Abstract
Background and Purpose
To understand how anandamide transport inhibition impacts the regulation of nausea and vomiting and the receptor level mechanism of action involved. In light of recent characterization of an anandamide transporter, fatty acid amide hydrolase-1-like anandamide transporter, to provide behavioural support for anandamide cellular reuptake as a facilitated transport process.
Experimental Approach
The systemic administration of the anandamide transport inhibitor ARN272 ([(4-(5-(4-hydroxy-phenyl)-3,4-diaza-bicyclo[4.4.0]deca-1(6),2,4,7,9-pentaen-2-ylamino)-phenyl)-phenylamino-methanone]) was used to evaluate the prevention of LiCl-induced nausea-induced behaviour (conditioned gaping) in rats, and LiCl-induced emesis in shrews (Suncus murinus). The mechanism of how prolonging anandamide availability acts to regulate nausea in rats was explored by the antagonism of cannabinoid 1 (CB1) receptors with the systemic co-administration of SR141716.
Key Results
The systemic administration of ARN272 produced a dose-dependent suppression of nausea-induced conditioned gaping in rats, and produced a dose-dependent reduction of vomiting in shrews. The systemic co-administration of SR141716 with ARN272 (at 3.0 mg·kg−1) in rats produced a complete reversal of ARN272-suppressed gaping at 1.0 mg·kg−1. SR141716 alone did not differ from the vehicle solution.
Conclusions and Implications
These results suggest that anandamide transport inhibition by the compound ARN272 tonically activates CB1 receptors and as such produces a type of indirect agonism to regulate toxin-induced nausea and vomiting. The results also provide behavioural evidence in support of a facilitated transport mechanism used in the cellular reuptake of anandamide.
Keywords: endocannabinoid, anandamide, nausea, gaping, vomiting, FAAH-1-like anandamide transporter, FLAT, CB1, ARN272, taste reactivity
Introduction
The Cannabis sativa plant has been known for centuries to exert therapeutic effects in the treatment of nausea and vomiting. More recently, cannabinoid agonists such as Δ9-tetrahydrocannabinol have been found to be as effective as anti-emetic dopamine antagonists in human clinical trials (Carey et al., 1983; Tramer et al., 2001). The anti-emetic properties of cannabinoid agonists have been found to extend beyond humans to other emetic species, attenuating vomiting in ferrets (Simoneau et al., 2001; Van Sickle et al., 2001), cats (McCarthy and Borison, 1981) and the house musk shrew Suncus murinus (Kwiatkowska et al., 2004; Parker et al., 2004). Despite this long history, knowledge of how the endogenous cannabinoid system mediates nausea and vomiting is still incomplete.
Comparable to the effects of plant-derived cannabinoids, there is a body of evidence among animal models implicating the endocannabinoid anandamide, as important in the regulation of nausea and vomiting. The administration of exogenous anandamide has been found to have anti-emetic properties in the least shrew (Darmani, 2002) and in ferrets (Van Sickle et al., 2005). Deactivation of anandamide occurs through intracellular hydrolysis and is known to be mediated by the enzyme fatty acid amide hydrolase, FAAH (Desarnaud et al., 1995; Cravatt et al., 1996; McKinney and Cravatt, 2005). As such, prolonging the activity of endogenous anandamide through the inhibition of its degradation has also been found to reduce vomiting, specifically in the house musk shrew (Parker et al., 2009) and in the ferret (Sharkey et al., 2007). FAAH inhibition has also been shown to attenuate nausea-induced responding, interfering with conditioned gaping reactions in rats (Cross-Mellor et al., 2007). These findings suggest that anandamide acts within the endocannabinoid system to regulate both nausea and vomiting.
Anandamide is known to be an endogenous agonist at cannabinoid 1 (CB1) receptors (Devane et al., 1992). The mechanism by which anandamide exerts its anti-emetic and anti-nausea effects is thought to be CB1 receptor mediated. The anti-emetic effects of exogenous anandamide administration have been found to be reversed by the CB1 antagonist, AM251 (Van Sickle et al., 2005), and the suppression of nausea by the FAAH inhibitor URB597 was reversed by the CB1 antagonists AM251 and SR141716 in rat (Cross-Mellor et al., 2007) and the house musk shrew respectively (Parker et al., 2009).
