Abstract
Purpose
Non-CML myeloproliferative neoplasms (MPN) include essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF). Reported median overall survival (OS) ranges from a few to several years for MF, a decade or more for ET and PV. The study objective was to compare US survival rates of ET, PV, and MF patients with matched non-MPN/non-cancer controls in a nationally representative database.
Patients and Methods
Data were taken retrospectively from the Survey, Epidemiology, and End Results (SEER)-Medicare linked database. Medicare enrollees with a new SEER MPN diagnosis between Jan 1, 2001 and Dec 31, 2007 were eligible. First MPN diagnosis was required at or after Medicare enrollment to allow for continuous follow-up. Non-MPN/non-cancer control groups were selected from Medicare separately for each MPN subtype and demographically matched to cases at a ratio of 5∶1. Survival was determined starting from the case diagnosis date using the Kaplan-Meier method.
Results
A total of 3,364 MPN patients (n = 1,217 ET; 1,625 PV; 522 MF) met the inclusion criteria and were matched to controls. Mean age was 78.4, 76.1, and 77.4 years for ET, PV, and MF, respectively, and percent female was 63, 50, and 41. Median OS was significantly (p<0.05) lower for MPN cases vs. controls (ET: 68 vs. 101 months; PV: 65 vs. 104; MF: 24 vs. 106).
Conclusions
In the US Medicare population, survival in MF patients was worse than that of patients with ET or PV and significantly worse than matched controls. Survival of patients with ET or PV was substantially inferior to matched controls. These findings have implications for the clinical management of MPN patients and underscore the need for effective therapies in all MPN subtypes.
Introduction
Myeloproliferative neoplasms (MPNs) are a group of hematologic malignancies characterized by the clonal or oligoclonal proliferation of one or more myeloid lineages that arise from a polyclonal stem cell pool. The World Health Organization classification of MPNs [1] includes chronic myelogenous leukemia, polycythemia vera (PV), essential thrombocythemia (ET), myelofibrosis (MF), chronic neutrophilic leukemia, chronic eosinophilic leukemia not otherwise specified, mastocytosis, and MPNs not otherwise specified (MPN-NOS). The non-chronic myeloid leukemia (CML) MPNs ET, PV, and MF are characterized by activation of JAK2 signaling and abnormal blood cell production [2]–[4]. MPNs are rare with an incidence ranging from approximately 0.5 to 3 per 100,000 persons depending on the subtype and geography [5]–[8].
The non-CML MPNs are distinguished from one another on the basis of effects on cell lineages and the involvement of fibrosis in the bone marrow compartment [1]. PV is characterized by an increase in red blood cell production, or red cell mass (RCM), occurring independently of normal regulatory mechanisms. In PV, increases in the RBC mass may occur in concert with an increase in platelet numbers as well. Major diagnostic criteria for PV include increases in hemoglobin (>18.5 g/dl in males, >16.5 g/dL in females; or other evidence of increased red cell volume) and presence of JAK2 V617F or similar mutation [9]. While not included in the current WHO diagnostic criteria, an elevated RCM may assist in identifying PV patients who do not meet the defined elevations in hemoglobin or hematocrit values [10]. In contrast, ET involves overproduction of platelets in the absence of an increased red blood cell mass [11], [12]. MF is characterized by a progressive evolution or worsening of bone marrow fibrosis and abnormal blood cell production, with many patients initially demonstrating hypercellularity, but changes in hematopoiesis can lead to the development of a hypocellular state later in the evolution of the disease [13].
Survival rates of non-CML MPN patients, by subtype, from a nation-wide US population have not been recently described. MPNs are more prevalent in the elderly, and therefore Medicare enrollees are a highly relevant source for US-based survival estimates in these diseases. To address this knowledge gap, this study compared survival rates of Medicare enrollees diagnosed with MPNs (ET, PV, and MF) with matched non-MPN/non-cancer controls.
