Abstract
Introduction
A new method for preparation of silver nanoparticles (AgNPs) based on using hydrazino-isatin derivatives in an aqueous methanol reaction medium is reported here. AgNPs were prepared using silver nitrate solubilized in a water core as the source of silver ions and 3-hydrazino-isatin derivatives (3-hydrazino-isatin [IsH] and 1-benzyl-3-hydrazino-isatin [BIsH]) solubilized in methanol core as a reducing agent. The proposed method is effective, rapid, and convenient. X-ray diffraction (XRD), energy dispersive X-ray analysis, scanning electron microscope (SEM) and transmission electron microscopy (TEM) were used for characterization of the AgNPs. The TEM micrographs confirmed that the nanopowders consist of well-dispersed agglomerates of grains with a narrow size distribution of 18–21 nm and 17–20 nm. The AgNPs, as well as BIsH, showed high antimicrobial and bactericidal activity against the Gram-positive Bacillus subtilis and Gram-negative Micrococcus luteus and Proteus vulgaris, as well as antifungal activities against Saccharomyces cerevisiae. On the other hand, they were not effective against the Gram-negative Escherichia coli.
Purpose
A simple, effective, rapid, and convenient chemical reduction method for the synthesis of AgNPs in an aqueous methanol reaction medium using hydrazino-isatin derivatives and studying their antibacterial effect.
Results
IsH and BIsH are remarkably powerful reductants for Ag+ ions in an aqueous methanol medium, which could be considered as a simple chemical reduction method for formation of AgNPs. The AgNP formation depends on the solubility of the hydrazino-isatin derivatives. BIsH gave more AgNPs than IsH, as observed from XRD. The formation of AgNPs is attributed to the adsorption of hydrazine derivatives and/or interparticle interaction on the surface of AgNP through electrostatic interactions between the lone pair electrons of the hydrazino group (C=N-NH2) and the positive surface of AgNPs. AgNPs and BIsH showed high antimicrobial and bacterial activity.
Conclusion
In summary, it is shown that IsH and BIsH are remarkably powerful reductants for Ag+ ions in an aqueous methanol medium. BIsH gave more AgNPs than IsH, as observed from XRD due to better solubility of the BIsH than IsH in aqueous-methanol. The formation of AgNPs is attributed to the adsorption of hydrazine derivatives and/or interparticle interaction on the surface of AgNPs through electrostatic interactions between the lone pair electrons of the hydrazino group (C=N-NH2) and the positive surface of AgNPs. The AgNps as well as BIsH ligand showed high antimicrobial and bactericidal activity.
Keywords: silver nanoparticles, 1-benzyl-3-hydrazinoisatin, SEM, TEM, antimicrobial
Introduction
Nanoparticles (NPs) are emerging materials that have been the subject of focused research with a broad range of applications, due to their unique physical and chemical properties that are significantly different from those of bulk materials.1–5 Silver nanoparticles (AgNPs) are one of the most attractive inorganic materials not only because of their broad application in catalysis,6 photography,7 biosensors,8 biomolecular detection,9 diagnostics,10 and antimicrobial activities.11–18 A number of methods are used for the synthesis of AgNPs, such as the electrochemical method,19,20 thermal decomposition,21 laser ablation,22 microwave irradiation,23 and sonochemical synthesis.24 The simplest and most commonly synthetic method for metal NPs is the chemical reduction of metal salts.25–30 The chemical reduction methods have been applied to synthesize stable and various shapes of AgNPs in water by the use of different reducing agents (eg, ascorbic acid,31 hydrazine,32 ammonium formate,33 dimethylformamide [DMF],34 and sodium borohydride).35 The shape, size, and size distribution depend on the tendency of organic substrates to reduce the silver ions. Compared with physical and chemical methods, biologic methods using microbes and plants are regarded as an ecofriendly process.36 Several biologic methods employing bacteria,37 fungi,38 yeast,39 or plant extracts40,41 have been used as an alternative to chemical methods. The presence of various natural products such as carbohydrates, alkaloids, steroids, proteins, and/or peptides in plant extract, and the tendency of silver ions to form a variety of complexes with them, makes the systems of considerable complexity. Using a chemical reduction method for the synthesis of different morphology NPs can be advantageous over other biosynthetic methods because it involves reduction of an ionic salt in the presence of a reducing agent, is cost-effective, is easily scaled up, and there is no need to use high pressure, energy, and temperature.
Recently,42 aniline was used as a stabilizer for controlling the morphology and particle size of AgNPs using hydrazine and sodium citrate as the reducing agents. Later, Hussain et al43 used aniline in the presence of cetyltrimethylammonium bromide as a simple chemical reduction aniline route for the synthesis of silver nanocrystals. The studies revealed that the reaction conditions (cetyltrimethylammonium bromide and aniline) have great influence on the morphology of AgNPs.
Because isatin derivatives have been the subject of extensive research, particularly in the pharmaceutical and agrochemical areas,44–52 the present work describes a simple chemical reduction employing 3-hydrazino-isatin (IsH) derivatives as reducing agent for the synthesis of AgNPs in aqueous methanol. The antimicrobial activity is also described for both of the reducing agents and AgNPs.
Materials and methods
Silver nitrate (AgNO3), hydrazine hydrate, and isatin were purchase from Sigma-Aldrich (St Louis, MO, USA). All chemicals were used as received. Double-distilled deionized water was used. The evaluation of crystal structure was achieved by X-ray diffractometer (XRD) (X’Pert PRO, PANalytical BV, Almelo, the Netherlands) using CuKα radiation. The studies of size, morphology, and composition of the NPs were performed by means of scanning electron microscope (SEM) and transmission electron microscopy (TEM), equipped with energy dispersive X-ray (EDX) analysis. Histograms of AgNPs’ size distribution were calculated from the TEM images by measuring the diameters of at least 50 particles. Samples for TEM studies were prepared by placing drops of the AgNP solutions on carbon-coated TEM grids. 1H- and 13C-NMR spectra of compounds were run on a JEOL-NMR spectrometer (400 MHz) (JEOL, Tokyo, Japan). The chemical shifts are expressed in ppm downfield from tetramethylsilane as the internal standard.
