Abstract
This review provides an overview on the incorporation of heparin into biomaterials with a focus on drug delivery and the use of heparin-based biomaterials for self-assembly of polymer networks. Heparin conjugation to biomaterials was originally explored to reduce the thrombogenicity of materials in contact with blood. Many of the conjugation strategies that were developed for these applications are still popular today for other applications. More recently heparin has been conjugated to biomaterials for drug delivery applications. Many of the delivery approaches have taken advantage of the ability of heparin to bind to a wide variety of growth factors, protect them from degradation and to potentiate their interactions with cell surface receptors. More recently, the use of heparin as a base polymer for scaffold fabrication has also been explored, often utilizing non-covalent binding of heparin with peptides or proteins to promote self-assembly of hydrogel networks. This review will highlight recent advances in each of these areas.
INTRODUCTION
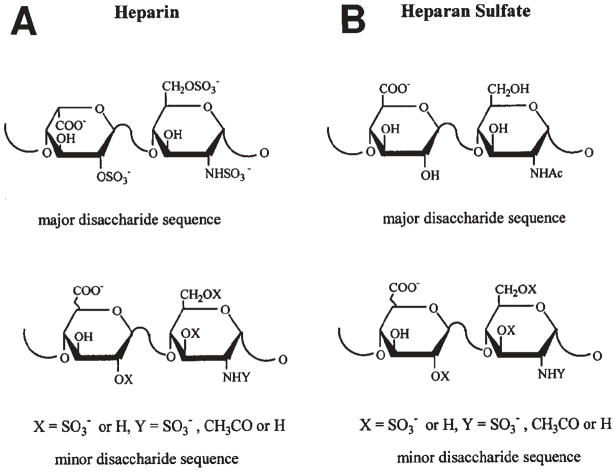
Heparin and heparin sulfate are linear polysaccharides. Both are synthesized from a common precursor proteoglycan. Heparin is only produced in mast cells, where it is cleaved from the core protein (serglycin) at the end of the synthesis [1]. Heparin sulfate (HS) is found in most tissues and remains attached to the core protein. Both are sulfated and also contain carboxylic acids, which contribute to an overall net negative charge [2]. Heparin/HS polymer chains are made up of repeating disaccharides, primarily uronic acid and glucosamine with varying degrees of sulfation and Nacetylation (Figure 1). While their interactions with proteins are largely electrostatic, there are clearly contributions from hydrophobic effects and hydrogen bonding, as well as promoting secondary structure in the proteins binding to heparin, which imparts some selectivity and specificity [3]. In addition to binding to growth factors, heparin also binds to a number of enzymes (e.g. antithrombin III), plasma proteins (platelet factor 4), and extracellular matrix (ECM) proteins (e.g. fibronectin, laminin) [4, 5]. In some cases, specific heparin sulfation codes have been identified that facilitate binding with growth factors (e.g. bFGF) or enzymes (e.g. antithrombin III) [6, 7].
Figure 1.
(A) Structure of the major and minor disaccharide sequences of heparin. (B) Structure of the major and minor disaccharide sequences of heparan sulfate. Reprinted with permission from Ref. [94]
Heparin Modification of Materials to Reduce Thrombogenecity
Heparin was discovered in 1916 and has been used as an anticoagulant clinically since 1935 [1]. Modification of biomaterials with heparin has been performed for over 50 years. Initially, heparin was immobilized via ionic interactions to reduce the thrombogenecity of materials in the 1960’s [8, 9]. This approach took advantage of electrostatic interactions with the negatively charge sulfate groups on heparin with the colloidal graphite and benzalkonium chloride (cation) in alternating layers [8]. Leininger et al. adapted this method for use on plastic surfaces by forming quaternary ammonium sites on the material surface to promote electrostatic interactions with the heparin [10].
In the 80’s, methods for covalent conjugation were developed that used end-point immobilization, in which a primary amine on the material of interest was reacted with an aldehyde group generated by heparin chain depolymerization [11]. The literature on heparin immobilization is vast and has been reviewed extensively elsewhere [12–15]. This end-point immobilization has been use to conjugate heparin to vascular grafts and has been commercialized for ePTFE and Dacron grafts [16, 17]. More recently work with heparin immobilization on vascular grafts has explored mechanisms other than antithrombotic effects that may be influence by heparin, including elastin synthesis [18]. Additional studies explored coating vascular stents with heparin, however more recent studies suggest that this may stimulate restenosis by sequestration of growth factors that promote smooth muscle cell proliferation [19].
HEPARIN MODIFICATION OF MATERIALS FOR DRUG DELIVERY
Many types of drug delivery systems have been developed for the control release of small molecule and protein-based drugs for biomedical applications [20]. For delivery of protein-based drugs, such as growth factors, there are many advantages to the use of affinity drug delivery systems, such as heparin-based delivery systems. These affinity delivery systems utilize specific non-covalent interactions to stabilize drugs and immobilize them within a biomaterial matrix, thus protecting their biological activity and slowing their diffusion from the matrix. The interactions with growth factors and affinity delivery systems can mimic those that naturally occur with native ECM proteoglycans.
Because a large number of growth factors bind to heparin with either moderate or high affinity (~10−6 – 10−9 M KD), heparin-based delivery systems have proven useful for the delivery of a wide range for growth factors for different biomedical applications [21]. In the case of heparin-based systems, these interactions can also modulate the binding of growth factor to the cell surface receptor. For some growth factors, such as basic fibroblast growth factor (bFGF), heparin facilitates this binding of bFGF to its receptor and actually increases activity [22]. However for other growth factors, such as bone morphogenetic protein 2 (BMP2), heparin can inhibit binding to the cell surface receptor [23]. The effect of heparin on BMP2 signaling are complex, as heparin has also been shown to block inhibition by noggin of the BMP2 pathway [24], thus demonstrating that heparin can have direct interactions with the growth factors and their receptors, as well as indirect and sometimes opposing effects on signaling cascades.