Patients and Methods
Data Source
Retrospective data were taken from the Survey, Epidemiology, and End Results (SEER)-Medicare linked database in the US, which combines clinical information from the SEER cancer registry (MPN reporting has been required since 2001) with medical and pharmacy claims for Medicare Part A and B enrollees. SEER areas have been shown to be nationally representative [14] and capture approximately one-quarter of the total US population [15]. For each incident cancer diagnosis reported in SEER, patient-level information is captured on demographics, date of diagnosis, clinical data about the malignancy (e.g., histology, morphology, topography, stage, grade), specific International Classification of Diseases for Oncology, Third Revision (ICD-O-3) codes, and survival. Medicare claims are currently linked through 2009 for Medicare-enrolled SEER patients with an incident cancer diagnosis between 1991 and 2007.
The research presented in this report was conducted with Institutional Review Board (IRB) approval in accordance with the Helsinki Declaration on the protection of human subjects. The research organization conducting this study, RTI Health Solutions, a business unit of RTI International, holds a Federal-Wide Assurance (FWA) from the US Department of Health and Human Services (DHHS) Office for Human Research Protections (OHRP) that allows for the review and approval of human subjects protocols through internal IRB committees. The ethics committee, Research Triangle Institute Committee for the Protection of Human Subjects (FWA #3331), reviewed this study to ensure adherence to appropriate regulations that govern human subjects research, including 45 CFR 46, 21 CFR 50 and 56, and all applicable International Conference on Harmonization provisions, including the Helsinki Declaration.
Because no new data were collected on human subjects (i.e., all data were retrospective, de-identified, and non-interventional), the study was exempted from patient consent requirements and was approved for conduct by the authorized IRB. Furthermore, this research followed rules of the SEER-Medicare Data Use Agreement (DUA) for external investigators that require suppression or combining of results fields comprising fewer than 11 patients in order to further ensure patient anonymity.
Patient Cohort
Medicare enrollees with a new SEER MPN diagnosis between January 1, 2001 and December 31, 2007 were selected and followed on survival in the linked Medicare claims from first MPN diagnosis date (as reported in SEER) until death or end of follow-up in the linked claims data (December 31, 2009). Patients were classified by MPN subtype based on the ICD-O-3 code recorded in SEER (9962/3 for ET, 9950/3 for PV, 9961/3 for MF). First MPN diagnosis date was required to occur on or after first Medicare enrollment to allow for continuous follow-up until death or censoring at the end of the database. Thus, patients with an MPN diagnosis prior to their Medicare enrollment date were excluded. Non-MPN/non-cancer control groups were selected from the national 5% Medicare sample for each MPN subtype and matched to cases 5∶1 based on year of birth, gender, race, geographic location, and reason for Medicare eligibility (age or disability). Patients were excluded if reason for Medicare eligibility was end-stage renal disease (Figure S1).
Survival Analyses
Survival for the matching controls was assessed from the diagnosis date of their respective match until death or until censoring at the end of the database. Survival was descriptively estimated and compared for MPN cases and controls using the Kaplan-Meier method. Separate Cox proportional hazards models were fit to each of the three diseased cohorts to investigate the prognostic impact of age group, gender, race and reason for Medicare eligibility on survival. Finally, using life table methods, the proportion of patients in each MPN subtype surviving at least 1, 3, 5, and 7 years after diagnosis was estimated and reported. All survival analyses were conducted in SAS (Version 9.3, Cary, NC) statistical software using the LIFETEST procedure.
Results
A total of 3,364 MPN patients (n = 1,217 ET, 1,625 PV, 522 MF) were identified for inclusion and assigned matching controls (Table 1). Mean [SD] age was 78.4 [8.2], 76.1 [10.5], and 77.4 [7.9] years for ET, PV, and MF, respectively, while percent female was 62.5, 50.3, and 41.4. Disability was the Medicare eligibility reason for 10% of ET, 18% of PV, and 12% of MF patients. Non-white ethnicity was 13% of ET, 11% of PV, and 10% of MF patients.