The specific mechanisms behind how anandamide signalling is terminated are still unfolding. How anandamide re-enters the post-synaptic cell appears to conform to a twofold process, cellular reuptake and subsequently cellular degradation. Previously, cellular reuptake has been hypothesized as occurring through either passive membrane diffusion driven by FAAH metabolism (Glaser et al., 2003), or by some previously unknown selective carrier system (Ligresti et al., 2004; Hillard et al., 2007). In support of the latter, Fu et al. (2012) recently reported the molecular identity of a facilitated anandamide transport mechanism, FAAH-1-like anandamide transporter (FLAT). As an isoform of the FAAH molecule, FLAT was found to bind anandamide selectively, but not other structurally similar molecules such as 2-AG, and without enacting any catalytic activity (Fu et al., 2012). Concurrently, Fu et al. (2012) identified an antagonist of anandamide transport, ARN272 ([(4-(5-(4-hydroxy-phenyl)-3,4-diaza-bicyclo[4.4.0]deca-1(6),2,4,7,9-pentaen-2-ylamino)-phenyl)-phenylamino-methanone]), which produced analgesic effects in rodent models of nociceptive and inflammatory pain in a CB1 dependent manner. The present study evaluated the potential of ARN272 to also reduce nausea and vomiting in animal models.
While emetic species are used to explore the regulation of vomiting, the subjective experience of nausea requires more consideration in animal models. Conditioned taste avoidance (CTA) is a measure that has often been used to evaluate the nauseating potential of drugs in rats (the extent to which a taste previously paired with an emetic agent is avoided through the amount a rat drinks in a consumption test). However, problematic evidence for CTA as a model of nausea in rats has been found. Anti-emetic drugs do not generally interfere with the establishment of CTA, (for review, see Parker et al., 2008). Also, rats not only avoid tastes paired with nauseating drugs, but they also avoid tastes paired with drugs they choose to self-administer (Berger, 1972; Reicher and Holman, 1977). Therefore, the CTA test cannot be considered a selective measure of nausea in rat. Physiological regulation of nausea can be studied, however, in the rat using their distinctive pattern of disgust reactions, most prominently conditioned gaping reactions (Parker et al., 2011). Despite the rat's inability to vomit, the detection mechanism of nausea is still present, with similar orofacial musculature being activated by the gaping reaction as the orofacial vomiting reaction in emetic species (Travers and Norgren, 1986). Conditioned gaping reactions occur both when intra-orally infused with a bitter-tasting solution of quinine, as well as when exposed to cues (taste or context) previously paired with a drug, which produces vomiting in emetic species (Parker et al., 2008). Moreover, and unlike CTA, only drugs with emetic properties produce conditioned gaping reactions when paired with a flavour or contextual stimulus, and anti-emetic drugs consistently prevent the establishment of nausea-induced conditioned gaping in rats (Limebeer and Parker, 2000;2003). Therefore, conditioned gaping can be used as a selective measure of nausea in rat.
The experiments reported here investigate the impact of anandamide transport inhibition on the endocannabinoid systems regulation of nausea and vomiting. Experiment 1 evaluated the potential of systemic administration of ARN272 to attenuate LiCl-induced conditioned gaping in rats and the potential of the CB1 receptor antagonist/inverse agonist SR141617 to reverse the ARN272-suppressed conditioned gaping response. The extent to which rats avoided a taste paired with the nausea inducing agent LiCl was also assessed using a CTA measure, to re-assert the selectivity of conditioned gaping in the measurement of nausea. Experiment 2 evaluated the potential of systemic administration of ARN272 to regulate LiCl-induced vomiting in the house musk shrew (S. murinus). Here, we provide behavioural support for anandamide reuptake occurring through a facilitated transport mechanism from the ARN272-suppressed gaping in rats and attenuated vomiting in shrews. As such, it is reasoned that prolonging the synaptic availability of endogenous anandamide augmented its action to tonically activate CB1 receptors and extend the anti-emetic and nausea attenuating properties of anandamide.
Methods
Animals
All animal care and experimental procedures complied with the recommendations of the Canadian Council on Animal Care and were approved by the Animal Care Committee of the University of Guelph. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 58 naïve male Sprague-Dawley rats (Charles River Lab, St. Constrant, QC, Canada) were used for assessment of anti-nausea-induced behaviour. Rats were single-housed in 48 × 26 × 20 cm shoebox cages in a colony room at an ambient temperature of 21°C. All animals were maintained on a reverse light/dark cycle (0700 h lights off; 1900 h lights on) with free access to food (Iams rodent chow, 18% protein) (Harlan 2014 Teklad Global Rodent Diet; Harlan, Indianapolis, ID, USA) and tap water, except during testing, which occurred during the dark cycle. All animals were provided with environmental enrichment from two clean paper towels (replenished weekly during cage changes) and a soft plastic container 14 cm long and 12 cm in diameter.
A total of 21 S. murinus, house musk shrews, were bred and raised in a colony at the University of Guelph. Shrews were single-housed in cages at an ambient temperature of 21°C on a 14/10 light dark schedule (lights off at 2100 h). Shrews were tested during their light cycle, between 0900 and 1400 h. Both males (42.9–53.0 g) and females (26.1–32.9 g) were used and equally distributed among the groups, with subjects ranging from 98 to 814 days of age. The sexes did not significantly differ in vomiting frequency in any analysis; therefore, males and females were pooled in all reported analyses. The shrews had previous emetic experience with the limitation of a minimum of 3 weeks recovery between treatments.