Growth factors that bind to heparin include commonly studied heparin-binding growth factors, such as bFGF and vascular endothelial growth factor (VEGF), as well as members of transforming growth factor (TGF, e.g. BMPs), platelet derived growth factor (PDGF), epidermal growth factor (EGF), and hepatocyte growth factor families [25–28]. Other morphogens, such as sonic hedgehog (Shh) and pathogens, such as B. pertussis, herpes simplex virus (HSV) and Plasmodium falciparum, also bind to heparin and can be delivered or sequestered using a similar approach [29, 30].
Covalent conjugation of heparin to biomaterials for delivery
Early work in demonstrating the utility of heparin-based delivery was performed by Edelman and Langer. Their initial system utilized heparin-conjugated Sepharose beads to bind bFGF within alginate microspheres. Their preliminary studies in vitro demonstrated that active bFGF could be released for at least two weeks and heparin enhanced growth factor activity [31]. They went on to demonstrate the delivery of bFGF from heparin-Sepharose bead in alginate stimulated angiogenesis and neointimal proliferation in a rat carotid artery model [32]. Later, a Phase I clinical trial using these materials showed that bFGF improved revascularization after coronary artery bypass in a small number of patients [33]. Additionally, a Phase II trial demonstrated improved revascularization with bFGF treatment and a trend toward increased left ventricular ejection fraction [34]. These studies demonstrate that heparin-based delivery can be used to provide sustained release of growth factors in a clinical model.
Another approach to covalently immobilize heparin to biomaterials was to covalently link it to a protein, such as collagen or albumin, using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) [35]. Heparin was conjugated to albumin using this method and emulsified to form microsphere that could then be covalently crosslinked with glutaraldehyde [36]. A similar approach was used crosslink heparin to collagen matrices for the delivery of bFGF, and bFGF delivery was found to enhance endothelial cell proliferation in vitro [37, 38]. Later in vivo studies demonstrated that bFGF delivery from similar collagen matrices increased vascularization for three weeks in a rat subcutaneous implant model [39, 40].
Similar EDC chemistry can be use to immobilize heparin onto poly(L-lactide-co-glycolide) (PLGA)-based materials. Jeon et al. developed heparin-conjugated PLGA nanospheres by first reacting PLGA with t-Boc protected-glycine and then deprotecting. Nanospheres of PLGA were formed using an oil/water emulsion and reacted with heparin in the presence of EDC/N-hydroxysuccinimide (NHS) [41]. Controlled release of bFGF and increased cell proliferation was observed over 28 days in vitro. They observed increased capillary density with bFGF and PLGA nanospheres versus controls in a mouse ischemic limb model. Similar heparin-conjugation methods have also been used with salt-leached PLGA scaffolds to deliver BMP2 and BMP2 delivery increased bone formation in an ectopic bone formation model compared to PLGA scaffolds without heparin [42].
Coupling via end point reductive amination has also been used to covalently attach heparin to various biomaterials (similar to strategy most commonly used for used for vascular graft coupling). Hyaluronic acid (HA) was modified to contain amine groups by Liu et al. (using periodate and then amine groups were added using ethylene diamine in the presence of cyanoborohydride). Aminated-HA was then reacted this with heparin-aldehyde via reductive amination [43]. Controlled release of bFGF from HA scaffolds via enzymatic degradation of the HA and biological activity of the released bFGF (cell proliferation) was observed in these studies. More recently novel methods for site selective aldehyde modification have been developed that allow modification at specific sites on heparin [44].
Modification of heparin to contain thiol groups is another method for adding an additional reactive functionality to heparin that can reacts with many existing polymer end groups (e.g. acrylates, vinyl sulfones). A method for thiolation of glycosaminoglycans (GAGs), such as HA and heparin was developed by the Prestwich lab [45] that utilizes EDC and dithiothreitol (DTT). The thiolated GAGs (HA and heparin) are then reacted with PEG diacrylate via Michael type addition to generate PEG-HA-heparin hydrogels [46]. The release of growth factor from these gels is dependent on hyaluronidase for degradation of gels containing HA (as part of the GAG component) and bFGF release was sustained for at least 28 days in vitro. Delivery of bFGF from these scaffolds was also found to promote neovascularization in vivo in a mouse subcutaneous implant model.
This approach for use with PEG diacrylate hydrogels in the absence of HA by Tae et al., and they demonstrated that fibroblasts could be encapsulated within these gels [47]. Delivery of human growth hormone (hGH) from hydrogels formed from thiolated heparin and PEG diacrylate by Michael type addition, rather than by photo-polymerization has also been explored [48]. These hydrogels were shown to promote increased vascularization in a subcutaneous mouse implant model when osteoprotegerin, a pro-angiogenic factor from the tumor necrosis factor (TNF) superfamily, was delivered using this method in vivo [49]. This thiolation method also shows great potential for a broad variety of applications, and the thiol chemistry is somewhat more selective due to the relatively low incidence (1–4% frequency) of thiols (cysteines) in protein sequences compared to amine groups [50].
Maleimide groups can also provide another unique functionality to react with polymer end groups for conjugation of heparin to materials. Heparin-containing PEG hydrogels can be made by reacting PEG-tetrathiol with low molecular weight heparin-maleimide. The PEG-heparin conjugates are then reacted with PEG-tetraHIP (heparin interacting peptide) [51] to form gels, and these gels can sequester bFGF. This approach can also be modified to react PEG-dithiol with high molecular weight heparin-maleimide and controlled bFGF can be obtained from these gels as well [52]. This approach can be modified to work well with the thiol-based approaches described above.