Table 1. Patient Characteristics, by MPN Subtype.
| ET (n = 1,217) | PV (n = 1,625) | MF (n = 522) | ||||
| N | % | N | % | N | % | |
| Age at MPN Diagnosis | ||||||
| ≤64 years | 62 | 5.1 | 184 | 11.3 | 22 | 4.2 |
| 65–74 years | 305 | 25.1 | 442 | 27.2 | 163 | 31.2 |
| 75–84 years | 608 | 50.0 | 730 | 44.9 | 271 | 51.9 |
| ≥85 years | 242 | 19.9 | 269 | 16.6 | 66 | 12.6 |
| Mean [SD] Age | 78.4 [8.2] | 76.1 [10.5] | 77.4 [7.9] | |||
| Gender | ||||||
| Male | 457 | 37.6 | 808 | 49.7 | 306 | 58.6 |
| Female | 760 | 62.5 | 817 | 50.3 | 216 | 41.4 |
| Race | ||||||
| White | 1059 | 87.0 | 1450 | 89.2 | 470 | 90.0 |
| Black | 96 | 7.9 | 92 | 5.7 | 31 | 5.9 |
| Other* | 19 | 1.6 | 23 | 1.4 | 21 | 4.0 |
| Asian | 29 | 2.4 | 42 | 2.6 | — | — |
| Hispanic | 14 | 1.2 | 18 | 1.1 | — | — |
| Reason for Medicare Eligibility | ||||||
| Age | 1095 | 90.0 | 1326 | 81.6 | 459 | 87.9 |
| Disability | 122 | 10.0 | 299 | 18.4 | 63 | 12.1 |
* Per SEER-Medicare privacy rules, cell sizes <11 have been supressed, requiring collapsed reporting of “Other” race as follows: ET: “Other” includes North American Native and Other race/ethnicity; PV: “Other” includes North American Native and Other race/ethnicity; MF: “Other” includes Asian, Hispanic, North American Native, and Other race/ethnicity.
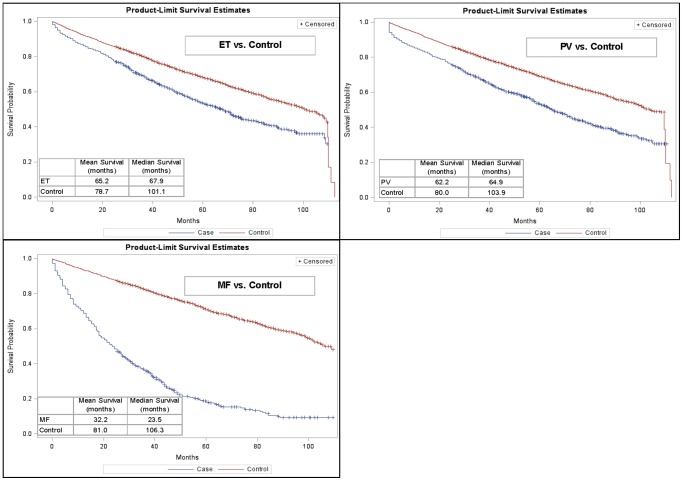
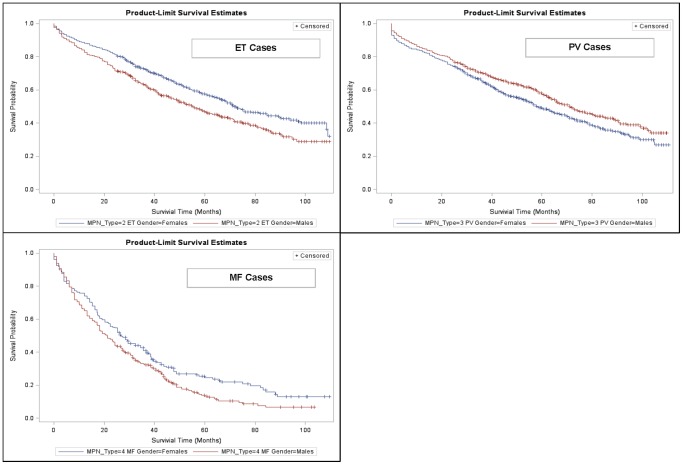
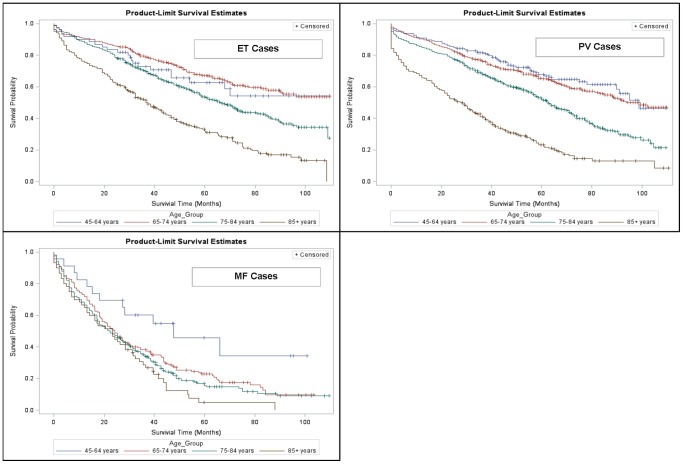
Median overall survival was significantly (p<0.05) lower for MPN cases vs. controls (ET: 68 vs. 101 months; PV: 65 vs. 104 months; MF: 24 vs. 106 months) (Figure 1). These results were also reflected in the 1-, 3-, 5- and 7-year survival rates presented in Table 2. The 7-year survival rate for MF patients, for example, was only 11% compared with 42% for patients with ET. Females with ET and MF appeared to have better survival than males with these MPN subtypes (Figure 2). Females with PV, however, were found to have a survival disadvantage relative to males with PV. Increasing age was generally found to be inversely associated with survival, except for patients with ET in whom younger patients (age 45–64 years) had worse survival than patients 65–74 years of age (Figure 3). Various factors, including age group, gender, region and race were significant in the Cox proportional hazard models with the most salient by far being advanced age (Table 3).
Figure 1. Kaplan-Meier Survival Estimates, by MPN Subtype.
ET = essential thrombocythemia, PV = polycythemia vera, MF = myelofibrosis.