Drugs
The anandamide transport inhibitor, ARN272 (Danieli Piomelli, University of California Irvine/Istituto Italiano di Tecnologia), was prepared in a vehicle solution (VEH) of 1:1:8 PEG400, Tween 80 and physiological saline, respectively, for all experiments. All systemic injections were administered i.p. In experiment 1, ARN272 was delivered to rats at concentrations of 0.1 mg·mL−1 (0.1 mg·kg−1 dose), 1 mg·mL−1 (1 mg·kg−1 dose) and 3 mg·mL−1 (3 mg·kg−1 dose), and at a volume of 1 mL·kg−1, chosen on the basis of previous experiments performed by Fu et al. (2012) where 1 mg·kg−1 increased plasma anandamide levels 2 h post-administration. In experiment 2, ARN272 was delivered to shrews at concentrations of 3.0 mg·mL−1 (9.0 mg·kg−1 dose) at a volume of 3 and 3.0 mg·mL−1 (18 mg·kg−1 dose) at a volume of 6 mL·kg−1, chosen on the basis of previous experiments where S. murinus required a dose increase by at least a factor of three times an effective dose in rats (Kwaitkowska et al., 2004; Parker et al., 2004).
The concentration of SR141716 (SR; Sequoia Research Products Ltd., Pangbourne, UK) in experiment 1 was delivered at 1.0 mg·mL−1 (1.0 mg·kg−1 dose) at a volume of 1 mL·kg−1. The dose of 1.0 mg·kg−1 SR141716 was chosen based on prior effectiveness in reversing the breakpoint and reinstatement of nicotine self-administration (Forget et al., 2009), while also being found to not to potentiate the effects of emetic agents unlike doses of SR141716 at 2.5 mg·kg−1 or higher (Parker et al., 2003).
The LiCl drug treatment 0.15 M (Sigma-Aldrich, St. Louis, MO, USA) used in all experiments was prepared in sterile water and administered at volumes of 20 mL·kg−1 (127 mg·kg−1) in rats in experiment 1 (Limebeer and Parker, 2000), and 60 mL kg−1 (390 mg·kg−1) in shrews in experiment 2 (Rock et al., 2011).
Apparatus
The taste reactivity (TR) chamber consisted of a clear Plexiglas box (29 × 29 × 10 cm) resting on a glass surface. Two 60 W lights suspended from the apparatus illuminated the chamber. A mirror mounted at a 45° angle below the glass surface facilitated viewing of the ventral surface of the rat, specifically any orofacial responses. Each rat, prior to being placed in the chamber, was connected to an infusion pump (KDS100; KD Scientific Inc., Holliston, MA, USA) via a section of PE 90 tubing attached to their intra-oral cannula, which ran through a hole in the lid of the TR chamber. All orofacial and somatic responses were recorded during the session via a video camera (Sony DCR-HC28 Handy Cam, New York, NY, USA) connected directly to a desktop PC using Roxio Videowave Premiere Suite 8 video capture program (Corel Corporation, Ottawa, ON, Canada).
Vomiting in shrews was measured in a clear Plexiglas chamber (22.5 × 26 × 20 cm) illuminated by a 60 W light suspended from the chamber's floor. A mirror was mounted at a 45° angle beneath the chamber floor, which allowed for clear viewing of the ventral surface of the shrew, and an observer counted the number of vomiting episodes.
Procedure
Experiment 1: potential of ARN272 to attenuate LiCl-induced conditioned gaping, and reversal of ARN272-suppressed gaping by SR141716
All rats were surgically implanted with intra-oral cannula under isoflurane anaesthesia as described by Limebeer et al. (2010). Following recovery from surgery (3 days), rats received a single adaptation trial to habituate them to the chamber and the infusion procedure. During the adaptation trial, rats were placed individually in the TR chamber and received a 2 min intra-oral infusion of water (reverse osmosis water infused at 1 mL min−1). On the following day, rats received the first of two conditioning trials (separated by 72 h). On each conditioning trial, rats received a pretreatment injection of ARN272 or VEH 120 min prior to the conditioning trials. During conditioning trails, rats were intra-orally infused with a saccharin solution (0.1%) for 2 min (1 mL min−1) and orofacial and somatic reactions were recorded on video. Immediately following the saccharin infusion, the rats were injected with LiCl (0.15 M) or saline, and then returned to their home cage. Two additional groups were added (after ARN272 at 3.0 mg·kg−1 attenuated gaping) where a pretreatment of ARN272 at 3.0 mg·kg−1 or VEH was given 120 min prior, and with SR141716 30 min prior, to each conditioning trial. The groups were VEH-Saline (VEH-SAL), n = 9; VEH-LiCl, n = 8; 0.1 mg·kg−1 ARN272-LiCl, n = 9; 1.0 mg·kg−1 ARN272-LiCl, n = 8; 3.0 mg·kg−1 ARN272-LiCl, n = 8; 1.0 mg·kg−1 SR-3.0 mg·kg−1 ARN272, n = 8; 1.0 mg·kg−1 SR-VEH, n = 8.
