhanced pain responses after i.pl. injection of formalin (Guan et al., 2010), CFA or carrageenan (Liu et al., 2009) in MrgC cluster knock-out mice. The increase in mRNA for MrgC receptors following the induction of inflammation may explain the observations that i.t. injection of BAM8-22 or MSH inhibits inflammatory pain (Chen et al., 2006; Guan et al., 2010), but not physiological pain (Cai et al., 2007b; Guan et al., 2010). The up-regulation of MrgC receptor mRNA may parallel the enhanced expression of BAM22, a putative endogenous ligand of MrgC receptors (Lembo et al., 2002), in the spinal cord and DRG in CFA-induced inflammation (Cai et al., 2007a). However, plastic changes that develop in pathological pain involve not only pro-nociceptive mediators but also anti-nociceptive molecules. For example, inflammation has been shown to enhance the expression of opioid receptors in peripheral nerve terminals (Stein and Zollner, 2009) and cannabinoid receptors in DRG and in hindpaws (Hsieh et al., 2011). Apparently, the significance of enhanced expression of MrgC receptors is an attempt to reduce pain severity (Liu et al., 2009; Guan et al., 2010), similar to other anti-nociceptive molecules (Rittner et al., 2008). Up-regulation of MrgC receptors may allow MrgC receptor agonists exert greater biological activity. Therefore, in our experiments, the inhibition of inflammatory pain by MrgC receptor agonists could be partially attributed to the up-regulation of MrgC receptors, following CFA injection.
In agreement with behavioural responses, i.t. BAM8-22 or MSH inhibited CFA-induced increase in the pro-nociceptive mediators, CGRP and nNOS, in DRG and/or the spinal cord. The induction of inflammatory pain has been attributed to the up-regulation of CGRP (Miletic and Tan, 1988; Ryu et al., 1988) and nNOS (Meller et al., 1994; Boettger et al., 2007). CGRP is synthesized in DRG (Nahin and Byers, 1994; Galeazza et al., 1995) and released from primary sensory nerve terminals (Kuraishi et al., 1988) during CFA-induced inflammation, leading to the sensitization of nociceptive neurons in the spinal cord (Miletic and Tan, 1988; Ryu et al., 1988) and DRG (Ryu et al., 1988). Accordingly, blockade of CGRP receptors by antagonists (Kawamura et al., 1989; Neugebauer et al., 1996; Sun et al., 2003) relieves inflammatory pain.
The production of NO by nNOS in neurons (Snyder, 1992) also plays a pivotal role in the induction of inflammatory hyperalgesia. Expression of nNOS is increased in the spinal cord of the CFA model of inflammatory hyperalgesia (Chu et al., 2005; Boettger et al., 2007), whereas administration of the nNOS inhibitor (Pozza et al., 1998) or the targeted disruption of the nNOS gene (Chu et al., 2005) markedly attenuates CFA-induced hyperalgesia. Moreover, nNOS is linked to the up-regulation and release of CGRP to induce inflammatory hyperalgesia because the nNOS inhibitors, L-NAME and 7-nitroindazole, reduced inflammation-associated CGRP release from spinal dorsal horn (Garry et al., 2000), and i.pl. injection of CFA failed to increase CGRP expression in DRG neurons in nNOS knock-out mice (Boettger et al., 2007). As reported before (Pozza et al., 1998; Chu et al., 2005; Hong et al., 2009), the present study showed that CFA injection increased the expression of nNOS and CGRP in the spinal dorsal horn or DRG. Importantly, these biochemical alterations were abolished following treatment with BAM8-22 or MSH. The present study demonstrated that nNOS was contained in MrgC receptor-expressing neurons in DRG. This might be the histological substrate for the modulation of CFA-induced nNOS expression by MrgC receptors. Based on the data discussed above, the suppression of nNOS by MrgC receptor activation is likely to result in inhibition of CGRP up-regulation. Consistent with the inhibition of nNOS and CGRP expression in DRG, c-Fos expression in the spinal cord was also abolished, reflecting the overall inhibition of spinal neuronal activation (Harris, 1998). Taken together, these results suggest that the mechanism underlying the effect of BAM8-22 or MSH on hypersensitivity is linked to the inhibition of CFA-induced up-regulation of nNOS and CGRP and spinal neuronal activation.