Table 2. 1-, 3-, 5- and 7-Year Survival Rates Post-Diagnosis, by MPN Subtype.
| Survival Time | ET (n = 1,217) | PV (n = 1,625) | MF (n = 522) |
| 1 year | 86.9% | 84.6% | 70.3% |
| 3 years | 69.1% | 67.9% | 36.0% |
| 5 years | 53.2% | 53.1% | 18.2% |
| 7 years | 42.0% | 40.2% | 10.7% |
ET = essential thrombocythemia, PV = polycythemia vera, MF = myelofibrosis,
Figure 2. Kaplan-Meier Survival Estimates, by MPN Subtype and Gender.
ET = essential thrombocythemia, PV = polycythemia vera, MF = myelofibrosis.
Figure 3. Kaplan-Meier Survival Estimates, by MPN Subtype and Age Group.
ET = essential thrombocythemia, PV = polycythemia vera, MF = myelofibrosis.
Table 3. Cox Proportional Hazard Regression Results by MPN Type.
| Table 3a: Cox Proportional Hazard Regression Results: MPN type - ET | ||||
| Hazard ratio | 95% CI | P Value | ||
| Age Groups (Ref: 45–64 years) | ||||
| 65–74 years | 1.00 | 0.57 | 1.73 | 0.986 |
| 75–84 years | 1.68 | 0.97 | 2.90 | 0.065 |
| ≥85 years | 3.21 | 1.85 | 5.57 | <.0001 |
| Gender (Ref: Females) | ||||
| Males | 1.43 | 1.21 | 1.68 | <.0001 |
| Race (Ref: White) | ||||
| Black | 1.63 | 1.23 | 2.15 | 0.001 |
| Other | 0.88 | 0.59 | 1.32 | 0.545 |
| Region (Ref: West) | ||||
| Northeast | 1.13 | 0.90 | 1.40 | 0.291 |
| Midwest | 0.91 | 0.73 | 1.13 | 0.389 |
| South | 1.16 | 0.91 | 1.47 | 0.248 |
| Reason for Medicare Eligibility (Ref: Normal enrollment) | ||||
| Disability | 1.14 | 0.80 | 1.62 | 0.467 |
Discussion
Table 4 briefly summarizes available studies on survival in MPN patients. Studies describing survival in US patients with MPNs are limited, and the few available feature small samples [16]–[18] or limited follow-up time [19]. Prior to 2012, only a limited number of observational studies on MPN survival were published from international populations. In Europe, country-specific studies have examined MPN survival in Spain [20], Italy [21], France [5], and Sweden [22]. A few pan-European studies also reported MPN survival [23], [24]. Additionally, one non-Caucasian report provided insight into the Chinese MPN population survival trends [25]. Lastly, potential reasons for survival disparity can also include limited geographic scope, referral bias, socio-economic differences, specificity for MPN (a single general category or by subtype), age and recency of data, all-comers in the US vs Medicare-only, treatment heterogeneity, inclusion of matched controls, study duration (one vs. several years) and oversampling for minorities.