Seventy-two hours following the second conditioning trial, the rats received a drug-free TR test. During the TR test, rats were re-exposed to a 2 min intra-oral infusion of saccharin solution and their orofacial and somatic responses again recorded. All video recordings were later scored by a rater blind to the experimental conditions using ‘The Observer’ (Noldus Information Technology Inc., Leesburg, VA, USA).
Following the TR test, the rats were returned to their home cages and at 16:00 h, their water bottles were removed to begin a water deprivation regime in preparation for the CTA test. At 08:00 h the following morning, the rats received a one-bottle test in which a graduated tube of 0.1% saccharin solution was placed on the home cage, and the amount consumed was recorded at 30 and 120 min intervals. A one-bottle test was used as there is evidence to suggest it is more sensitive in detecting between group differences in strength of taste avoidance than a two-bottle test where both water and saccharin are made available, (Batsell and Best, 1993).
Experiment 2: effect of systemic administration of ARN272 on LiCl-induced vomiting in shrews
Each shrew was offered four meal worms (Tenebrio sp.) in its home cage 15 min prior to pretreatment injections. The shrews received pretreatment injection of ARN272 120 min prior to behavioural testing (VEH, n = 10; 9.0 mg·kg−1, n = 6; 18.0 mg·kg−1, n = 5). Immediately prior to behavioural testing, the shrews were injected with LiCl (0.15 M) and then placed in the TR chamber for 45 min. An observer counted the number of vomiting episodes. A vomiting episode is defined as abdominal contractions and expulsion of gastric fluid.
Behavioural measures
In experiment 1, video recordings were scored for the number of gaping reactions (rapid, large amplitude opening of the mandible with retraction of the corners of the mouth) during the 2 min infusions. During the CTA test, the mean cumulative amount of saccharin consumed was measured at 30 and 120 min. In experiment 2, the frequency of vomiting episodes was scored live during the 45 min period post-LiCl administration.
Data analysis
In experiment 1, the number of gapes exhibited by rats on the drug-free test trial was entered into a one-way anova and analysed with the group as the between-subjects factor. For the CTA measure, the mean cumulative volume of saccharin consumed across drug pretreatment groups was analysed using two separate one-way anovas at each of the two time points, 30 and 120 min. Bonferroni post hoc comparison tests were conducted for all statistically significant effects. In experiment 2, the number of vomiting episodes was entered into a one-way anova and analysed with the drug pretreatment as the between-subjects factor. Planned comparisons were conducted. Statistical significance was defined as P < 0.05.
Results
Experiment 1: systemic ARN272 suppressed LiCl-induced conditioned gaping in rats, and was reversed by the CB1 receptor antagonist SR141716
Gaping measure
The systemic administration of ARN272 produced a dose-dependent suppression in nausea-induced conditioned gaping in rats, effects that were reversed by pretreatment with the CB1 receptor antagonist SR141716. Figure 1 presents the mean number of gapes on the drug-free test day by drug pretreatment group. The one-way anova revealed a significant effect of drug pretreatment, F(6, 51) = 10.83, P < 0.001; subsequent post hoc Bonferroni tests revealed that ARN272 3.0 significantly attenuated gaping as compared with all groups other than VEH-SAL (Ps < 0.01), which also differed from all other groups (Ps < 0.01).
Figure 1.