Synthesis of 1-benzylisatin (3)52
A mixture of isatin (5 mmol) and potassium carbonate (8 mmol) in DMF (10 mL) was stirred for 10 minutes at room temperature. Benzyl bromide (6 mmol) was added dropwise to the reaction mixture and then the reaction was microwave irradiated using a multimode reactor (Synthos 3000, Anton Paar GmbH, Graz, Austria) (1,400 W maximum magnetron). The vessel was placed in the corresponding rotor, fixed by screwing down the upper rotor place, and finally the rotor was closed with a protective hood. The vessel was heated for 5 minutes at 80°C and held at the same temperature for a further 5 minutes (~2 bar pressure, 400 W). Cooling was accomplished by a fan (5 minutes). The final product was dried and recrystallized from ethanol to give orange red crystals in yield 88%, mp: 134°C–136°C; 1H-NMR (CDCl3) δ (ppm): 4.93 (s, 2H, C6H5CH2), 6.77 (d, J=7.7 Hz, 1H), 7.08 (t, J=7.35 Hz, 1H), 7.33 (s, 5H, Ar), 7.47 (t, J=8.07, 1H), 7.61 (d, J=8.07, 1H). 13C-NMR (CDCl3 δ (ppm): 42.94, 109.90, 122.74, 124.31, 126.31, 127.04, 127.93, 133.37, 137.18.
General method for preparation of the hydrazino-isatin derivatives 4 and 5
Hydrazine hydrate (3 mL) was added to a solution of isatin (1, 5 mmol) or 1-benzylisatin (3, 5 mmol) in ethanol (10 mL). The reaction mixture was microwave irradiated using a multimode reactor (Synthos 3000, Anton Paar GmbH, 1,400 W maximum magnetron). The vessels were placed in the corresponding rotor, fixed by screwing down the upper rotor place, and finally the rotor was closed with a protective hood. The vessel was heated for 3 minutes at 80°C and held at the same temperature for a further 3 minutes (~2 bar pressure, 400 W). Cooling was accomplished by a fan (5 minutes). The final product was dried and recrystallized from ethanol to give a yellow solid.
1-Benzyl-3-hydrazino-isatin (4)
The product obtained was a yellow crystal from ethanol, yield 93%, mp: 114°C–115°C. 1H-NMR (dimethyl sulfoxide [DMSO]-d6) δ (ppm): 4.97 (s, 2H, C6H5CH2), 6.96 (d, J=8.04 Hz, 1H), 7.02 (t, J=7.32 Hz, 1H), 7.17 (t, J=8.08 Hz, 1H), 7.32 (s, 5H, Ar), 7.43 (d, J=7.36 Hz, 1H), 9.77 (d, J=14.64 Hz, 1NH), 10.58 (d, J=13.96 Hz, NH); 13C-NMR (DMSO-d6) δ (ppm): 42.8, 109.8, 117.9, 122.13, 122.6, 125.6, 127.5, 127.8, 127.9, 129.2, 137.3, 139.5, 161.3. Elemental analysis calculated. for C15H13N3O: C, 71.70; H, 5.21; N, 16.72. Found: C, 71.91; H, 5.19; N, 16.98.
3-hydrazino-isatin (5)
The product obtained was a yellow crystal from ethanol, yield 90%, mp: 240°C–242°C. 1H-NMR (DMSO-d6) δ (ppm): 6.86 (d, J=8.08 Hz, 1H), 6.98 (t, J=7.32 Hz, 1H), 7.14 (t, J=7.32 Hz, 1H), 7.35 (d, J=8.08 Hz, 1H), 9.55 (d, J=13.96 Hz, 1NH), 10.54 (d, J=14.68 Hz, NH); 13C-NMR (DMSO-d6) δ (ppm): 110.5, 118.01, 121.9, 122.8, 126.8, 127.6, 139.2, 163.3. Elemental analysis calculated for C8H7N3O: C, 59.62; H, 4.38; N, 26.07. Found: C, 59.88; H, 4.52; N, 26.31.
Preparation of AgNPs
For the synthesis of AgNPs, silver nitrate solution (0.1 mmol in 50 mL H2O) was used as a metal salt precursor and added slowly (5–10 minutes) to a solution of IsH derivatives (1 mmol in 50 mL methanol). The reaction mixture was stirred at 25°C–30°C for 1 hour and then at room temperature overnight. The transparent, colorless solution was converted to the characteristic pale yellow and then finally to black with formation of a silver mirror on the wall. The AgNPs were collected and purified by centrifugation and then washed three times with deionized water. A dried powder of the AgNPs was obtained by drying under vacuum. The dried particles were used to carry out all characterization methods and interaction of the AgNPs with bacteria.
Micro-organisms
The microbial organisms used in this study included the Gram-positive bacterium Bacillus subtilis NRRL B-543; the Gram-negative bacteria Escherichia coli JM 105 DSM 3949, Proteus Vulgaris, and Micrococcus luteus; as well as the fungal yeast Saccharomyces cerevisiae.