As demonstrated before (Chen et al., 2006; 2008; Guan et al., 2010), the anti-nociceptive effect of BAM8-22 and MSH was exerted immediately (within 20min) after their administration. Interestingly, attenuation of hyperalgesia was also observed at 4, 24 and 48 h following the administration of BAM8-22 or MSH at 0 and 24 h. This delayed long-lasting effect is more likely to be due to downstream mechanisms activated by MrgC receptors because BAM8-22 and MSH are degraded within 10–30 min in the spinal cord (Grazzini et al., 2004). This possibility was supported by the results showing that the selective MOR antagonist CTAP (Pelton et al., 1985), administered at 4 and 48 h, abolished the anti-nociceptive effects produced by i.t. administration of BAM8-22 at 0 and 24 h, respectively, suggesting that MOR-mediated mechanism(s) was involved in the delayed anti-hyperalgesia induced by BAM8-22. Consistent with these results, treatment with CTAP also abolished BAM8-22-induced inhibition of nNOS and CGRP (mRNA) expressions in DRG. As both BAM8-22 and MSH do not demonstrate any affinity with μ-, δ-or κ-opioid receptors (Lembo et al., 2002), we explored next the potential production of endogenous opioid peptides. In accordance with previous studies (Puehler et al., 2004; Shaqura et al., 2004; Obara et al., 2009), the present study showed no changes in the expression of MORs in both the spinal cord and DRG following CFA injection. Importantly, treatment with i.t. BAM8-22 increased MOR mRNA levels. Similarly, the CFA injection did not alter POMC mRNA levels in the spinal dorsal horn and DRG (Obara et al., 2009). However, POMC mRNA level and β-endorphin content in the spinal dorsal horn and/or DRG were increased following the i.t. administration of BAM8-22. Many non-opioid receptors produce anti-nociceptive effects partly via interactions with the endogenous opioid system. Responses to activation of oxytocin (Russo et al., 2012), sphingosine-1-phosphate (Welch et al., 2012) and CB2 (Negrete et al., 2011) receptors can be abolished by MOR antagonists. Activation of CB2 receptors also increased the synthesis of POMC as well as β-endorphin (Su et al., 2011) and stimulated the release of β-endorphin (Katsuyama et al., 2013). Particularly relevant in this context are the reciprocal interactions involving the release of opioid peptides by cannabinoids or of endocannabinoids by opioids (Parolaro et al., 2010). The data in the present study suggest that the activation of MrgC receptors in CFA-induced inflammation can up-regulate the expression of both MOR and POMC mRNAs and β-endorphin content in the spinal cord and/or DRG, resulting in delayed but long-lasting anti-nociception. Further experimentation is, however, needed to elucidate the mechanisms underlying this recruitment by MrgC receptor agonists of endogenous activation of MORs.
The treatment of various types of chronic pain still constitutes a real challenge as most analgesics display serious side effects that are often associated with unwanted actions in the CNS (Benyamin et al., 2008). One of the main reasons is that receptors targeted by these analgesics are widely distributed in the CNS. Besides anti-nociception, the inhibition or activation of these receptors also alters other brain functions. Such unwanted CNS effects should be minimized following activation of MrgC receptors because of their highly restricted distribution in DRG. Endogenous μ-opioid agonists at the spinal level recruited by MrgC receptor activation should only exhibit analgesic activity and not the adverse effects of exogenously applied opiates (Viet and Schmidt, 2012). Therefore, the present study suggests that targeting MrgC receptors should be considered as a novel approach for the treatment of inflammatory pain.
Acknowledgments
This study was supported by the grants from the Natural Science Foundation (30970985, 31171072), Education Ministry of China (20113503110001) and the Canadian Institutes of Health Research. The authors wish to thank Mira Thakur for proof-reading the manuscript.