Heparin can also be modified by the addition of hydrazide groups to carboxylic acids on heparin for covalent attachment to polymers using a method develop by Bulpitt and Aeschlimann [53]. Tae et al. used this method to reacted hydrazide modified heparin with NHS ester of PEG-bis-butanoic acid (SBA-PEG-SBA) to form hydrogels. They demonstrated sustained VEGF release in vitro and increased angiogenesis in vivo in a subcutaneous mouse implant model [54].
Non-covalent immobilization of heparin for delivery
A novel method for immobilization of heparin within biomaterials was developed by Sakiyama-Elbert and Hubbell, using non-covalent (primarily electrostatic) interactions rather than covalent immobilization [3]. The three-component delivery system consisted of a heparin-binding peptide covalently immobilized to the biomaterial, heparin and a heparin-binding growth factor. To demonstrate the feasibility of this approach, a peptide containing a modified version of the heparin-binding domain of antithrombin III was crosslinked into fibrin matrices using the transglutaminase activity of Factor XIIIa. They demonstrated that bFGF could be released in a controlled manner from this delivery system [55]. They also demonstrated that this approach can also be used with growth factors that possess only moderate heparin binding affinity (KD~10−6) via short basic domains that are accessible in protein surfaces, such as nerve growth factor (NGF), neurotrophin 3 (NT-3) and brain derived neurotrophic factor (BDNF) [56].
Others have shown that this approach can be expanded to other materials, such as polyethylene glycol (PEG) hydrogels, for the delivery of heparin-binding growth factors. Pratt et al. demonstrated that BMP2 can be delivered from plasmin-degradable PEG hydrogels via a heparin-binding peptide/heparin complex and promotes improved bone healing in a rat cranial defect model [57]. This approach has also been used for delivery of PDGF-BB to enhance gliding after mid-substance injury in a canine flexor tendon model [58–60].
The effect of binding site affinity on the rate of release has also been explored. To identify peptide sequences with a broader range of affinities for heparin, a phage display library was screened to identify sequences with varying affinity for heparin [61]. The role of heparin-binding affinity for heparin was evaluated for electrostatic immobilization of heparin, and increasing heparin-binding affinity has been shown to provide a longer duration of release [62]. Recently, Wieduwild et al. explored the effect on sequence/affinity on assembly of peptide-heparin networks and effects on gelation time and mechanical properties [63].
This approach for delivery of heparin-binding growth factors has proven useful in a number of applications. Delivery of NGF and glial derived neurotrophic factor (GDNF) using fibrin scaffolds containing a heparin-binding delivery system enhanced peripheral nerve regeneration in a rat sciatic nerve injury model at both 6 and 12 weeks (see Figure 2) [64, 65]. In spinal cord injury models, delivery of NT-3 was found to promote neural fiber sprouting after acute and sub-acute (2 week delayed) treatment [66, 67].
Figure 2.
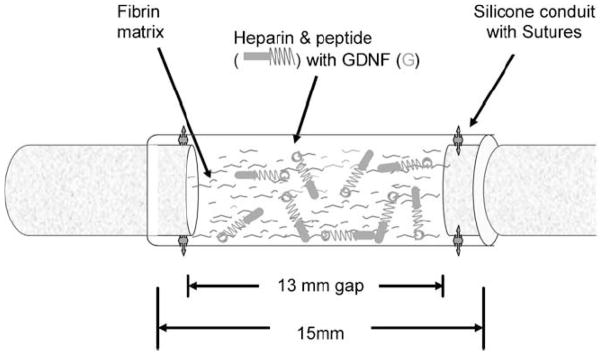
Schematic representation of surgical implantation of nerve guidance conduit containing the affinity-based delivery system. A 13 mm nerve gap was repaired with a 15 mm silicone conduit containing fibrin matrices with or without delivery system and growth factor and sutured to the transected proximal and distal stumps, incorporating 1 mm of nerve on either end. The delivery system consisted of a bi-domain peptide crosslinked into the fibrin matrix at one domain while the other binds heparin by electrostatic interactions. The growth factor can then bind to the bound heparin, creating a matrix-bound, non-diffusible complex, which can be retained for cell-mediated degradation of the fibrin matrix. Reprinted with permission from Ref. [65].
This approach can also be expanded to allow not only delivery of exogenous growth factors, but for use as a method to sequester endogenous growth factors secreted in vitro or in vivo. Hudalla et al. have demonstrated that this approach can be used sequester endogenous growth factors from serum in culture [68] and to direct the differentiation of stem cells on self assembled monolayers [69]. Recently Seif-Naraghi et al. demonstrated that decelluarlized ECM can also serve as a vehicle for sequestration and delivery of endogenous heparin-binding growth factors [70].
HEPARIN MIMETIC POLYMERS FOR DRUG DELIVERY
Due to the heterogeneous structure of heparin, it would be beneficial to develop synthetic analogs that can bind to heparin-binding growth factors and provide more selective synthetic control of structure and thus allow better control of binding affinity. Maynard et al. used sulfated amino acids and combinatorial chemistry to identify artificial peptide sequences that function as heparin mimetics and can bind to heparin-binding growth factors [71]. They found a tetra-peptide sequence of sulfated amino acids that can bind to VEGF with micromolar affinity. This approach can be extended for nanopatterning applications using e-beam lithography to provide patterns of heparin-binding growth factors on a surface [72]. Nguyen et al. extended this work to demonstrate that co-polymers of styrene sulfonate and methyl methacrylate bearing PEG side chains could be used to stabilize bFGF and preserved its activity in the presence of environmental stressors, such as heat and acidic conditions [73]. This approach holds potential for developing sequences that are selective for an individual growth factors and that could potentially be resistant to enzymatic degradation by heparinases.