Assay for the antimicrobial activity
The prepared silver complexes were evaluated for their antimicrobial activity by the modified agar diffusion technique of Perez et al.53 The compounds were dissolved in DMSO (Panreac Sintesis, Barcelona, Spain) to a final concentration of 1 mg/mL working solution. The test bacterial and fungal strains were maintained on nutrient agar (Merck Millipore, Billerica, MA, USA) and ISP-2 mediums at 37°C and 26°C, respectively. Prior to the assay, the cultured strains were cultivated on their corresponding broth mediums for at least 12 hours on a reciprocal shaker at 150 rpm. Samples in the form of 1 mL were taken each hour and the growth was determined by measuring the optical density of the growing cells at 623 nm against control growth medium. When the growth entered the exponential phase, the cells were used to inoculate agar media to achieve a cell concentration of 0.015 mL−1. After that, the medium was poured on to 9 mm agar plates to provide thin agar layers with a thickness of 3.5–4.5 mm. After solidification, wells of 10 mm diameter were cut using a sterile cork-borer, and 0.1 mL of the prepared stock solution was poured into the wells. For comparison, 1% DMSO was used as a negative control. Additionally, standard tetracycline and erythromycin sensitivity discs at 15 μg/disc were used as positive controls. The agar plates were then incubated at 4°C for 1 hour to ensure the diffusion of the tested compounds, and they were then incubated for 24 hours at 37°C and 26°C for bacterial and yeast strains, respectively. After incubation, the diameter of the resulted inhibition zone was measured.
Results and discussion
N-benzylisatin 3 was prepared by reaction of isatin 1 with benzyl bromide 2 in the presence of K2CO3 and DMF as solvent under microwave irradiation for 10 minutes (Figure 1). Reaction of 3 with hydrazine hydrate (80%) under microwave irradiation for 5 minutes afforded 1-benzyl-3-hydrazino-isatin (4, BIsH), yield 92% (Figure 1). Isatin (1) was reacted with hydrazine hydrate (80%) under the same conditions described to afford IsH (5), yield 90% (Figure 1). The structures of the synthesized compound were confirmed by spectral data.
Figure 1.
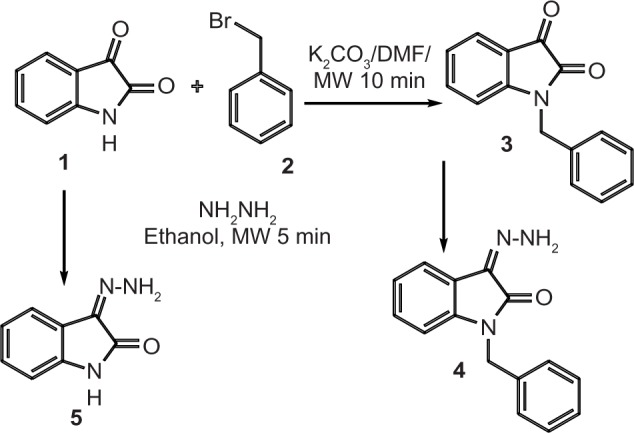
Synthesis of 1-benzyl-3-hydrazino-isatin 4 and 3-hydrazino-isatin 5.
Abbreviations: MW, microwave irradiation; DMF, dimethylformamide.
The synthesized compounds 4 and 5 were used as a reducing agent for preparation of AgNPs in aqueous methanol at room temperature. A solution of AgNO3 in water was added to a solution of hydrazino derivative 4 or 5 in methanol. The reaction mixture was slowly stirred at room temperate for 24 hours. The color of the reaction mixture was changed from colorless to yellow and finally to black, indicating that AgNPs were formed. The precipitate was collected and dried under vacuum.
The elemental analysis of the AgNPs was performed using EDX on the TEM. EDX analysis confirmed the presence of the elementary silver signal of the prepared AgNPs, as shown in Figure 2A and B. Signal peaks in the range of 2.5–4 keV were observed, which correspond to the binding energies of crystalline silver.54–56 Also, a strong signal peak near 0.2 keV corresponded to carbon in the ligand connected to AgNPs. On the other hand, several peaks for CuKα and CuKβ showed, which correspond to the TEM holding grid. These results were confirmed by previous reports that showed that AgNPs are crystalline in nature with the same EDX results.
Figure 2.
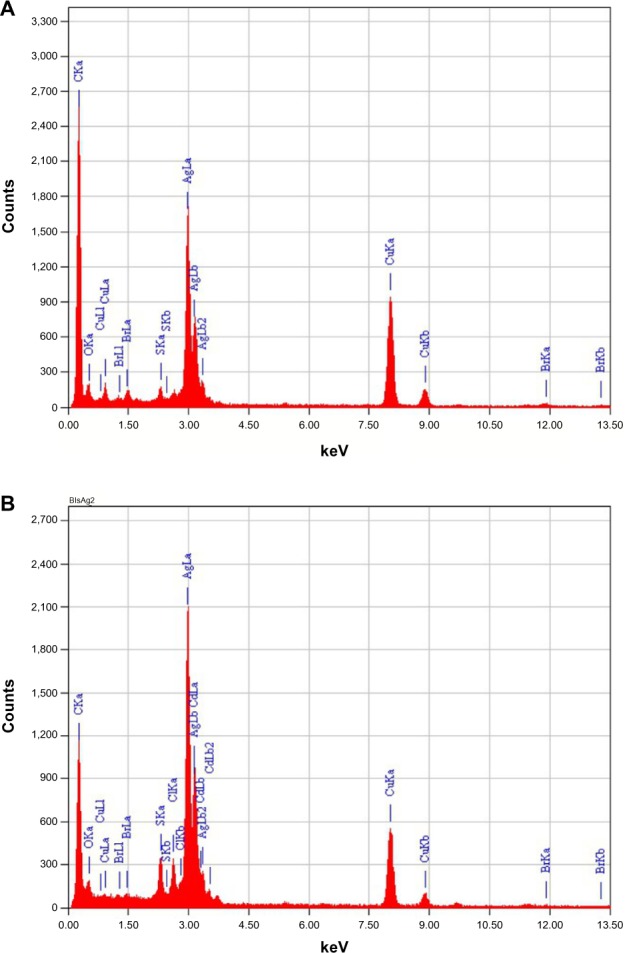
Energy dispersive X-ray spectra of the prepared silver nanoparticles from 3-hydrazino-isatin (A) and 1-benzyl-3-hydrazino-isatin (B) composite powders.