Glossary
- BAM8-22
bovine adrenal medulla 8-22
- CFA
complete Freund's adjuvant
- DRG
dorsal root ganglia
- i.pl
intraplantar
- i.t
intrathecal
- MOR
μ-opioid receptor
- Mrg
Mas oncogene-related gene
- MSH
melanocyte-stimulating hormone;
- nNOS
neuronal NOS
- POMC
pro-opiomelanocortin
- SNSR
sensory neuron-specific receptors
Conflict of interest
The authors have no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site: http://dx.doi.org/10.1111/bph.12326
Methods for percutaneous lumbar puncture, quantification of IR for CGRP and nNOS in DRG, Western blots for nNOS and c-Fos in spinal dorsal horn, RT-PCR for mRNAs of CGRP, MrgC receptors, MOR and POMC in DRG and/or spinal dorsal horn and ELISA for β-endorphin in spinal dorsal horn.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- Boettger MK, Uceyler N, Zelenka M, Schmitt A, Reif A, Chen Y, et al. Differences in inflammatory pain in nNOS-, iNOS-and eNOS-deficient mice. Eur J Pain. 2007;11:810–818. doi: 10.1016/j.ejpain.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Burstein ES, Ott TR, Feddock M, Ma JN, Fuhs S, Wong S, et al. Characterization of the Mas-related gene family: structural and functional conservation of human and rhesus MrgX receptors. Br J Pharmacol. 2006;147:73–82. doi: 10.1038/sj.bjp.0706448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot PJ, Carter L, Schafer M, Stein C. Methionine-enkephalin-and Dynorphin A-release from immune cells and control of inflammatory pain. Pain. 2001;93:207–212. doi: 10.1016/S0304-3959(01)00322-0. [DOI] [PubMed] [Google Scholar]
- Cai M, Chen T, Quirion R, Hong Y. The involvement of spinal bovine adrenal medulla 22-like peptide, the proenkephalin derivative, in modulation of nociceptive processing. Eur J Neurosci. 2007a;26:1128–1138. doi: 10.1111/j.1460-9568.2007.05755.x. [DOI] [PubMed] [Google Scholar]
- Cai Q, Jiang J, Chen T, Hong Y. Sensory neuron-specific receptor agonist BAM8-22 inhibits the development and expression of tolerance to morphine in rats. Behav Brain Res. 2007b;178:154–159. doi: 10.1016/j.bbr.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Chen T, Cai Q, Hong Y. Intrathecal sensory neuron-specific receptor agonists bovine adrenal medulla 8-22 and (tyr(6))-gamma2-msh-6-12 inhibit formalin-evoked nociception and neuronal fos-like immunoreactivity in the spinal cord of the rat. Neuroscience. 2006;141:965–975. doi: 10.1016/j.neuroscience.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Chen T, Hu Z, Quirion R, Hong Y. Modulation of NMDA receptors by intrathecal administration of the sensory neuron-specific receptor agonist BAM8-22. Neuropharmacology. 2008;54:796–803. doi: 10.1016/j.neuropharm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Choi SS, Lahn BT. Adaptive evolution of MRG, a neuron-specific gene family implicated in nociception. Genome Res. 2003;13:2252–2259. doi: 10.1101/gr.1431603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu YC, Guan Y, Skinner J, Raja SN, Johns RA, Tao YX. Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund's adjuvant-induced persistent pain. Pain. 2005;119:113–123. doi: 10.1016/j.pain.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–141. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Galeazza MT, Garry MG, Yost HJ, Strait KA, Hargreaves KM, Seybold VS. Plasticity in the synthesis and storage of substance P and calcitonin gene-related peptide in primary afferent neurons during peripheral inflammation. Neuroscience. 1995;66:443–458. doi: 10.1016/0306-4522(94)00545-g. [DOI] [PubMed] [Google Scholar]
- Garry MG, Walton LP, Davis MA. Capsaicin-evoked release of immunoreactive calcitonin gene-related peptide from the spinal cord is mediated by nitric oxide but not by cyclic GMP. Brain Res. 2000;861:208–219. doi: 10.1016/s0006-8993(99)02448-8. [DOI] [PubMed] [Google Scholar]
- Grazzini E, Puma C, Roy MO, Yu XH, O'Donnell D, Schmidt R, et al. Sensory neuron-specific receptor activation elicits central and peripheral nociceptive effects in rats. Proc Natl Acad Sci U S A. 2004;101:7175–7180. doi: 10.1073/pnas.0307185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Liu Q, Tang Z, Raja SN, Anderson DJ, Dong X. Mas-related G-protein-coupled receptors inhibit pathological pain in mice. Proc Natl Acad Sci U S A. 2010;107:15933–15938. doi: 10.1073/pnas.1011221107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Dong X, Hwang JI, Zylka MJ, Anderson DJ, Simon MI. Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci U S A. 2002;99:14740–14745. doi: 10.1073/pnas.192565799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Hong Y, Liu Y, Chabot JG, Fournier A, Quirion R. Upregulation of adrenomedullin in the spinal cord and dorsal root ganglia in the early phase of CFA-induced inflammation in rats. Pain. 2009;146:105–113. doi: 10.1016/j.pain.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Hong Y, Jiang J, Chen T, Wang D, Huo Y, Zhang W, et al. 2012. Intrathecal administration of the MrgC receptor agonist BAM8-22 or MSH inhibits CFA-induced hyperalgesia via suppression of nNOS and CGRP as well as recruiting endogenous μ-opioid action in rats. The 14th World Congress of Pain Stud, 12516.
- Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, et al. Central and peripheral sites of action for CB receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol. 2011;162:428–440. doi: 10.1111/j.1476-5381.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuyama S, Mizoguchi H, Kuwahata H, Komatsu T, Nagaoka K, Nakamura H, et al. Involvement of peripheral cannabinoid and opioid receptors in beta-caryophyllene-induced antinociception. Eur J Pain. 2013;17:664–675. doi: 10.1002/j.1532-2149.2012.00242.x. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Kuraishi Y, Minami M, Satoh M. Antinociceptive effect of intrathecally administered antiserum against calcitonin gene-related peptide on thermal and mechanical noxious stimuli in experimental hyperalgesic rats. Brain Res. 1989;497:199–203. doi: 10.1016/0006-8993(89)90990-6. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Nanayama T, Ohno H, Minami M, Satoh M. Antinociception induced in rats by intrathecal administration of antiserum against calcitonin gene-related peptide. Neurosci Lett. 1988;92:325–329. doi: 10.1016/0304-3940(88)90611-8. [DOI] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Groblewski T, O'Donnell D, Roy MO, Zhang J, et al. Proenkephalin A gene products activate a new family of sensory neuron – specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Pate KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcangio M, Bowery NG. Spinal cord SP release and hyperalgesia in monoarthritic rats: involvement of the GABAB receptor system. Br J Pharmacol. 1994;113:1561–1566. doi: 10.1111/j.1476-5381.1994.tb17174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller ST, Cummings CP, Traub RJ, Gebhart GF. The role of nitric oxide in the development and maintenance of the hyperalgesia produced by intraplantar injection of carrageenan in the rat. Neuroscience. 1994;60:367–374. doi: 10.1016/0306-4522(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Miletic V, Tan H. Iontophoretic application of calcitonin gene-related peptide produces a slow and prolonged excitation of neurons in the cat lumbar dorsal horn. Brain Res. 1988;446:169–172. doi: 10.1016/0006-8993(88)91310-8. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Byers MR. Adjuvant-induced inflammation of rat paw is associated with altered calcitonin gene-related peptide immunoreactivity within cell bodies and peripheral endings of primary afferent neurons. J Comp Neurol. 1994;349:475–485. doi: 10.1002/cne.903490311. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Hylden JL, Iadarola MJ, Dubner R. Peripheral inflammation is associated with increased dynorphin immunoreactivity in both projection and local circuit neurons in the superficial dorsal horn of the rat lumbar spinal cord. Neurosci Lett. 1989;96:247–252. doi: 10.1016/0304-3940(89)90386-8. [DOI] [PubMed] [Google Scholar]
- Ndong C, Pradhan A, Puma C, Morello JP, Hoffert C, Groblewski T, et al. Role of rat sensory neuron-specific receptor (rSNSR1) in inflammatory pain: contribution of TRPV1 to SNSR signaling in the pain pathway. Pain. 2009;143:130–137. doi: 10.1016/j.pain.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Negrete R, Hervera A, Leanez S, Martin-Campos JM, Pol O. The antinociceptive effects of JWH-015 in chronic inflammatory pain are produced by nitric oxide-cGMP-PKG-KATP pathway activation mediated by opioids. PLoS One. 2011;6:e26688. doi: 10.1371/journal.pone.0026688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Rumenapp P, Schaible HG. Calcitonin gene-related peptide is involved in the spinal processing of mechanosensory input from the rat's knee joint and in the generation and maintenance of hyperexcitability of dorsal horn-neurons during development of acute inflammation. Neuroscience. 1996;71:1095–1109. doi: 10.1016/0306-4522(95)00473-4. [DOI] [PubMed] [Google Scholar]
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, et al. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain. 2009;141:283–291. doi: 10.1016/j.pain.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Parolaro D, Rubino T, Vigano D, Massi P, Guidali C, Realini N. Cellular mechanisms underlying the interaction between cannabinoid and opioid system. Curr Drug Targets. 2010;11:393–405. doi: 10.2174/138945010790980367. [DOI] [PubMed] [Google Scholar]