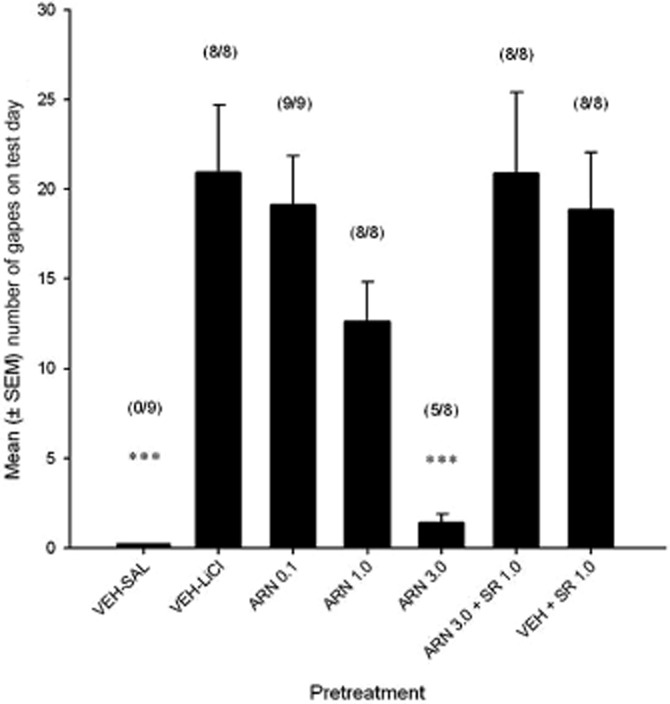
Mean (+SEM) number of gapes by rats on drug-free test day, in experiment 1, by each of the groups. VEH-SAL (n = 9), VEH-LiCl (n = 8), ARN272 0.1 mg·kg−1 (n = 9), ARN272 1.0 mg·kg−1 (n = 8), ARN272 3.0 mg·kg−1 (n = 8), ARN272 3.0 mg·kg−1 + SR 1.0 mg·kg−1 (n = 8), VEH + SR 1.0 mg·kg−1 (n = 8). ***P < 0.001 indicates that group ARN272 3.0 gaped less than VEH, ARN272 0.1, SR 1.0 and ARN272 3.0 + SR 1.0, and that group VEH-SAL gaped less than all other groups. The number of rats that gaped in each group is indicated above each bar.
CTA measure
All pretreatment groups demonstrated greater taste avoidance than the VEH-SAL group at both time intervals (30, 120 min) in that less saccharin was consumed. There were no saccharin consumption differences specifically between the pretreatment conditions that received LiCl, at any of the time intervals. Figure 2 presents the mean cumulative amount of saccharin consumed by the various drug pretreatment groups. The one-way anovas revealed significant differences in the mean cumulative amount of saccharin consumed at 30 min, F(6, 51) = 34.66, P < 0.001, and at 120 min, F(6, 51) = 27.66, P < 0.001. Subsequent post hoc Bonferroni tests revealed the VEH-SAL group as drinking significantly more saccharin than all other groups (Ps < 0.001) at each time period.
Figure 2.
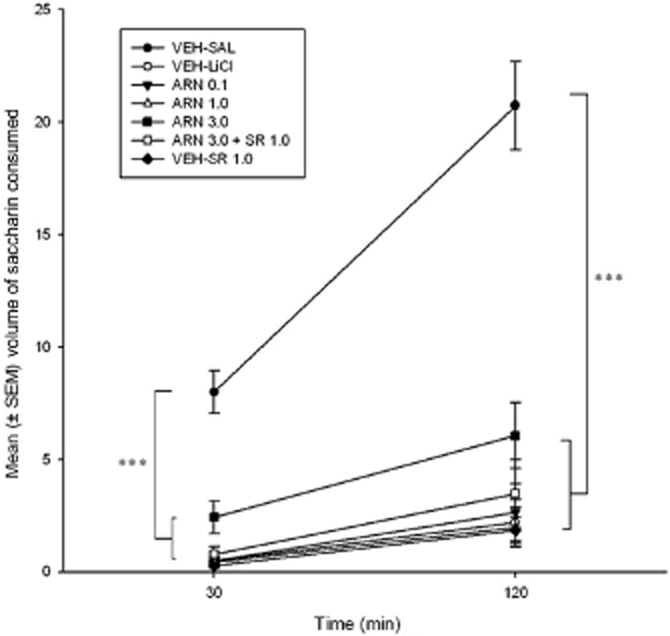
Mean (±SEM) cumulative volume of saccharin consumed by rats 24 h after TR test day and immediately following water restriction, at 30 and 120 min, in experiment 1. ***P < 0.001 indicates that all pretreatment groups consumed less saccharin than the VEH-SAL group at both time intervals, and there were no saccharin consumption differences between the LiCl pretreatment conditions at any of the time intervals.
Experiment 2: systemic ARN272 reduced LiCl-induced vomiting in shrews
Vomiting measure
The systemic administration of ARN272 produced a dose-dependent reduction of vomiting in shrews. Figure 3 present the mean number of LiCl-induced vomiting episodes for shrews pretreated with VEH, ARN272 9.0 and ARN272 18.0. The one-way anova revealed a significant effect of drug pretreatment, F(2, 18) = 3.75, P < 0.05, with planned comparisons revealing that ARN272 18.0 significantly attenuated vomiting as compared with VEH (P < 0.05).
Figure 3.