The typical powder XRD patterns of the prepared AgNPs from IsH (IsHAg) and from BIsH (BIsHAg) are shown in Figure 3. The XRD patterns (Figure 3) showed peaks at about 38.1°C, 44.09°C, 64.36°C, 77.29°C, and 81.31°C for both the prepared materials, which corresponded to 111, 200, 220, 311, 222, 400, 331, and 420 planes, respectively, to indicate a typical face-centered cubic structure of silver as per the available literature (Joint Committee on Powder Diffraction Standards, JCPDS file No 04-0783).57,58 The high peaks intensity observed in BIsHAg indicates that a large amount of AgNPs were produced compared with IsHAg. This is attributed to the higher solubility of BIsH in an aqueous methanol medium.
Figure 3.
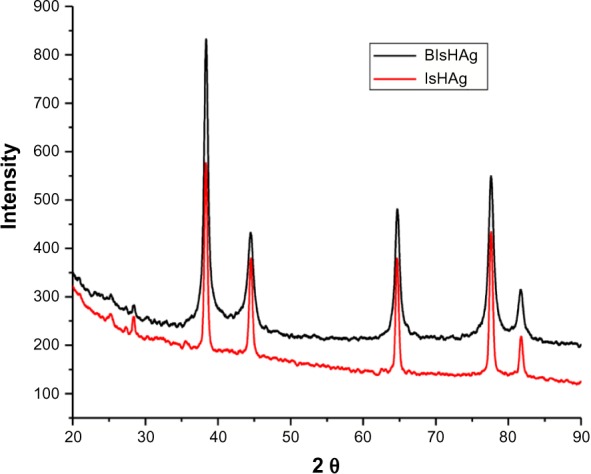
X-ray diffraction pattern of the silver nanoparticles produced from 3-hydrazino-isatin (IsHAg) and 1-benzyl-3-hydrazino-isatin (BIsHAg).
A scanning electron microscope (JSM-5800 JEOL) was employed to study the structure of the prepared silver–ligand composite, as shown in Figures 4 and 5. The composite that formed was uniform in structure, and a homogenous distribution of AgNPs over ligand surface is observed as shown in Figure 4.
Figure 4.
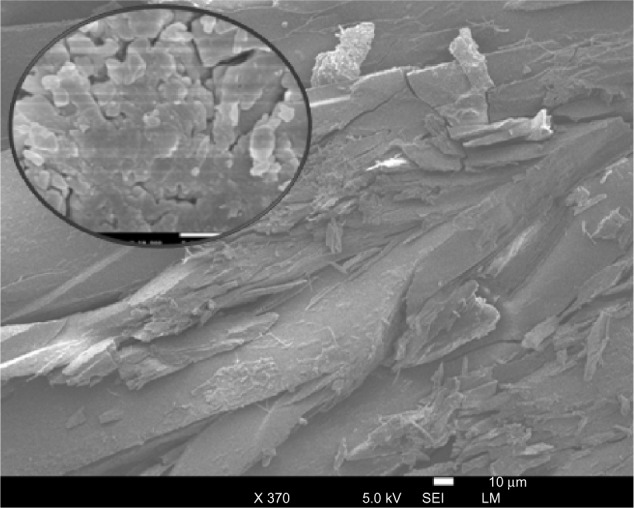
Low-resolution scanning electron microscope (SEM) micrograph of silver-ligand composite using secondary electron imaging mode (SEI).
Figure 5.
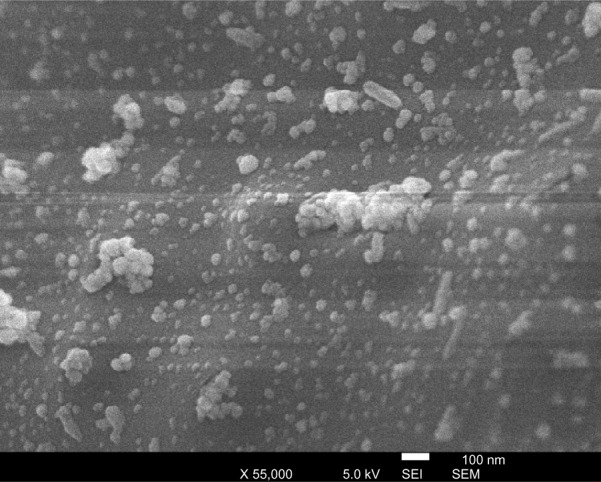
High-resolution electron microscope (SEM) micrograph of silver-ligand composite using secondary electron imaging mode (SEI).
The TEM image of the synthesized silver is represented in Figure 6A and B. Analysis of TEM imaging shows that prepared AgNPs are monodispersed spheres in the range of 18–21 nm and 17–20 nm in size for IsHAg and BIsHAg, respectively. Nanosized silver particles were formed by nucleation and growth mechanism after reduction by the lone pair of electrons of the hydrazino group (C=N-NH2) located at the ligand structure. On the other hand, some particles whose diameters were longer than 40 nm were formed because of aggregation during preparation of the TEM holding grid.
Figure 6.