Table 4. Summary of Available MPN Survival Estimates.
| MPN Subtype | Author | Source | Population | Median OS (Years) | OS/RS/RSR |
| All MPN | D Rollison et al | Blood 2008; 112:45–52 | 3916 from US cancer registries | — | 80% OS at 3 yrs |
| ET | M Hultcrantz et al | JCO 2012; 30(24):2995–3001 | 2559 pts from SW 1973–2008 | — | .68 RSR at 10 yrs |
| ET | Maynadie M et al | Haematologica 2012; Sept 14 (epub) | 1230 pts in 48 registries in 20 EU countries | — | 89.9% RS at 5 yrs |
| ET | Maynadie M et al | Haematologica 2011;96(1):55–61 | 229 pts from Cote d'Or in France | — | 60% OS at 10 yrs |
| ET | Mesa RA et al | Am J Hemat 1999;61:10–15 | 39 pts from Olmstead County, MN | 10.8 | — |
| ET | Passamonti F et al | Am J Med 2004;117:755–761 | 435 pts from two general hosp in IT | 22.6 | — |
| ET | S Malak S et al | Blood Cells Mol Dis 2012;49(3–4):170–6 | 105 pts from FR, BE 1998–2010 | — | 83% OS at 10 yrs |
| ET | Wolanskyj AP et al | Mayo Clin Proc 2006; 81(2):159–166 | 322 pts seen at Mayo Clinic in MN | 18.9 | — |
| PV | Ania BJ et al | Am J Hemat 1994;47(2):89–93 | 50 pts from Olmstead County, MN | 7.2 | — |
| PV | M Hultcrantz et al | JCO 2012; 30(24):2995–3001 | 4389 pts from SW 1973–2008 | — | .64 RSR at 10 yrs |
| PV | Maynadie M et al | Haematologica 2012; Sept 14 (epub) | 1382 pts in 48 registries in 20 EU countries | — | 84.8% RS at 5 yrs |
| PV | Maynadie M et al | Haematologica 2011;96(1):55–61 | 116 pts from Cote d'Or in France | — | 56% OS at 10 yrs |
| PV | Passamonti F et al | Am J Med 2004;117:755–761 | 396 pts from two general hosp in IT | 20 | — |
| PV | S Malak S et al | Blood Cells Mol Dis 2012;49(3–4):170–6 | 97 pts from FR, BE 1998–2010 | — | 83% OS at 10 yrs |
| MF | Cervantes F et al | JCO 2012;30(24):2981–7 | 434 pts from FR, IT, SP, UK | 4.6 | — |
| MF | Cervantes F et al | JCO 2012;30(24):2981–7 | 368 pts from FR, IT, SP, UK | 6.5 | — |
| MF | M Hultcrantz et al | JCO 2012; 30(24):2995–3001 | 1048 pts from SW 1973–2008 | — | .21 RSR at 10 yrs |
| MF | S Malak S et al | Blood Cells Mol Dis 2012;49(3–4):170–6 | 14 pts from FR, BE 1998–2010 | — | 46% OS at 10 yrs |
| MF | Xu Z et al | Blood 2012; 119(11):2469–73 | 642 pts from a single hospital in China | 6.6 | — |
| MF | Mesa RA et al | Am J Hemat 1999;61:10–15 | 21 pts from Olmstead County, MN | 3 | — |
| MF | Maynadie M et al | Haematologica 2012; Sept 14 (epub) | 249 pts in 48 registries in 20 EU countries | — | 34.6% RS at 5 yrs |
| MF | Maynadie M et al | Haematologica 2011;96(1):55–61 | 43 pts from Cote d'Or in France | — | 21% OS at 10 yrs |
| MPN-NOS | Maynadie M et al | Haematologica 2012; Sept 14 (epub) | 1311 pts in 48 registries in 20 EU countries | — | 55.3% RS at 5 yrs |
| MPN-NOS | M Hultcrantz et al | JCO 2012; 30(24):2995–3001 | 1388 pts from SW 1973–2008 | — | .49 RSR at 10 yrs |
| MPN-NOS | Maynadie M et al | Haematologica 2011;96(1):55–61 | 25 pts from Cote d'Or in France | — | 25% OS at 10 yrs |
ET = essential thrombocythemia, MF = myelofibrosis, MPN-NOS = myeloproliferative disorder not otherwise specified, OS = overall survival, pts = patients, PV = polycythemia vera, RSR = relative survival rate.