- Pelton JT, Gulya K, Hruby VJ, Duckles SP, Yamamura HI. Conformationally restricted analogs of somatostatin with high mu-opiate receptor specificity. Proc Natl Acad Sci U S A. 1985;82:236–239. doi: 10.1073/pnas.82.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogatzki EM, Zahn PK, Brennan TJ. Lumbar catheterization of the subarachnoid space with a 32-gauge polyurethane catheter in the rat. Eur J Pain. 2000;4:111–113. doi: 10.1053/eujp.1999.0157. [DOI] [PubMed] [Google Scholar]
- Pozza M, Bettelli C, Magnani F, Mascia MT, Manzini E, Calza L. Is neuronal nitric oxide involved in adjuvant-induced joint inflammation? Eur J Pharmacol. 1998;359:87–93. doi: 10.1016/s0014-2999(98)00618-9. [DOI] [PubMed] [Google Scholar]
- Puehler W, Zollner C, Brack A, Shaqura MA, Krause H, Schafer M, et al. Rapid upregulation of mu opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience. 2004;129:473–479. doi: 10.1016/j.neuroscience.2004.06.086. [DOI] [PubMed] [Google Scholar]
- Rittner HL, Brack A, Stein C. Pain and the immune system. Br J Anaesth. 2008;101:40–44. doi: 10.1093/bja/aen078. [DOI] [PubMed] [Google Scholar]
- Russo R, D'Agostino G, Mattace RG, Avagliano C, Cristiano C, Meli R, et al. Central administration of oxytocin reduces hyperalgesia in mice: implication for cannabinoid and opioid systems. Peptides. 2012;38:81–88. doi: 10.1016/j.peptides.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Ryu PD, Gerber G, Murase K, Randic M. Calcitonin gene-related peptide enhances calcium current of rat dorsal root ganglion neurons and spinal excitatory synaptic transmission. Neurosci Lett. 1988;89:305–312. doi: 10.1016/0304-3940(88)90544-7. [DOI] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Gehrke BJ, Shippenberg TS. Endogenous kappa-opioid receptor systems inhibit hyperalgesia associated with localized peripheral inflammation. Pain. 2008;138:423–439. doi: 10.1016/j.pain.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold VS, Galeazza MT, Garry MG, Hargreaves KM. Plasticity of calcitonin gene related peptide neurotransmission in the spinal cord during peripheral inflammation. Can J Physiol Pharmacol. 1995;73:1007–1014. doi: 10.1139/y95-141. [DOI] [PubMed] [Google Scholar]
- Shaqura MA, Zollner C, Mousa SA, Stein C, Schafer M. Characterization of mu opioid receptor binding and G protein coupling in rat hypothalamus, spinal cord, and primary afferent neurons during inflammatory pain. J Pharmacol Exp Ther. 2004;308:712–718. doi: 10.1124/jpet.103.057257. [DOI] [PubMed] [Google Scholar]
- Simonin F, Kieffer BL. Two faces for an opioid peptide – and more receptors for pain research. Nat Neurosci. 2002;5:185–186. doi: 10.1038/nn0302-185. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Nitric oxide: first in a new class of neurotransmitters. Science. 1992;257:494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Stein C, Zollner C. Opioids and sensory nerves. Handb Exp Pharmacol. 2009;194:495–518. doi: 10.1007/978-3-540-79090-7_14. [DOI] [PubMed] [Google Scholar]
- Su TF, Zhang LH, Peng M, Wu CH, Pan W, Tian B, et al. Cannabinoid CB2 receptors contribute to upregulation of beta-endorphin in inflamed skin tissues by electroacupuncture. Mol Pain. 2011;7:98. doi: 10.1186/1744-8069-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. doi: 10.1016/s0304-3959(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Viet CT, Schmidt BL. Biologic mechanisms of oral cancer pain and implications for clinical therapy. J Dent Res. 2012;91:447–453. doi: 10.1177/0022034511424156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch SP, Sim-Selley LJ, Selley DE. Sphingosine-1-phosphate receptors as emerging targets for treatment of pain. Biochem Pharmacol. 2012;84:1551–1562. doi: 10.1016/j.bcp.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Zeng J, Thomson LM, Aicher SA, Terman GW. Primary afferent NMDA receptors increase dorsal horn excitation and mediate opiate tolerance in neonatal rats. J Neurosci. 2006;26:12033–12042. doi: 10.1523/JNEUROSCI.2530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Taylor N, Xie Y, Ford R, Johnson J, Paulsen JE, et al. Cloning and expression of MRG receptors in macaque, mouse, and human. Brain Res Mol Brain Res. 2005;133:187–197. doi: 10.1016/j.molbrainres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A. 2003;100:10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods for percutaneous lumbar puncture, quantification of IR for CGRP and nNOS in DRG, Western blots for nNOS and c-Fos in spinal dorsal horn, RT-PCR for mRNAs of CGRP, MrgC receptors, MOR and POMC in DRG and/or spinal dorsal horn and ELISA for β-endorphin in spinal dorsal horn.