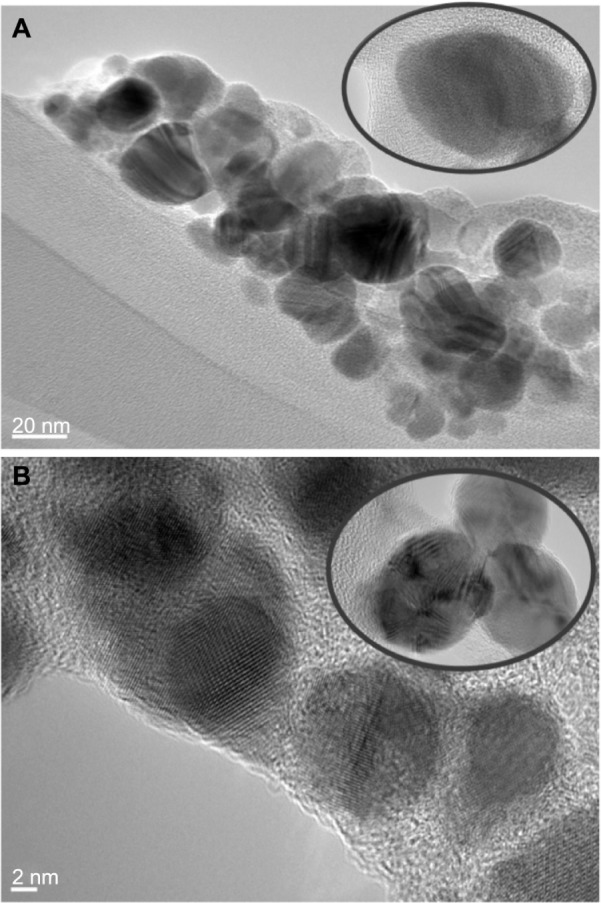
High-resolution transmission electron microscopy micrographs of prepared nanosilver from 3-hydrazino-isatin (IsHAg) (A) and 1-benzyl-3-hydrazino-isatin (BIsHAg) (B).
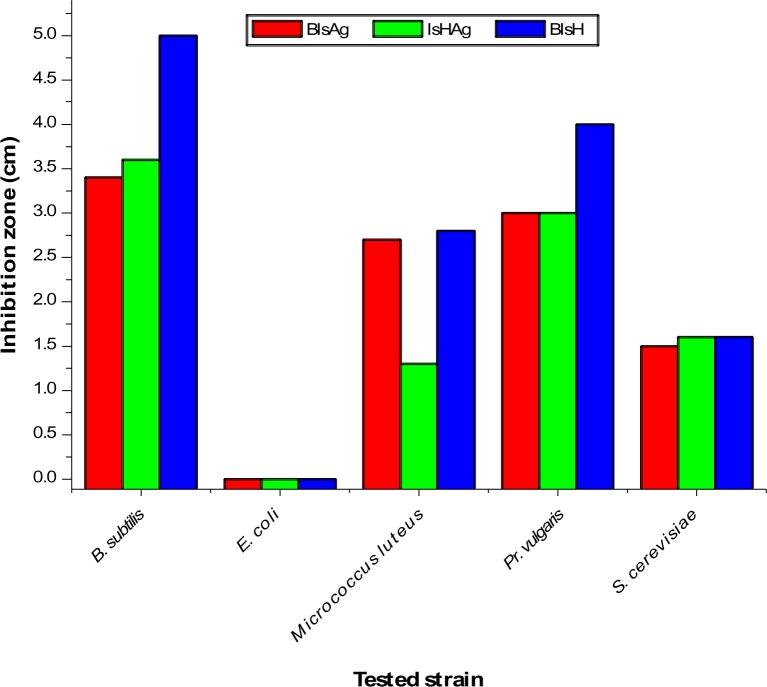
Finally, the antimicrobial susceptibility of synthesized AgNPs was investigated. The obtained results showed that prepared AgNPs from BIsH (BIsHAg) and from IsH (IsHAg) exhibited both antibacterial and antifungal activities (Figure 7). The nanosilver compounds were active against Gram-positive B. subtilis and Gram-negative M. luteus and Pr. vulgaris, though they were not effective against E. coli. The prepared compounds showed similar antifungal activities against S. cerevisiae, where the reported inhibition zone ranged from 1.5 cm to 1.6 cm. The inhibition zone reported for the Gram-positive B. subtilis was 5 cm, 3.6 cm, and 3.4 cm for BIsH, IsHAg, and BIsHAg, respectively. The Gram-negative Pr. vulgaris was similarly affected by IsHAg and BIsHAg, where both compounds gave an inhibition zone of 3 cm, representing 75% of the activity recorded for BIsH. On the contrary, the growth of all tested microorganisms was not inhibited upon the addition of 1% DMSO. The standard tetracycline antibiotic showed inhibition zones of 1.3 cm and 1.8 cm for B. subtilis and M. luteus, respectively, whereas the erythromycin showed inhibition of 2.8 cm and 3.2 cm for the same two microbes, respectively. Hence, the activity of the prepared NPs showed comparable activities with standard antibiotics.
Figure 7.
Antimicrobial activity of silver nanoparticles against Gram-positive, Gram-negative, and fungal micro-organisms.
Abbreviations: Ag, silver; BIsH, 1-benzyl-3-hydrazinoisatin; IsH, 3-hydrazinoisatin; B. subtilis, Bacillus subtilis; E. Coli, Escherichia coli; Pr. vulgaris, Proteus vulgaris; S. cerevisiae, Saccharomyces cerevisiae.