Across most of the available studies, patients with MF were consistently reported to have a substantially reduced life expectancy, while patients with PV or ET were generally observed to have a good prognosis with only a slight reduction or no change in expected survival. In one of the studies, however, survival in ET patients was observed to be similar to controls only in the first decade after diagnosis and then significantly worsened thereafter [18]. A recent study in Sweden reported substantially reduced survival in all MPN subtypes over four calendar periods compared to the general population, while noting that survival improved in PV and ET patients after 1993 [22].
In our large population-based study including more than 3,300 Medicare enrollees with MPNs diagnosed between 2001 and 2007, survival in MF patients was significantly worse than that of patients with ET or PV. This finding is consistent with the previously summarized studies comparing survival in MPN patients versus non-MPN patients. Also consistent with findings by Hulcrantz et al. (2012) in a Swedish population [22], and contrary to previous reports that ET and PV patient's experience near-normal life expectancy, survival of patients with ET or PV was substantially inferior to matched controls in the Medicare population examined here.
Previous studies suggest that survival in MPN patients can be influenced by several factors. Consistent with many other cancer types, increased age is associated with decreased survival in MPNs [19], [22]–[24], while female gender is associated with improved survival [22]. MPN subtype influences survival, with PV and ET generally showing significantly longer survival that MF [22], [26]. JAK2 V617F mutation status may also impact survival [27], but this factor is rarely examined or captured outside of clinical trial settings, particularly for PV and ET patients. Geography and ethnicity can also impact survival. Maynadie et al. (2012) reported regional survival differences between Northern Europe and Eastern Europe of 74% and 27%, respectively [23]. Xu et al. (2012) compared MPN patients in China with literature reports based primarily on Caucasian populations, finding that Chinese MPN patients were significantly younger, with fewer having palpable spleens or constitutional symptoms, and had significantly better survival as compared with Caucasian MPN patients [25].
Our survival findings based on age group were consistent with these prior reports. Interestingly, ET and PV patients 85 years of age or older in our study were well-separated from the younger three cohorts in terms of lower survival. However, in the MF group, the youngest cohort (45–64) separated with far better survival from the older three age groups, which had relatively similar survival. Improved survival based on female gender was mostly consistent with prior MPN research, with one exception for PV males, who had better survival than PV females. Compared to white patients with PV and ET, black patients in both subtypes experienced a statistically significant higher risk of death, possibly related to health care access challenges for patients with longer term MPN disease. Across the three MPN subtypes, approximately half of the MPN patients were eligible for the JAK2 V617F testing based on their diagnosis date, but very few results for this test were recorded in SEER. The reasons for this lack of data are unclear and preclude further survival analysis based on JAK2 V617F status. Disease associated risk factors such as age greater than 65 years, hemoglobin level, white blood cell count, peripheral blood blasts, constitutional symptoms, and abnormal karyotypes have been shown to have predictive value for assessment of survival using the Dynamic International Prognostic Scoring System for Primary Myelofibrosis [28], [29]. Except for age, the information was not available so that analysis based on risk was not conducted.