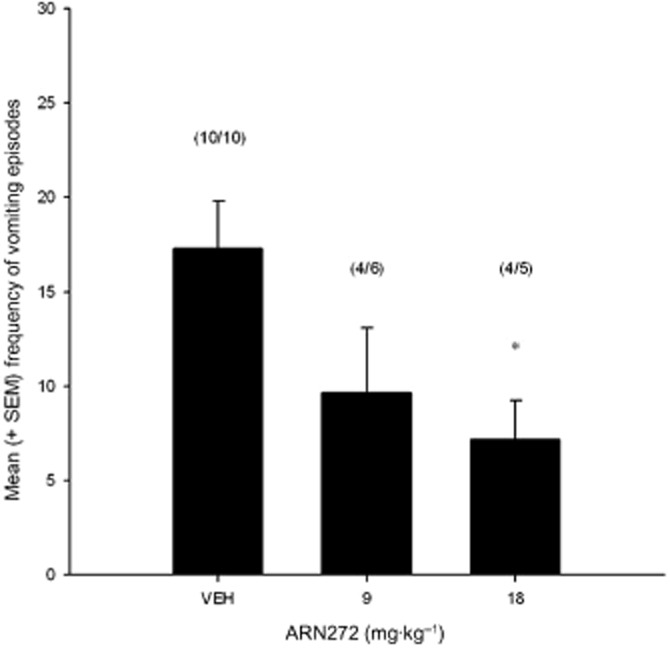
Mean (±SEM) number of vomiting episodes displayed by S. murinus during the 45 min post-LiCl administration observation period. S. murinus were given the following pretreatments prior to LiCl treatment administration: VEH (n = 10), ARN272 9.0 mg·kg−1 (n = 6), ARN272 18.0 mg·kg−1 (n = 5). *P < 0.05 indicates that group ARN272 18.0 vomited significantly less than VEH. The number of shrews that vomited in each group is indicated above each bar.
Discussion
Inhibition of anandamide transport by pretreatment with ARN272 reduced both LiCl-induced conditioned gaping in rats and LiCl-induced vomiting in the house musk shrew. The present study's findings were consistent with existing evidence that increased anandamide availability through FAAH inhibition also attenuates nausea-induced gaping in rats (Cross-Mellor et al., 2007). Both the attenuation of nausea by FAAH inhibition (Cross-Mellor et al., 2007) and the attenuation of nausea by ARN272 were reversed by pretreatment with a CB1 antagonist. As well, consistent with the reported anti-emetic effects of increasing anandamide availability through FAAH inhibition in shrews (Parker et al., 2009) and ferrets (Sharkey et al., 2007), anandamide transport inhibition by ARN272 reduced vomiting in the house musk shrew. Although the mechanism for this reversal was not specifically evaluated here, the suppression of vomiting by FAAH inhibition was reversed by the CB1 antagonist, SR141716 (Parker et al., 2009).
The increased doses required to attenuate vomiting as compared with those required to suppress conditioned gaping are likely due to the extremely high basal metabolism of the shrew. The common European white-toothed shrew Crocidura russula (from the same subfamily as the house musk shrew, Crocidurinae) has been found to have a metabolic quotient higher than that required by its small body weight (Vogel, 1976). In comparison to the rat, Durrer (1982) found the common European white-toothed shrew to exhibit an increased number of cells per cm3 of liver tissue, an increase in mitochondrial volume and a relative larger liver volume. Such differences in the cytoarchitecture of liver hepatocytes between shrew and rat likely account for the higher dose requirements in shrew due to greater clearance by increased liver metabolism.
Fu et al. (2012) previously reported greater anandamide accumulation in cells expressing FLAT as compared with controls, and an elevation in extracellular anandamide in cells, which overexpress FLAT, suggesting a bidirectional mechanism of anandamide translocation. Endocannabinoids have a short duration of action (Di Marzo, 2008) and a highly localized form of neural communication (Wilson and Nicoll, 2001), being produced on demand as required. As such, it is logical that the termination of endocannabinoid signalling would need to be as efficient a process as the one required for its activation. The suggested involvement of FLAT in both the release and the reuptake of anandamide may impact how FLAT inhibition would alter the synaptic availability of anandamide in regions responsible for the control of nausea and vomiting. Should FLAT inhibition favour reuptake, synaptic availability would increase. Conversely, if export is favoured, synaptic availability of anandamide would decrease. If FLAT inhibition occurs without favour bidirectionally, it would be expected to prolong the presence of any available anandamide within the synapse. It is hypothesized then that FLAT inhibition acted to regulate nausea in rats and vomiting in shrews by prolonging the synaptic availability of endogenously produced anandamide. This extended agonist action of anandamide at CB1 receptors was evidenced by the reversal of ARN-suppressed gaping by a CB1 receptor antagonist. Here, the behavioural evidence suggests that the efficiency of anandamide synaptic removal occurs via a facilitated transport system, through FLAT. Further investigation of the mechanism by which ARN272 acts to regulate nausea and vomiting is however warranted.