The resulting antimicrobial effects of the prepared AgNPs can be attributed to the fact that AgNPs can interact with the microbial cell surface, which will lead to the rupture of the cell membranes. Many mechanisms have been proposed to explain the antimicrobial effect of AgNPs. One mechanism suggests the incorporation of AgNPs in the cell membrane, leading to the leakage of intracellular components and eventually resulting in cell death.59 Another hypothesis suggests that the NPs will be oxidized, dissolve, and generate reactive oxygen species, which will, in turn, enter the cytoplasm, causing bacterial death.60–62 Moreover, small-sized nanoparticles may accumulate intracellularly and lead to cell malfunction.63
Our results showed varying responses of different microbial micro-organisms as affected by the prepared AgNPs. This can be explained based on the fact that the antimicrobial activity of AgNPs differs according to the microbial strain tested. Yoon et al64 showed that E. coli and B. subtilis have different susceptibility to silver and copper NPs. This is due to the differences in the membrane structure of different microbial strains, mainly the thickness of the peptidoglycan layer of the cell membrane.65 On the other hand, our results showed that E. coli was insensitive to the prepared AgNPs. Although these results contradict most of the work presented in the literature showing positive effects against the same microbe, few reports reported the same finding. Rawani et al66 found that their two investigated human pathogens, E. coli and Pseudomonas aeruginosa, were not affected by their prepared NPs. Recently, this was attributed to the finding that AgNPs stimulated the activation of anaerobic respiration-related regulators and enzymes, which play an important role in the resistance of E. coli to AgNPs.67
Conclusion
In summary, it is shown that IsH and BIsH are remarkably powerful reductants for Ag+ ions in an aqueous methanol medium, which could be considered as a simple effective, rapid, and convenient chemical reduction method for formation of AgNPs. The NP formation depends on the solubility of the hydrazino-isatin derivatives. BIsH gave more AgNPs than IsH, as observed from XRD. The formation of AgNPs is attributed to the adsorption of hydrazine derivatives and/or interparticle interaction on the surface of AgNP particles through electrostatic interactions between the lone pair electrons of the hydrazino group (C=N-NH2) and the positive surface of AgNPs. The TEM micrographs indicate that the nanopowders consist of well-dispersed agglomerates of grains with a narrow size distribution of 18–21 nm and 17–20 nm. The AgNPs, as well as BIsH ligand, showed high antimicrobial and bactericidal activity against the Gram-positive B. subtilis and Gram-negative M. luteus and Pr. Vulgaris for both BIsH and BIsHAg, as well as antifungal activities against S. cerevisiae. On the other hand, they were not effective against the Gram-negative E. coli.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia, for funding this work through research group “RGP-VPP-234.”
Footnotes
Author contributions
Ayman El-Faham and Ahmed A Elzatahry carried out the preparation, designed the proposed methods, and statistically analyzed the data together. Zeid A Al-Othman carried out the analysis and characterization for silver nanoparticles. Elsayed Ahmed Elsayed carried out the biologic studies. All authors took part in drafting the article or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mazur M. Electrochemically prepared silver nanoflakes and nanowires. Electrochem Commun. 2004;6:400–403. [Google Scholar]
- 2.Kim TN, Feng QL, Kim JO, et al. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J Mater Sci Med. 1998;9(3):129–134. doi: 10.1023/a:1008811501734. [DOI] [PubMed] [Google Scholar]
- 3.Roosta M, Ghaedi M, Shokri N, Daneshfar A, Sahraei R, Asghar A. Optimization of the combined ultrasonic assisted/adsorption method for the removal of malachite green by gold nanoparticles loaded on activated carbon: experimental design. Spectrochim Acta A Mol Biomol Spectrosc. 2014;118:55–65. doi: 10.1016/j.saa.2013.08.082. [DOI] [PubMed] [Google Scholar]
- 4.Ghaedi M, Ansari A, Habibi MH, Asghari AR. Removal of malachite green from aqueous solution by zinc oxide nanoparticle loaded on activated carbon: kinetics and isotherm study. J Indust Eng Chem. 2014;20(1):17–28. [Google Scholar]
- 5.Roosta M, Ghaedi M, Shokri N, Daneshfar A, Sahraei R, Asghari A. Optimization of the ultrasonic assisted removal of methylene blue by gold nanoparticles loaded on activated carbon using experimental design methodology. Ultrason Sonochem. 2014;21(1):242–252. doi: 10.1016/j.ultsonch.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Sun T, Seff K. Silver clusters and chemistry in zeolites. Chem Rev. 1994;94:857–870. [Google Scholar]
- 7.Albrecht MA, Evans CW, Raston CL. Green chemistry and the health implications of nanoparticles. Green Chem. 2006;8:417–432. [Google Scholar]
- 8.Xiong DJ, Chen mL, Li H. Synthesis of para-sulfonatocalix[4] arene-modified silver nanoparticles as colorimetric histidine probes. Chem Commun. 2008:880–882. doi: 10.1039/b716270g. [DOI] [PubMed] [Google Scholar]