Our study was subject to several limitations. First, our study was limited to enrollees in the US Medicare system, which consists primarily of older patients. Thus, our findings may not be generalizable to other populations, including those enrolled in commercial managed care plans or patients in other public payer systems such as Medicaid. Second, although MPN incidence is highest in elderly persons, our Medicare sample was not representative of the true age distribution of all MPN cases in the US, and was specifically subject to underrepresentation of younger patients. Third, MPN patients in our study were required to have been first diagnosed with an MPN on or after Medicare entry. This inclusion criterion ensured that all patients could be followed continuously until death or censoring, but it resulted in the exclusion of some patients diagnosed with an MPN at younger ages who survived until Medicare entry. Our study sample may therefore be older than the general Medicare population living with these diseases. Lastly, while the SEER cancer registry is considered an authoritative source and system for the reporting of both solid tumor and hematologic malignancies in the US, recent evidence suggests that MPNs may be underreported in US cancer registries [30]. It is unknown whether and to what extent MPN cases captured by SEER may differ from unreported cases.
Despite these limitations, our study provides new data on expected survival in Medicare enrollees with MPNs and supports new findings in a recent study of Swedish MPN patients that ET and PV do not carry as favorable a prognosis as once thought. These findings affirm a changing thought paradigm that recognizes all MPN subtypes as diseases that reduce life expectancy. Our findings may therefore have implications for the clinical management of MPN patients and underscore the need for improved therapies of all MPN subtypes. Further research is needed to not only assess MPN survival in a more generalized US population over a longer period of time, but to also formally examine potential patient and clinical factors that affect survival. Such information may further aid clinicians in the provision of optimal care for MPN patients and in the development of more effective therapies for all MPN subtypes.
Supporting Information
Sample Attrition. ET = essential thrombocythemia, PV = polycythemia vera, MF = myelofibrosis, HMO = health maintenance organization, *MPN-NOS = 1,664 patients were identified in the study time period with a new diagnosis of MPN-NOS (myeloproliferative neoplasm-not otherwise specified) and excluded from this report.
(PDF)
Funding Statement
The authors have no support or funding to report.
References
- 1. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, et al. (2011) The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 117: 5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, et al. (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352: 1779–1790. [DOI] [PubMed] [Google Scholar]
- 3. Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, et al. (2005) Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7: 387–397. [DOI] [PubMed] [Google Scholar]
- 4. James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, et al. (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434: 1144–1148. [DOI] [PubMed] [Google Scholar]
- 5. Maynadié M, Girodon F, Manivet-Janoray I, Mounier M, Mugneret F, et al. (2011) Twenty-five years of epidemiological recording on myeloid malignancies: Data from the specialized registry of hematologic malignancies of Cote d'Or (Burgundy, France). Haematologica 96: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leukemia and Lymphoma Society. Disease Information and Support: Myeloproliferative Neoplasms. Available: http://www.lls.org/#/diseaseinformation/myeloproliferativediseases/.
- 7.Leukemia and Lymphoma Society. Disease Information and Support: Myeloproliferative Neoplasms, Incidence. Available: http://www.lls.org/#/diseaseinformation/myeloproliferativediseases/incidence/.