HEPARIN-MODIFIED MATERIALS TO DIRECT CELL DIFFERENTIATION
In addition to growth factor delivery, heparin-modified materials can also be used to direct cell differentiation of stem cell populations. For example, heparin-based delivery of growth factors (NT-3 and PDGFAA) was used to direct differentiation of embryonic stem cell (ESC)-derived neural progenitor cells in vitro and in vivo after spinal cord injury [74, 75]. Lam et al. used immobilization of bFGF and EGF via heparin on nanofibers to direct neuronal differentiation and axon growth of human ESC-derived neural stem cells in vitro [76]. Heparin-mimicking polymers made of polystyrene sulfonate (PSS) have been shown to promote myogenic differentiation of C2C12 muscle progenitor cells, see Figure 3 [77]. Delivery of bFGF from heparin/peptide amphiphiles was shown to promote survival, insulin secretion from and angiogenesis toward islets [78]. Heparin grafting onto biomaterials (scaffolds composed of polycaprolactone and polyhydroxybuterate) have been shown to increase differentiation of induced pluripotent stem cells (iPSCs) into neuronal cells [79]. In other cases, the use of heparin-mimetic (PSS) surfaces can be used to increase the pluripotency of embryonic stem cell under the appropriate culture conditions [80]. Heparin-based hydrogels can also be used for the expansion of adipose-derived and bone marrow-derived stem cells [81]. In total, these studies suggest that heparin-containing or heparin-mimetic materials can play an important role in modulating the differentiation and pluripotency of stem cells.
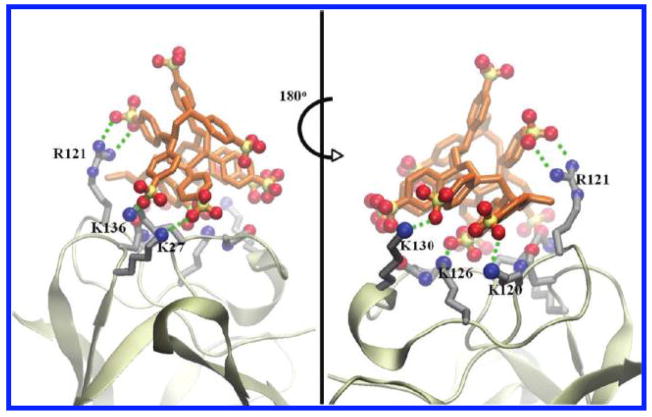
Figure 3.
Front and side views of the most favorable docked configuration of heparin mimetic polymer PSS on bFGF, illustrating salt bridges and hydrogen bonding interactions (green dashed lines) between the PSS and bFGF residues. Reprinted with permission from Ref. [77]. Copyright 2010 American Chemical Society.
HEPARIN-BASED MATERIALS
In addition to modification of materials with heparin covalently or based on affinity, heparin has been used more recently as a base polymer for the formation of hydrogels, and its ability to bind to basic peptides and cationic polymers has been exploited to promote self assembly of gels. Seal and Panitch demonstrated self assembly of PEG hydrogels by coupling heparin-binding peptides to each arm of a 4 arm PEG and mixing with heparin [82]. Zhang et al. built upon this approach and used a 4-arm PEG modified with either heparin-binding peptides from PF4 or low molecular weight heparin (as described above [51]) to form gels that assemble non-covalently and deliver bFGF [83]. Rajangam et al. used basic peptide amphiphiles and heparin to generate self assembling materials that provided controlled delivery of bFGF in vitro and angiogenesis in a rat corneal implant model [84]. This approach has been expanded to generate heparin/HA membranes with peptide amphiphiles to deliver VEGF, and these materials promote angiogenesis in a chick chorioallentoic membrane model [78, 85]. Some caution must be used with this approach, as the hydrogels formed using this method are generally weak mechanically, but may be useful for applications that require materials to gel after injection in the absence of any crosslinking agent or where materials are later crosslinked covalently by enzymes or via photo-polymerization.
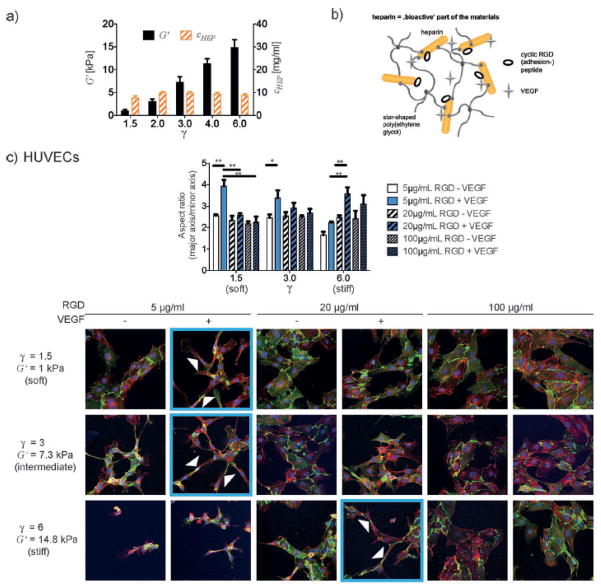
This approach to drug delivery can be expanded to use heparin in combination with another polymer as the base material for the scaffold. Freudenberg used amine-terminated PEG star polymers to form hydrogels for growth factor delivery with heparin using EDC/NHS crosslinking. They also coupled Arginine-Glycine- Aspartic Acid (RGD) peptides to the heparin to enhance cell adhesion to the scaffolds. The addition of bFGF and RGD promoted improved cell survival and differentiation of mesencephalic neural stem cells from embryonic day 13.5 mice [86]. This work has continued on through a number of elegant studies that demonstrate the use of heparin-based materials to design Michael addition crosslinked and enzymatically degradable materials based on heparin-PEG star polymers that also facilitated delivery of growth factors [63, 87–92]. Recently, Freudenberg et al. have also demonstrated an elegant method for using modeling to predict and decouple the biomolecular and mechanical properties of heparin-based materials, see Figure 4 [93].