- 9.Duran N, Marcato PD, Alves OL, De Souza GIH, Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnology. 2005;3:8–15. doi: 10.1186/1477-3155-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu S, Jana S, Pande S, Pal T. Interaction of DNA bases with silver nanoparticles: assembly quantified through SPRS and SERS. J Colloid and Interface Sci. 2008;321:288–293. doi: 10.1016/j.jcis.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 12.Guzman MG, Dille J, Godet S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. World Acad Sci Eng Technol. 2008;43:357–364. [Google Scholar]
- 13.Kandile NG, Zaky HT, Mohamed MI, Mohamed HM. Silver nanoparticles effect on antimicrobial and antifungal activity of new heterocycles. Bull Korean Chem Soc. 2010;31:3530–3538. [Google Scholar]
- 14.Shahverdi AR, Fakhimi A. Synthesis and effect of silver nanoparticles of the anti-bacterial activity of different antibiotic against Staphylococcus aureus and Escherichia coli. Nonomedicine. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panacek A, Kvitek L, Prucek R, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110:16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 17.Mock JJ, Barbic M, Smith DR, Schultz DA, Schultz S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J Chem Phys. 2002;116:6755–6759. [Google Scholar]
- 18.Reddy GAK, Joy JM, Mitra T, Shabnam S, Shilpa T. Nanosilver review. Int J Advanc Pharm. 2012;2:9–15. [Google Scholar]
- 19.Tang Z, Liu SH, Dong SJ, Wang EK. Electrochemical synthesis of Ag nanoparticles on functional carbon surfaces. J Electroanal Chem. 2001;502:146–151. [Google Scholar]
- 20.Jian Z, Xiang Z, Yongchang W. Electrochemical synthesis and fluorescence spectrum properties of silver nanospheres. Mictoelectronic Eng. 2005;77:58–62. [Google Scholar]
- 21.Kim YH, Lee DK, Kang YS. Synthesis and characterization of Ag and Ag–SiO2nanoparticles. Colloids and Surfaces A. 2005:257–258. 273–276. [Google Scholar]
- 22.Bae CH, Nam SH, Park SM. Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Appl Surf Sci. 2002:197–198. 628–634. [Google Scholar]
- 23.Patel K, Kapoor S, Dave DP, Mukherjee T. Synthesis of Pt, Pd, Pt/Ag and Pd/Ag nanoparticles by microwave-polyol method. J Chem Sci. 2005;117:311–316. [Google Scholar]
- 24.Zhang J, Chen P, Sun C, Hu X. Sonochemical synthesis of colloidal silver catalysts for reduction of complexing silver in DTR system. Appl Catal A. 2004;266:49–54. [Google Scholar]
- 25.Chaudhari VR, Haram SK, Kulshreshtha SK. Micelle assisted morphological evaluation of silver nanoparticles. Colloids and Surf A. 2007;301:475–480. [Google Scholar]
- 26.Pal A, Shah S, Devi S. Synthesis of Au, Ag, and Au-Ag alloy nanoparticles in aqueous polymer solution. Colloids Surf A Physicochem Eng Asp. 2007;302:51–57. [Google Scholar]
- 27.Chen Z, Gao L. A facile and novel way for the synthesis of nearly monodisperse silver nanoparticles. Mater Res Bull. 2007;42:1657–1661. [Google Scholar]
- 28.Kumar A, Joshi H, Pasricha R, Mandale AB, Sastry M. Phase transfer of silver nanoparticles from aqueous organic solution using fatty amine molecules. J Colloid Interface Sci. 2003;264:396–401. doi: 10.1016/S0021-9797(03)00567-8. [DOI] [PubMed] [Google Scholar]
- 29.Li DG, Chen SH, Zhao SY, Hou XM, Ma HY, Yang XG. Simple method for preparation of cubic Ag 8 nanoparticles and their self-assembled films. Thin Solid Films. 2004;460:78–82. [Google Scholar]
- 30.Kim M, Byun JW, Shin DS, Lee YS. Spontaneous formation of silver nanoparticles on polymeric supports. Mater Res Bull. 2009;44:334–338. [Google Scholar]
- 31.Al-Thabaiti SA, Al-Nawaiser FM, Obaid AY, Al-Youbi AO, Khan Z. Formation and characterization of surfactant stabilized silver nanoparticles: a kinetic study. Colloids Surf B Biointerfaces. 2008;67:230–237. doi: 10.1016/j.colsurfb.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Khan Z, Al-Thabaiti SA, El-Mossalamy EH, Obaid AY. Studies on the kinetics of growth of silver nanoparticles in different surfactant solutions. Colloids Surf B Biointerfaces. 2009;73:284–288. doi: 10.1016/j.colsurfb.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Won H, II, Nersisyan H, Won CW, Lee J-M, Hwang J-S. Preparation of porous silver particles using ammonium formate and its formation mechanism. Chem Eng J. 2010;156:459–464. [Google Scholar]
- 34.Pastoriza I, Liz-marzan LM. Reduction of silver nanoparticles in DMF. Formation of monolayers and stable colloids. Pure Appl Chem. 2000;72:83–90. [Google Scholar]
- 35.Solomon SD, Bahadory M, Jeyarajasingam AV, Rutkowsky SA, Boritz C. Synthesis and study of silver nanoparticles. J Chem Edu. 2007;84:322–325. [Google Scholar]
- 36.He S, Zhang Y, Guo Z, Gu N. Biological synthesis of gold nanowires using extract of Rhodopseudomonas capsulate. Biotechnol Prog. 2008;24:476–480. doi: 10.1021/bp0703174. [DOI] [PubMed] [Google Scholar]
- 37.Saifuddin N, Wong CW, Nur Yasumira AA. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. Eur J Chem. 2009;6:61–70. [Google Scholar]
- 38.Duran N, Marcato PD, De Souza GIH, Alves OL, Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Biomed Nanotechnol. 2007;3:203–208. doi: 10.1186/1477-3155-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kowshik M, Ashtaputre S, Kharraz S, Vogel WJ, Kulkarni SK, Paknikar KM. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 2003;14:95–100. [Google Scholar]
- 40.Haverkamp RG, Marshall AT. The mechanism of metal nanoparticle formation in plants: limits on accumulation. J Nanoparticle Res. 2009;11:1453–1463. [Google Scholar]