The challenges associated with the many available compounds that pharmacologically target anandamide transport are their diverse off-target effects, most notably at higher concentrations, FAAH inhibition (Hillard et al., 2007). The inhibition of anandamide transport by ARN272 does appear to be selective in that the compound produced only a weak and incomplete inhibition of FAAH in vitro, and had little to no inhibitory effect on other endocannabinoid metabolizing enzymes such as monoacylglycerol lipase, (Fu et al., 2012). There are further benefits to using indirect agonism by a selective transport inhibitor as compared with FAAH inhibition to understand the role of anandamide within the endocannabinoid system. The catalytic activity of the FAAH enzyme has been found to impact other N-acylethanolamides as well as anandamide; such as oleoylethanolamide and palmitoylethanolamide (Bracey et al., 2002; Kathuria et al., 2003), bioactive molecules that act on non-cannabinoid receptors. As such, the use of FAAH inhibition to isolate anandamide-mediated effects remains problematic.
Existing evidence to suggest that anti-nausea treatments do not interfere with CTA learning in rats (Rabin and Hunt, 1983; Rudd et al., 1998; Limebeer and Parker, 2000; Limebeer et al., 2012) was supported in that pretreatment with ARN272 did not attenuate CTA responding. Only conditioned disgust, the gaping reaction specifically produced by the nausea induced by LiCl, was blocked by pretreatment with ARN272. As such, the present findings suggest that ARN272 is not interfering with learning per se, instead, it interfered with LiCl-induced nausea selectively necessary for the production of gaping reactions, but not CTA (see Parker et al., 2009).
The use of endocannabinoid transport inhibitors have potential not only as a means to further elucidate the role and function of the endocannabinoid system, but also as therapeutic agents. The indirect agonism produced by anandamide transport inhibition seemingly augments the body's own regulatory processes. Indirectly enhancing anandamide action in tissue where synthesis, release and degradation is already occurring, could provide a safer and more selective action than direct agonists (Di Marzo, 2008).
The present experiments suggest that anandamide transport inhibition tonically activates CB1 receptors to regulate nausea and vomiting, and provides in vivo support for a facilitated transport mechanism used in the cellular reuptake of anandamide.
Acknowledgments
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC-92057) to L. A. P., and the National Institute on Drug Abuse (DA012413) to D. P.
Glossary
- ARN272
[(4-(5-(4-hydroxy-phenyl)-3,4-diaza-bicyclo[4.4.0]deca-1(6),2,4,7,9-pentaen-2-ylamino)-phenyl)-phenylamino-methanone]
- CB1
cannabinoid 1
- CTA
conditioned taste avoidance
- FAAH
fatty acid amide hydrolase
- FLAT
FAAH-1-like anandamide transporter
- TR
taste reactivity
Conflict of interest
None.
References
- Batsell WR, Best MR. One bottle too many? Method of testing determines the detection of overshadowing and retention of taste aversions. Anim Learn Behav. 1993;2:154–158. [Google Scholar]
- Berger B. Conditioning of food aversions by injections of psychoactive drugs. J Comp Physiol Psychol A. 1972;81:21–26. doi: 10.1037/h0033316. [DOI] [PubMed] [Google Scholar]
- Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science. 2002;298:1793–1796. doi: 10.1126/science.1076535. [DOI] [PubMed] [Google Scholar]
- Carey MP, Burish TG, Brenner DE. Delta 9-tetrahydrocannabinol in cancer chemotherapy: research problems and issues. Ann Intern Med. 1983;99:106–114. doi: 10.7326/0003-4819-99-1-106. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cross-Mellor SK, Klaus-Peter O, Piomelli D, Parker LA. Effects of the FAAH inhibitor, URB597, and anandamide on lithium-induced taste reactivity responses: a measure of nausea in the rat. Psychopharmacology (Berl) 2007;190:135–143. doi: 10.1007/s00213-006-0589-7. [DOI] [PubMed] [Google Scholar]
- Darmani NA. The potent emetogenic effects of the cannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by delta (9)-tetrahydrocannabinol and other cannabinoids. J Pharmacol Exp Ther. 2002;300:34–42. doi: 10.1124/jpet.300.1.34. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Durrer H. Quantitative cytoarchitecture of the liver of the shrew Crocidura russula (Fam. Soricidae) ultrastructural and morphometric comparison with rat liver. Cell Tissue Res. 1982;224:421–439. doi: 10.1007/BF00216884. [DOI] [PubMed] [Google Scholar]
