Abstract
Previous results suggested that the hobo transposable element is active predominantly in the germline of Drosophila. We investigate germline restriction of hobo transposition by testing in vitro modified elements for their ability to mobilize marked elements in vivo. Although intact hobo elements are germline specific, an hsp70 promoter-hobo transposase fusion is active in the soma. Analysis of the hsp70-promoted transcript does not provide evidence for splicing. Moreover, the hobo promoter confers germline bias to a highly sensitive reporter, delta 2-3 P transposase. These results indicate that hobo transposition is germline specific due to regulation of transposase production at the level of transcription. Thus, although hobo is similar to the P transposable element in organization and tissue specificity, it differs in the underlying mechanism governing germline specific activity.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Atia G. R., Fruscoloni P., Jacobs-Lorena M. Translational regulation of mRNAs for ribosomal proteins during early Drosophila development. Biochemistry. 1985 Oct 8;24(21):5798–5803. doi: 10.1021/bi00342a017. [DOI] [PubMed] [Google Scholar]
- Blackman R. K., Grimaila R., Koehler M. M., Gelbart W. M. Mobilization of hobo elements residing within the decapentaplegic gene complex: suggestion of a new hybrid dysgenesis system in Drosophila melanogaster. Cell. 1987 May 22;49(4):497–505. doi: 10.1016/0092-8674(87)90452-1. [DOI] [PubMed] [Google Scholar]
- Blackman R. K., Koehler M. M., Grimaila R., Gelbart W. M. Identification of a fully-functional hobo transposable element and its use for germ-line transformation of Drosophila. EMBO J. 1989 Jan;8(1):211–217. doi: 10.1002/j.1460-2075.1989.tb03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Bucheton A. I transposable elements and I-R hybrid dysgenesis in Drosophila. Trends Genet. 1990 Jan;6(1):16–21. doi: 10.1016/0168-9525(90)90044-7. [DOI] [PubMed] [Google Scholar]
- Bucheton A., Paro R., Sang H. M., Pelisson A., Finnegan D. J. The molecular basis of I-R hybrid dysgenesis in Drosophila melanogaster: identification, cloning, and properties of the I factor. Cell. 1984 Aug;38(1):153–163. doi: 10.1016/0092-8674(84)90536-1. [DOI] [PubMed] [Google Scholar]
- Calvi B. R., Hong T. J., Findley S. D., Gelbart W. M. Evidence for a common evolutionary origin of inverted repeat transposons in Drosophila and plants: hobo, Activator, and Tam3. Cell. 1991 Aug 9;66(3):465–471. doi: 10.1016/0092-8674(81)90010-6. [DOI] [PubMed] [Google Scholar]
- Chaboissier M. C., Busseau I., Prosser J., Finnegan D. J., Bucheton A. Identification of a potential RNA intermediate for transposition of the LINE-like element I factor in Drosophila melanogaster. EMBO J. 1990 Nov;9(11):3557–3563. doi: 10.1002/j.1460-2075.1990.tb07566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R. A trans-acting product needed for P factor transposition in Drosophila. Science. 1984 Dec 7;226(4679):1194–1196. doi: 10.1126/science.6095450. [DOI] [PubMed] [Google Scholar]
- Engels W. R., Benz W. K., Preston C. R., Graham P. L., Phillis R. W., Robertson H. M. Somatic effects of P element activity in Drosophila melanogaster: pupal lethality. Genetics. 1987 Dec;117(4):745–757. doi: 10.1093/genetics/117.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R. Extrachromosomal control of mutability in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4011–4015. doi: 10.1073/pnas.76.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Fitzgerald S. D., McGuire W. L. A simple polymerase chain reaction method for detection and cloning of low-abundance transcripts. Biotechniques. 1990 Aug;9(2):206–211. [PubMed] [Google Scholar]