- 41.Shankar SS, Ahmad A, Pasricha R, Khan MI, Kumar R, Sastry M. Immobilization of biogenic gold nanoparticles in thermally evaporated fatty acid and amine thin films. J Colloid Interface Sci. 2004;274:69–75. doi: 10.1016/j.jcis.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Khan Z, Al-Thabaiti SA, Obaid AY, Al-Youbi AO. Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf B Biointerfaces. 2011;82(2):513–517. doi: 10.1016/j.colsurfb.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Hussain JI, Kumar S, Hashmi AA, Khan Z. Silver nanoparticles: preparation, characterization, and kinetics. Adv Mat Lett. 2011;2(3):188–194. [Google Scholar]
- 44.Pandeya SN, Sriram D, Yogeeswari P, Ananthan S. Antituberculous activity of norfloxacin mannich bases with isatin derivatives. Chemotherapy. 2001;47:266–269. doi: 10.1159/000048533. [DOI] [PubMed] [Google Scholar]
- 45.Rastogi N, Kant P, Harrison DA, Sethi R, Tripathi D. Synthesis of 1-aminoethyl-5-substituted-3-[4′(2″-chlorobenzylloxy) benzylhydrazono]indolin-2-ones as antifungal agents. Indian J Heterocycl Chem. 2009;18:263–266. [Google Scholar]
- 46.Pandeya SN, Smitha S, Jyoti M, Sridhar SK. Biological activities of isatin and its derivatives. Acta Pharm. 2005;55:27–46. [PubMed] [Google Scholar]
- 47.Jarrahpour A, Khalili D, Clereq ED, Salmi C, Brunel JM. Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules. 2007;12(8):1720–1730. doi: 10.3390/12081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar N, Sharma PK, Singh P. Synthesis and anticonvulsant activity of novel substituted phenyl indoloimidazole derivatives. Current Res Chem. 2011;3(2):114–120. [Google Scholar]
- 49.Verma M, Pandeya SN, Singh KN, Stables JP. Anticonvulsant activity of schiff bases of isatin derivatives. Acta Pharm. 2004;54:49–56. [PubMed] [Google Scholar]
- 50.Smitha S, Pandeya SN, Stables JP, Ganpathy S. Anticonvulsant and sedative-hypnotic activities of N-acetyl/methyl isatin derivatives. Sci Pharm. 2008;76:621–636. [Google Scholar]
- 51.Nagarajan G, Balasubramaniam V, Manjunath KS, et al. Synthesis and in vitro cytotoxic activity of isatin derivatives. Asian J Chem. 2008;30(3):1665–1669. [Google Scholar]
- 52.Thanh ND, Giang NTK. Reaction of N-alkylisatin 4-(2,3,4,6-tetra-O-B-D-glucopyranosyl) thiosemicarbazide) J Chem. 2013;2013:1–5. [Google Scholar]
- 53.Perez C, Pauli M, Bazevque P. An antibiotic assay by the agar well diffusion method. Acta Biologiae et Medicinal Experimentalis. 1990;15:113–115. [Google Scholar]
- 54.Elavazhagan T, Arunachalam KD. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomedicine. 2011;6:1265–1278. doi: 10.2147/IJN.S18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Logeswari P, Silambarasan S, Abraham J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J Saudi Chem Soc. 2012 May 1; Epub. [Google Scholar]
- 56.Narayanan KB, Sakthivel N. Facile green synthesis of gold nanostructures by NADPH-dependent enzyme from the extract of Sclerotium rolfsii. Colloids Surf A Physicochem Eng Asp. 2011;380:156–161. [Google Scholar]
- 57.Liu C, Yang X, Yuan H, Zhou ZD. Preparation of silver nanoparticle and its application to the determination of ct-DNA. Sensors. 2007;7:708–718. [Google Scholar]
- 58.Lanje AS, Sharma SJ, Pode RB. Synthesis of silver nanoparticles: a safe alternative to conventional antimicrobial and antibacterial agents. J Chem Pharm Res. 2010;2(3):478–483. [Google Scholar]
- 59.Raja K, Saravanakumar A, Vijayakumar R. Efficient synthesis of silver nanoparticles from Prosopis juliflora leaf extract and its antimicrobial activity using sewage. Spectrochim Acta A Mol Biomol Spectrosc. 2012;97:490–494. doi: 10.1016/j.saa.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 60.Doiron K, Pelletier E, Lemarchand K. Impact of polymer-coated silver nanoparticles on marine microbial communities: a microcosm study. Aquatic Toxicol. 2012:124–125. 22–27. doi: 10.1016/j.aquatox.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Maio A-J, Luo Z, Chen C-S, Chin W-C, Santschi PH, Quigg A. Intracellular uptake: a possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS One. 2010:e15196. doi: 10.1371/journal.pone.0015196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi O, Deng KK, Kim N-J, Ross L, Jr, Surampalli RY, Hu Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008;42:3066–3074. doi: 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 63.Kloepfer JA, Mielke RE, Nadeua JL. Uptake of CdSe and CdSe/CnS quantum dots into bacteria via purine-dependent mechanisms. Appl Environ Microbiol. 2005;71:2548–2557. doi: 10.1128/AEM.71.5.2548-2557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon K-Y, Byeon JH, Park J-H, Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ. 2007;373:572–575. doi: 10.1016/j.scitotenv.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Kim JS, Kuk E, Yu K, et al. Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol Biol Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Rawani A, Chosh A, Chandra G. Mosquito larvicidal and antimicrobial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of Solanum nigrum L. (Solanaceae: Solanales) Acta Tropica. 2013;128:613–622. doi: 10.1016/j.actatropica.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Du H, Lo T-M, Sitompul J, Chang MW. Systems-level analysis of Escherichia coli response to silver nanoparticles: the roles of anaerobic respiration in microbial resistance. Biochem Biophys Res Commun. 2012;424:657–662. doi: 10.1016/j.bbrc.2012.06.134. [DOI] [PubMed] [Google Scholar]