- 8. Visser O, Trama A, Maynadié M, Stiller C, Marcos-Gragera R, et al. (2012) Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer 48: 3257–3266. [DOI] [PubMed] [Google Scholar]
- 9.Steven H. Swerdlow, International Agency for Research on Cancer, World Health Organization. (2008) WHO Classification of Tumours of Haematopoietic and Lymphatic Tissues, 4th ed. International Agency for Research on Cancer. Page 40–41. [Google Scholar]
- 10. Silver RT, Chow W, Orazi A, Arles SP, Goldsmith SJ (2013) Evaluation of WHO criteria for diagnosis of polycythemia vera: a prospective analysis. Blood 122: 1881–1886. [DOI] [PubMed] [Google Scholar]
- 11. Thiele J, Kvasnicka HM, Diehl V, Fischer R, Michiels J (1999) Clinicopathological diagnosis and differential criteria of thrombocythemias in various myeloproliferative disorders by histopathology, histochemistry and immunostaining from bone marrow biopsies. Leuk Lymphoma 33: 207–218. [DOI] [PubMed] [Google Scholar]
- 12. Murray J (2005) Myeloproliferative disorders. Clin Med 5: 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koopmans SM, van Marion AM, Schouten HC (2012) Myeloproliferative neoplasia: a review of clinical criteria and treatment. Neth J Med 70: 159–167. [PubMed] [Google Scholar]
- 14. Nattinger AB, McAuliffe TL, Schapira MM (1997) Generalizability of the surveillance, epidemiology, and end results registry population: factors relevant to epidemiologic and health care research. J Clin Epidemiol 50: 939–45. [DOI] [PubMed] [Google Scholar]
- 15. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF (2002) Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 40 suppl 4: S3–S18. [DOI] [PubMed] [Google Scholar]
- 16. Anía BJ, Suman VJ, Sobell JL, Codd MB, Silverstein MN, et al. (1994) Trends in the incidence of polycythemia vera among Olmsted County, Minnesota residents, 1935–1989. Am J Hematol 47: 89–93. [DOI] [PubMed] [Google Scholar]
- 17. Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A (1999) Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: An Olmsted County study, 1976–1995. Am J Hematol 61: 10–15. [DOI] [PubMed] [Google Scholar]
- 18. Wolanskyj AP, Schwager SM, McClure RF, Larson DR, Tefferi A (2006) Essential thrombocythemia beyond the first decade: Life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 81: 159–166. [DOI] [PubMed] [Google Scholar]
- 19. Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, et al. (2008) Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 112: 45–52. [DOI] [PubMed] [Google Scholar]
- 20. Rozman C, Giralt M, Feliu E, Rubio D, Cortés MT (1991) Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer 67: 2658–2663. [DOI] [PubMed] [Google Scholar]
- 21. Palandri F, Catani L, Testoni N, Ottaviani E, Polverelli N, et al. (2009) Longterm follow-up of 386 consecutive patients with essential thrombocythemia: Safety of cytoreductive therapy. Am J Hematol 84: 215–220. [DOI] [PubMed] [Google Scholar]
- 22. Hultcrantz M, Kristinsson SY, Andersson TM, Landgren O, Eloranta S, et al. (2012) Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 30: 2995–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maynadié M, De Angelis R, Marcos-Gragera R, Visser O, Allemani C, et al. (2013) Survival of European patients diagnosed with myeloid malignancies: a HAEMACARE study. Haematologica 98: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cervantes F, Dupriez B, Passamonti F, Vannucchi AM, Morra E, et al. (2012) Improving survival trends in primary myelofibrosis: an international study. J Clin Oncol 30: 2981–2987. [DOI] [PubMed] [Google Scholar]
- 25. Xu Z, Gale RP, Zhang Y, Qin T, Chen H, et al. (2012) Unique features of primary myelofibrosis in Chinese. Blood 119: 2469–2473. [DOI] [PubMed] [Google Scholar]
- 26. Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, et al. (2004) Life Expectancy and Prognostic Factors for Survival inpatients with Polycythemia Vera and Essential Thrombocythemia. Am J Med 117: 755–761. [DOI] [PubMed] [Google Scholar]
- 27. Tefferi A, Lasho TL, Huang J, Finke C, Mesa RA, et al. (2008) Low JAK2V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia 22: 756–761. [DOI] [PubMed] [Google Scholar]
- 28. Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, et al. (2010) A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 115: 1703–8. [DOI] [PubMed] [Google Scholar]
- 29. Gangat N, Caramazza D, Vaidya R, George G, Begna K, et al. (2011) DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 29: 392–7. [DOI] [PubMed] [Google Scholar]
- 30. Craig BM, Rollison DE, List AF, Cogle CR (2012) Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol Biomarkers Prev 21: 474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample Attrition. ET = essential thrombocythemia, PV = polycythemia vera, MF = myelofibrosis, HMO = health maintenance organization, *MPN-NOS = 1,664 patients were identified in the study time period with a new diagnosis of MPN-NOS (myeloproliferative neoplasm-not otherwise specified) and excluded from this report.
(PDF)