Figure 4.
a) Independent tuning of mechanical (indicated by the storage modulus) and biochemical (indicated by the constant heparin concentration) properties with varying γ. b) Heparin is the bioactive component of the hydrogel material mediating cell adhesion and provision of growth factors. c) HUVECs elongate to form a network of tube-like structures (arrows indicate cells with a high aspect ratio as a representative example) on starPEG-heparin hydrogels with independently varying VEGF and RGD incorporation and storage modulus. Images shown are confocal immunofluorescence images of CD31 (green, endothelial cell marker), actin (red), and DAPI (blue) of HUVECs plated for 20–24 h and are representative of results from 3 independent experiments. Mean values and standard error of the mean of three experiments in which > 20 cells each is shown (* p < 0.05 (significant), ** p < 0.01 (highly significant), p is the p -value of probability). Reprinted with permission from Ref. [93].
SUMMARY
Heparin modification of biomaterials have used for a broad spectrum of applications, including reducing material thrombogenecity, drug delivery, cell differentiation and promoting material self assembly. Incorporation of heparin into materials can be accomplished either via covalent conjugation or via non-covalent interactions with cationic polymers, including peptides and proteins. Methods for immobilization and conjugation have proven useful across many applications and have yielded a wide array of materials for biomaterial applications. In particular, non-covalent immobilization of heparin-binding growth factors, either exogenous or endogenous, in affinity-based delivery systems has proven high beneficial for many injury models and diseases.
Footnotes
Disclosure of Conflict of Interest:
SSE is an inventor on patents regarding the use of heparin binding drug delivery and these patents are licensed to Kuros Therapeutics. SSE may receive royalties from Kuros from sub-licensing revenue. SSE is not in any way involved in consulting or operations at Kuros.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabenstein DL. Heparin and heparan sulfate: structure and function. Natural Product Reports. 2002;19:312–31. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- 2.Lodish H, Berk A, Kaiser C, Krieger M, Scott M, Bretcher A, et al. Molecular Cell Biology. New York: WH Freeman and Co; 2008. [Google Scholar]
- 3.Sakiyama-Elbert S. Drug Delivery via Heparin Conjugates. In: Ducheyne PKEH, Hutmacher DW, Grainger DW, Kirkpatrick CJ, editors. Comprehensive Biomaterials. Elsevier; 2011. pp. 333–8. [Google Scholar]
- 4.Edgar D, Timpl R, Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984;3:1463–8. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers SL, McCarthy JB, Palm SL, Furcht LT, Letourneau PC. Neuron-specific interactions with two neurite-promoting fragments of fibronectin. J Neurosci. 1985;5:369–78. doi: 10.1523/JNEUROSCI.05-02-00369.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishihara M, Shaklee PN, Yang Z, Liang W, Wei Z, Stack RJ, et al. Structural features in heparin which modulate specific biological activities mediated by basic fibroblast growth factor. Glycobiology. 1994;4:451–8. doi: 10.1093/glycob/4.4.451. [DOI] [PubMed] [Google Scholar]
- 7.Choay J, Petitou M, Lormeau JC, Sinay P, Casu B, Gatti G. Structure-activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Commun. 1983;116:492–9. doi: 10.1016/0006-291x(83)90550-8. [DOI] [PubMed] [Google Scholar]
- 8.Gott VL, Whiffen JD, Dutton RC. Heparin Bonding on Colloidal Graphite Surfaces. Science. 1963;142:1297–8. doi: 10.1126/science.142.3597.1297. [DOI] [PubMed] [Google Scholar]
- 9.Grode GA, Anderson SJ, Grotta HM, Falb RD. Nonthrombogenic materials via a simple coating process. Trans Am Soc Artif Intern Organs. 1969;15:1–6. [PubMed] [Google Scholar]
- 10.Leininger RI, Cooper CW, Falb RD, Grode GA. Nonthrombogenic plastic surfaces. Science. 1966;152:1625–6. doi: 10.1126/science.152.3729.1625. [DOI] [PubMed] [Google Scholar]
- 11.Larm O, Larsson R, Olsson P. A new non-thrombogenic surface prepared by selective covalent binding of heparin via a modified reducing terminal residue. Biomater Med Devices Artif Organs. 1983;11:161–73. doi: 10.3109/10731198309118804. [DOI] [PubMed] [Google Scholar]
- 12.Hubbell JA, Muir WW, Gaynor JS. Cardiovascular effects of thoracic compression in horses subjected to euthanasia. Equine veterinary journal. 1993;25:282–4. doi: 10.1111/j.2042-3306.1993.tb02964.x. [DOI] [PubMed] [Google Scholar]
- 13.Olsson P, Sanchez J, Mollnes TE, Riesenfeld J. On the blood compatibility of endpoint immobilized heparin. J Biomater Sci Polym Ed. 2000;11:1261–73. doi: 10.1163/156856200744192. [DOI] [PubMed] [Google Scholar]
- 14.Tanzi MC. Bioactive technologies for hemocompatibility. Expert review of medical devices. 2005;2:473–92. doi: 10.1586/17434440.2.4.473. [DOI] [PubMed] [Google Scholar]
- 15.Jordan SW, Chaikof EL. Novel thromboresistant materials. Journal of vascular surgery. 2007;45 (Suppl A):A104–15. doi: 10.1016/j.jvs.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 16.Begovac PC, Thomson RC, Fisher JL, Hughson A, Gallhagen A. Improvements in GORE-TEX vascular graft performance by Carmeda BioActive surface heparin immobilization. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2003;25:432–7. doi: 10.1053/ejvs.2002.1909. [DOI] [PubMed] [Google Scholar]