- Forget B, Coen KM, Le Foll B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration – comparison with CB1 receptor blockade. Psychopharmacology (Berl) 2009;205:613–624. doi: 10.1007/s00213-009-1569-5. [DOI] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, et al. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci. 2012;15:64–69. doi: 10.1038/nn.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci U S A. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Shi L, Tuniki VR, Falck JR, Campbell WB. Studies of anandamide accumulation inhibitors in cerebellar granule neurons: comparison to inhibition of fatty acid amide hydrolase. J Mol Neurosci. 2007;33:18–24. doi: 10.1007/s12031-007-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowska M, Parker LA, Burton P, Mechoulam R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew) Psychopharmacology (Berl) 2004;174:254–259. doi: 10.1007/s00213-003-1739-9. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Morera E, Stelt van der M, Monory K, Lutz B, Ortar G, et al. Further evidence for the existence of a specific process for the membrane transport of anandamide. J Biochem. 2004;380:265–272. doi: 10.1042/BJ20031812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA. The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. J Exp Psychol Anim Behav Process. 2000;26:371–384. doi: 10.1037//0097-7403.26.4.371. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA. The 5-HT1A agonist 8-OH-DPAT dose-dependently interferes with the establishment and the expression of lithium-induced conditioned rejection reactions in rats. Psychopharmacology (Berl) 2003;166:120–126. doi: 10.1007/s00213-002-1309-6. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Vemuri VK, Bedard H, Lang ST, Osenkopp KP, Makriyannis A, et al. Inverse agonism of cannabinoid CB1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. Br J Pharmacol. 2010;161:336–349. doi: 10.1111/j.1476-5381.2010.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limebeer CL, Rock EM, Mechoulam R, Parker LA. The anti-nausea effects of CB1 agonists are mediated by an action at the visceral insular cortex. Br J Pharmacol. 2012;167:1126–1136. doi: 10.1111/j.1476-5381.2012.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy LE, Borison HL. Anti-emetic activity of N-methyllevonantrobil and naboline in cisplatin treated cats. J Clin Pharmacol. 1981;21:30S–37S. doi: 10.1002/j.1552-4604.1981.tb02570.x. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- Parker LA, Mechoulam R, Schlievert C, Abbott L, Fudge ML, Burton P. Effects of cannabinoids on lithium-induced conditioned rejection reactions in a rat model of nausea. Psychopharmacology (Berl) 2003;166:156–162. doi: 10.1007/s00213-002-1329-2. [DOI] [PubMed] [Google Scholar]
- Parker LA, Kwaitkowska M, Burton P, Mechoulam R. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew) Psychopharmacology (Berl) 2004;171:156–161. doi: 10.1007/s00213-003-1571-2. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rana SA, Limebeer CL. Conditioned nausea in rats: assessment by conditioned disgust reactions, rather than conditioned taste avoidance. Can J Exp Psychol. 2008;62:198–209. doi: 10.1037/a0012531. [DOI] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Rock EM, Litt DL, Kwiatkowska M, Piomelli D. The FAAH inhibitor URB-597 interferes with cisplatin- and nicotine-induced vomiting in the Suncus murinus (house musk shrew) Physiol Behav. 2009;97:121–124. doi: 10.1016/j.physbeh.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Rock EM, Limebeer CL. Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol. 2011;163:1411–1422. doi: 10.1111/j.1476-5381.2010.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin BM, Hunt WA. Effects of anti-emetics on the acquisition and recall of radiation and lithium chloride induced conditioned taste aversions. Pharmacol Biochem Behav. 1983;18:629–636. doi: 10.1016/0091-3057(83)90292-7. [DOI] [PubMed] [Google Scholar]
- Reicher MA, Holman EW. Location preference and flavor aversion reinforced by amphetamine in rats. Anim Learn Behav. 1977;5:343–346. [Google Scholar]
- Rock EM, Goodwin JM, Limebeer CL, Breuer A, Pertwee RG, Mechoulam R, et al. Interaction between non-psychotropic cannabinoids in marihuana: effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology (Berl) 2011;215:505–512. doi: 10.1007/s00213-010-2157-4. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Ngan MP, Wai MK. 5-HT3 Receptors are not involved in conditioned taste aversions induced by 5-hydroxytryptamine, ipecacuanha or cisplatin. Eur J Pharmacol. 1998;352:143–149. doi: 10.1016/s0014-2999(98)00359-8. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Cristino L, Oland LD, Van Sickle MD, Starowicz K, Pittman QJ, et al. Arvanil, anandamide and N-arachidonolyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosci. 2007;25:2773–2782. doi: 10.1111/j.1460-9568.2007.05521.x. [DOI] [PubMed] [Google Scholar]
- Simoneau II, Hamza MS, Mata HP, Siegel EM, Vanderah TW, Porreca F, et al. The cannabinoid agonist WIN 55,212-2 suppresses opioid-induced emesis in ferrets. Anesthesiology. 2001;94:882–886. doi: 10.1097/00000542-200105000-00029. [DOI] [PubMed] [Google Scholar]
- Tramer MR, Carroll D, Campbell FA, Reynolds DJM, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323:1–8. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Norgren R. Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav Neurosci. 1986;100:544–555. doi: 10.1037//0735-7044.100.4.544. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, et al. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vogel ER. Energy consumption of European and African shrews. Acta Theriol. 1976;21:195–206. [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]