- 17.Devine C, McCollum C. Heparin-bonded Dacron or polytetrafluorethylene for femoropopliteal bypass: five-year results of a prospective randomized multicenter clinical trial. Journal of vascular surgery. 2004;40:924–31. doi: 10.1016/j.jvs.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Saitow C, Kaplan DL, Castellot JJ., Jr Heparin stimulates elastogenesis: application to silk-based vascular grafts. Matrix biology : journal of the International Society for Matrix Biology. 2011;30:346–55. doi: 10.1016/j.matbio.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson SR, deSouza NM, Allison DJ. Endovascular stents and stent-grafts: is heparin coating desirable? Cardiovascular and interventional radiology. 2000;23:252–5. doi: 10.1007/s002700010064. [DOI] [PubMed] [Google Scholar]
- 20.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 21.Cardin AD, Jackson RL, Sparrow DA, Sparrow JT. Interaction of glycosaminoglycans with lipoproteins. Ann N Y Acad Sci. 1989;556:186–93. doi: 10.1111/j.1749-6632.1989.tb22503.x. [DOI] [PubMed] [Google Scholar]
- 22.Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992;12:240–7. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanzaki S, Takahashi T, Kanno T, Ariyoshi W, Shinmyouzu K, Tujisawa T, et al. Heparin inhibits BMP-2 osteogenic bioactivity by binding to both BMP-2 and BMP receptor. Journal of cellular physiology. 2008;216:844–50. doi: 10.1002/jcp.21468. [DOI] [PubMed] [Google Scholar]
- 24.Bramono DS, Murali S, Rai B, Ling L, Poh WT, Lim ZX, et al. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2) Bone. 2012;50:954–64. doi: 10.1016/j.bone.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klagsbrun M. The affinity of fibroblast growth factors (FGFs) for heparin; FGF-heparan sulfate interactions in cells and extracellular matrix. Curr Opin Cell Biol. 1990;2:857–63. doi: 10.1016/0955-0674(90)90084-r. [DOI] [PubMed] [Google Scholar]
- 26.Lin LF, Zhang TJ, Collins F, Armes LG. Purification and initial characterization of rat B49 glial cell line-derived neurotrophic factor. J Neurochem. 1994;63:758–68. doi: 10.1046/j.1471-4159.1994.63020758.x. [DOI] [PubMed] [Google Scholar]
- 27.Lyon M, Rushton G, Gallagher JT. The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific. J Biol Chem. 1997;272:18000–6. doi: 10.1074/jbc.272.29.18000. [DOI] [PubMed] [Google Scholar]
- 28.Tessler S, Rockwell P, Hicklin D, Cohen T, Levi BZ, Witte L, et al. Heparin modulates the interaction of VEGF165 with soluble and cell associated flk-1 receptors. J Biol Chem. 1994;269:12456–61. [PubMed] [Google Scholar]
- 29.Lee JJ, Ekker SC, von Kessler DP, Porter JA, Sun BI, Beachy PA. Autoproteolysis in hedgehog protein biogenesis. Science. 1994;266:1528–37. doi: 10.1126/science.7985023. [DOI] [PubMed] [Google Scholar]
- 30.Rostand KS, Esko JD. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12:619–26. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- 32.Edelman ER, Nugent MA, Smith LT, Karnovsky MJ. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest. 1992;89:465–73. doi: 10.1172/JCI115607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laham RJ, Sellke FW, Edelman ER, Pearlman JD, Ware JA, Brown DL, et al. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled trial. Circulation. 1999;100:1865–71. doi: 10.1161/01.cir.100.18.1865. [DOI] [PubMed] [Google Scholar]
- 34.Ruel M, Laham RJ, Parker JA, Post MJ, Ware JA, Simons M, et al. Long-term effects of surgical angiogenic therapy with fibroblast growth factor 2 protein. J Thorac Cardiovasc Surg. 2002;124:28–34. doi: 10.1067/mtc.2002.121974. [DOI] [PubMed] [Google Scholar]
- 35.Hennink WE, Feijen J, Ebert CD, Kim SW. Covalently bound conjugates of albumin and heparin: synthesis, fractionation and characterization. Thromb Res. 1983;29:1–13. doi: 10.1016/0049-3848(83)90120-2. [DOI] [PubMed] [Google Scholar]
- 36.Cremers HF, Kwon G, Bae YH, Kim SW, Verrijk R, Noteborn HP, et al. Preparation and characterization of albumin-heparin microspheres. Biomaterials. 1994;15:38–48. doi: 10.1016/0142-9612(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 37.Wissink MJ, Beernink R, Poot AA, Engbers GH, Beugeling T, van Aken WG, et al. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J Control Release. 2000;64:103–14. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 38.Wissink MJ, Beernink R, Scharenborg NM, Poot AA, Engbers GH, Beugeling T, et al. Endothelial cell seeding of (heparinized) collagen matrices: effects of bFGF pre-loading on proliferation (after low density seeding) and pro-coagulant factors. J Control Release. 2000;67:141–55. doi: 10.1016/s0168-3659(00)00202-9. [DOI] [PubMed] [Google Scholar]
- 39.van Wachem PB, Plantinga JA, Wissink MJ, Beernink R, Poot AA, Engbers GH, et al. In vivo biocompatibility of carbodiimide-crosslinked collagen matrices: Effects of crosslink density, heparin immobilization, and bFGF loading. J Biomed Mater Res. 2001;55:368–78. doi: 10.1002/1097-4636(20010605)55:3<368::aid-jbm1025>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Pieper JS, Hafmans T, van Wachem PB, van Luyn MJ, Brouwer LA, Veerkamp JH, et al. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res. 2002;62:185–94. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 41.Jeon O, Kang SW, Lim HW, Hyung Chung J, Kim BS. Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly(L-lactide-co-glycolide) nanospheres and fibrin gel. Biomaterials. 2006;27:1598–607. doi: 10.1016/j.biomaterials.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 42.Jeon O, Song SJ, Kang SW, Putnam AJ, Kim BS. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(L-lactic-co-glycolic acid) scaffold. Biomaterials. 2007;28:2763–71. doi: 10.1016/j.biomaterials.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Liu LS, Ng CK, Thompson AY, Poser JW, Spiro RC. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2) J Biomed Mater Res. 2002;62:128–35. doi: 10.1002/jbm.10238. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Oommen OP, Yan H, Varghese OP. Mild and Efficient Strategy for Site-selective Aldehyde Modification of Glycosaminoglycans: Tailoring Hydrogels with Tunable Release of Growth Factor. Biomacromolecules. 2013 doi: 10.1021/bm400612h. [DOI] [PubMed] [Google Scholar]
- 45.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304–11. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 46.Cai SS, Liu YC, Shu XZ, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–67. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Tae G, Kim YJ, Choi WI, Kim M, Stayton PS, Hoffman AS. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules. 2007;8:1979–86. doi: 10.1021/bm0701189. [DOI] [PubMed] [Google Scholar]
- 48.Choi WI, Kim M, Tae G, Kim YH. Sustained release of human growth hormone from heparin-based hydrogel. Biomacromolecules. 2008;9:1698–704. doi: 10.1021/bm701391b. [DOI] [PubMed] [Google Scholar]
- 49.McGonigle JS, Tae G, Stayton PS, Hoffman AS, Scatena M. Heparin-regulated delivery of osteoprotegerin promotes vascularization of implanted hydrogels. J Biomater Sci Polym Ed. 2008;19:1021–34. doi: 10.1163/156856208784909381. [DOI] [PubMed] [Google Scholar]
- 50.McCaldon P, Argos P. Oligopeptide biases in protein sequences and their use in predicting protein coding regions in nucleotide sequences. Proteins. 1988;4:99–122. doi: 10.1002/prot.340040204. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi N, Kiick KL. Polysaccharide-poly(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules. 2005;6:1921–30. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nie T, Baldwin A, Yamaguchi N, Kiick KL. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J Control Release. 2007;122:287–96. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–69. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 54.Tae G, Scatena M, Stayton PS, Hoffman AS. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomater Sci Polym Ed. 2006;17:187–97. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 55.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 56.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69:149–58. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 57.Pratt AB, Weber FE, Schmoekel HG, Muller R, Hubbell JA. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol Bioeng. 2004;86:27–36. doi: 10.1002/bit.10897. [DOI] [PubMed] [Google Scholar]
- 58.Thomopoulos S, Das R, Silva MJ, Sakiyama-Elbert S, Harwood FL, Zampiakis E, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009;27:1209–15. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomopoulos S, Zaegel M, Das R, Harwood FL, Silva MJ, Amiel D, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358–68. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- 60.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, Das R, Silva MJ. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg Am. 2007;32:373–9. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Maxwell DJ, Hicks BC, Parsons S, Sakiyama-Elbert SE. Development of rationally designed affinity-based drug delivery systems. Acta Biomater. 2005;1:101–13. doi: 10.1016/j.actbio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Wood MD, Sakiyama-Elbert SE. Release rate controls biological activity of nerve growth factor released from fibrin matrices containing affinity-based delivery systems. J Biomed Mater Res A. 2008;84:300–12. doi: 10.1002/jbm.a.31269. [DOI] [PubMed] [Google Scholar]
- 63.Wieduwild R, Tsurkan M, Chwalek K, Murawala P, Nowak M, Freudenberg U, et al. Minimal peptide motif for non-covalent peptide-heparin hydrogels. J Am Chem Soc. 2013;135:2919–22. doi: 10.1021/ja312022u. [DOI] [PubMed] [Google Scholar]
- 64.Lee AC, Yu VM, Lowe JB, 3rd, Brenner MJ, Hunter DA, Mackinnon SE, et al. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184:295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 65.Wood MD, Moore AM, Hunter DA, Tuffaha S, Borschel GH, Mackinnon SE, et al. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomater. 2009;5:959–68. doi: 10.1016/j.actbio.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson PJ, Parker SR, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin-based tissue engineering scaffolds enhances neural fiber sprouting following subacute spinal cord injury. Biotechnol Bioeng. 2009;104:1207–14. doi: 10.1002/bit.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113:226–35. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hudalla GA, Koepsel JT, Murphy WL. Surfaces that sequester serum-borne heparin amplify growth factor activity. Adv Mater. 2011;23:5415–8. doi: 10.1002/adma.201103046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hudalla GA, Kouris NA, Koepsel JT, Ogle BM, Murphy WL. Harnessing endogenous growth factor activity modulates stem cell behavior. Integrative biology : quantitative biosciences from nano to macro. 2011;3:832–42. doi: 10.1039/c1ib00021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seif-Naraghi SB, Horn D, Schup-Magoffin PJ, Christman KL. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012;8:3695–703. doi: 10.1016/j.actbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maynard HD, Hubbell JA. Discovery of a sulfated tetrapeptide that binds to vascular endothelial growth factor. Acta Biomater. 2005;1:451–9. doi: 10.1016/j.actbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Christman KL, Vazquez-Dorbatt V, Schopf E, Kolodziej CM, Li RC, Broyer RM, et al. Nanoscale growth factor patterns by immobilization on a heparin-mimicking polymer. J Am Chem Soc. 2008;130:16585–91. doi: 10.1021/ja803676r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nguyen TH, Kim SH, Decker CG, Wong DY, Loo JA, Maynard HD. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nature chemistry. 2013;5:221–7. doi: 10.1038/nchem.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willerth SM, Rader A, Sakiyama-Elbert SE. The effect of controlled growth factor delivery on embryonic stem cell differentiation inside fibrin scaffolds. Stem Cell Res. 2008;1:205–18. doi: 10.1016/j.scr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson PJ, Tatara A, Shiu A, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 and platelet-derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell Transplant. 2010;19:89–101. doi: 10.3727/096368909X477273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lam HJ, Patel S, Wang A, Chu J, Li S. In vitro regulation of neural differentiation and axon growth by growth factors and bioactive nanofibers. Tissue engineering Part A. 2010;16:2641–8. doi: 10.1089/ten.tea.2009.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sangaj N, Kyriakakis P, Yang D, Chang CW, Arya G, Varghese S. Heparin mimicking polymer promotes myogenic differentiation of muscle progenitor cells. Biomacromolecules. 2010;11:3294–300. doi: 10.1021/bm101041f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chow LW, Wang LJ, Kaufman DB, Stupp SI. Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets. Biomaterials. 2010 doi: 10.1016/j.biomaterials.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuo YC, Wang CT. Neuronal differentiation of induced pluripotent stem cells in hybrid polyester scaffolds with heparinized surface. Colloids and surfaces B, Biointerfaces. 2012;100:9–15. doi: 10.1016/j.colsurfb.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 80.Chang CW, Hwang Y, Brafman D, Hagan T, Phung C, Varghese S. Engineering cell-material interfaces for long-term expansion of human pluripotent stem cells. Biomaterials. 2013;34:912–21. doi: 10.1016/j.biomaterials.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim M, Kim YH, Tae G. Human mesenchymal stem cell culture on heparin-based hydrogels and the modulation of interactions by gel elasticity and heparin amount. Acta Biomater. 2013;9:7833–44. doi: 10.1016/j.actbio.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 82.Seal BL, Panitch A. Physical polymer matrices based on affinity interactions between peptides and polysaccharides. Biomacromolecules. 2003;4:1572–82. doi: 10.1021/bm0342032. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L, Furst EM, Kiick KL. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide-polysaccharide interactions. Journal of Controlled Release. 2006;114:130–42. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rajangam K, Behanna HA, Hui MJ, Han XQ, Hulvat JF, Lomasney JW, et al. Heparin binding nanostructures to promote growth of blood vessels. Nano Letters. 2006;6:2086–90. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 85.Chow LW, Bitton R, Webber MJ, Carvajal D, Shull KR, Sharma AK, et al. A bioactive self-assembled membrane to promote angiogenesis. Biomaterials. 2011;32:1574–82. doi: 10.1016/j.biomaterials.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Freudenberg U, Hermann A, Welzel PB, Stirl K, Schwarz SC, Grimmer M, et al. A star-PEG-heparin hydrogel platform to aid cell replacement therapies for neurodegenerative diseases. Biomaterials. 2009;30:5049–60. doi: 10.1016/j.biomaterials.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 87.Tsurkan MV, Chwalek K, Prokoph S, Zieris A, Levental KR, Freudenberg U, et al. Defined Polymer-Peptide Conjugates to Form Cell-Instructive starPEG-Heparin Matrices In Situ. Adv Mater. 2013;25:2606–10. doi: 10.1002/adma.201300691. [DOI] [PubMed] [Google Scholar]
- 88.Zieris A, Chwalek K, Prokoph S, Levental KR, Welzel PB, Freudenberg U, et al. Dual independent delivery of pro-angiogenic growth factors from starPEG-heparin hydrogels. J Control Release. 2011;156:28–36. doi: 10.1016/j.jconrel.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 89.Tsurkan MV, Levental KR, Freudenberg U, Werner C. Enzymatically degradable heparin-polyethylene glycol gels with controlled mechanical properties. Chem Commun (Camb) 2010;46:1141–3. doi: 10.1039/b921616b. [DOI] [PubMed] [Google Scholar]
- 90.Zieris A, Prokoph S, Levental KR, Welzel PB, Grimmer M, Freudenberg U, et al. FGF-2 and VEGF functionalization of starPEG-heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials. 2010;31:7985–94. doi: 10.1016/j.biomaterials.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 91.Tsurkan MV, Chwalek K, Levental KR, Freudenberg U, Werner C. Modular StarPEGH-eparin Gels with Bifunctional Peptide Linkers. Macromolecular rapid communications. 2010;31:1529–33. doi: 10.1002/marc.201000155. [DOI] [PubMed] [Google Scholar]
- 92.Baumann L, Prokoph S, Gabriel C, Freudenberg U, Werner C, Beck-Sickinger AG. A novel, biased-like SDF-1 derivative acts synergistically with starPEG-based heparin hydrogels and improves eEPC migration in vitro. J Control Release. 2012;162:68–75. doi: 10.1016/j.jconrel.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 93.Freudenberg U, Sommer J-U, Levental KR, Welzel PB, Zieris A, Chwalek K, et al. Using Mean Field Theory to Guide Biofunctional Materials Design. Advanced Functional Materials. 2012;22:1391–8. [Google Scholar]
- 94.LeBrun L, Linhardt RJ. Degradation of Heparan Sulfate with Heparin Lyases. In: Iozzo R, editor. Proteoglycan Protocols. Totowa, NJ: Humana Press; 2001. pp. 353–61. [DOI] [PubMed] [Google Scholar]