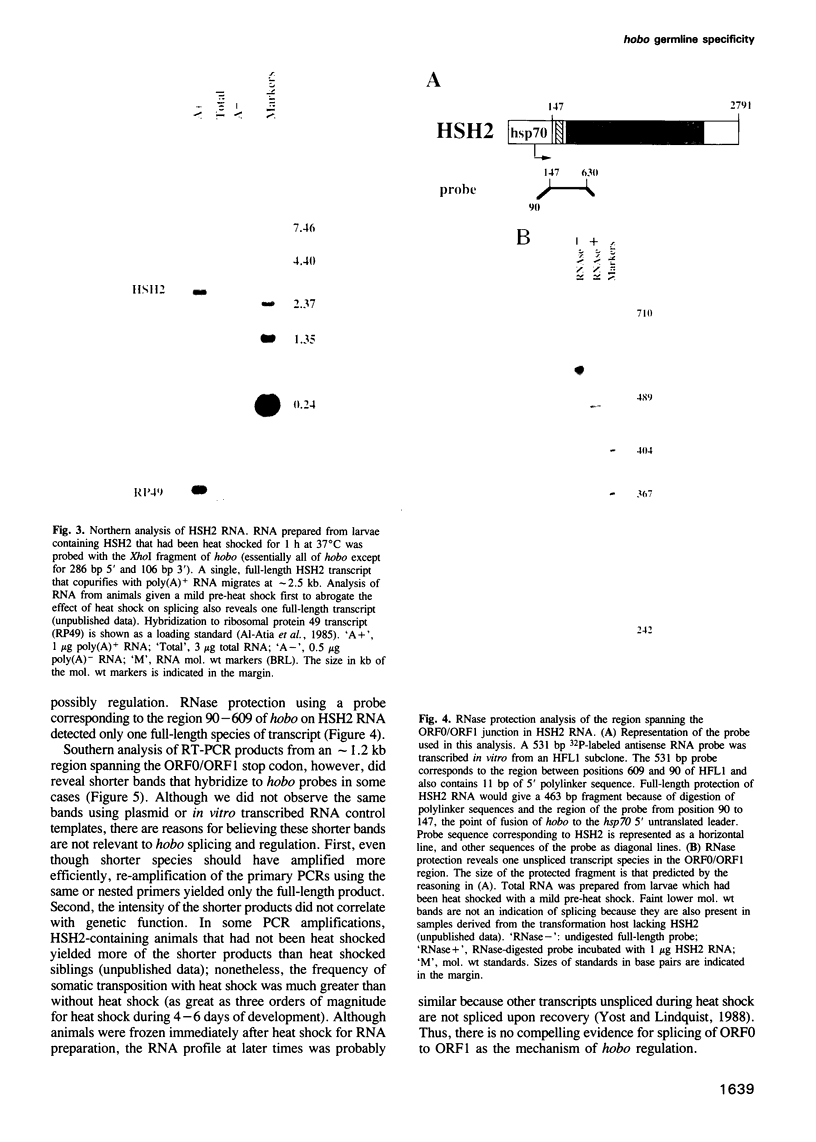
- Ho Y. T., Weber S. M., Lim J. K. Interacting hobo transposons in an inbred strain and interaction regulation in hybrids of Drosophila melanogaster. Genetics. 1993 Jul;134(3):895–908. doi: 10.1093/genetics/134.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyin Y. V., Lyubomirskaya N. V., Kim A. I. Retrotransposon Gypsy and genetic instability in Drosophila (review). Genetica. 1991;85(1):13–22. doi: 10.1007/BF00056102. [DOI] [PubMed] [Google Scholar]
- Kaufman P. D., Rio D. C. Drosophila P-element transposase is a transcriptional repressor in vitro. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2613–2617. doi: 10.1073/pnas.88.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. D., Rio D. C. P element transposition in vitro proceeds by a cut-and-paste mechanism and uses GTP as a cofactor. Cell. 1992 Apr 3;69(1):27–39. doi: 10.1016/0092-8674(92)90116-t. [DOI] [PubMed] [Google Scholar]
- Kellum R., Schedl P. A group of scs elements function as domain boundaries in an enhancer-blocking assay. Mol Cell Biol. 1992 May;12(5):2424–2431. doi: 10.1128/mcb.12.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. I., Belyaeva E. S. Transposition of mobile elements gypsy (mdg4) and hobo in germ-line and somatic cells of a genetically unstable mutator strain of Drosophila melanogaster. Mol Gen Genet. 1991 Oct;229(3):437–444. doi: 10.1007/BF00267467. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Regulation of transposition in bacteria. Annu Rev Cell Biol. 1990;6:297–327. doi: 10.1146/annurev.cb.06.110190.001501. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Lachaume P., Bouhidel K., Mesure M., Pinon H. Spatial and temporal expression of the I factor during oogenesis in Drosophila melanogaster. Development. 1992 Jul;115(3):729–735. doi: 10.1242/dev.115.3.729. [DOI] [PubMed] [Google Scholar]
- Laski F. A., Rio D. C., Rubin G. M. Tissue specificity of Drosophila P element transposition is regulated at the level of mRNA splicing. Cell. 1986 Jan 17;44(1):7–19. doi: 10.1016/0092-8674(86)90480-0. [DOI] [PubMed] [Google Scholar]
- Laski F. A., Rubin G. M. Analysis of the cis-acting requirements for germ-line-specific splicing of the P-element ORF2-ORF3 intron. Genes Dev. 1989 May;3(5):720–728. doi: 10.1101/gad.3.5.720. [DOI] [PubMed] [Google Scholar]
- Lathe R., Vilotte J. L., Clark A. J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57(2-3):193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lim J. K. Intrachromosomal rearrangements mediated by hobo transposons in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9153–9157. doi: 10.1073/pnas.85.23.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. K. Site-specific intrachromosomal rearrangements in Drosophila melanogaster: cytogenetic evidence for transposable elements. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):553–560. doi: 10.1101/sqb.1981.045.01.071. [DOI] [PubMed] [Google Scholar]
- McLean C., Bucheton A., Finnegan D. J. The 5' untranslated region of the I factor, a long interspersed nuclear element-like retrotransposon of Drosophila melanogaster, contains an internal promoter and sequences that regulate expression. Mol Cell Biol. 1993 Feb;13(2):1042–1050. doi: 10.1128/mcb.13.2.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mismer D., Rubin G. M. Analysis of the promoter of the ninaE opsin gene in Drosophila melanogaster. Genetics. 1987 Aug;116(4):565–578. doi: 10.1093/genetics/116.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Steller H., Bozzetti M. P. Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 1985 Dec 16;4(13A):3501–3508. doi: 10.1002/j.1460-2075.1985.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinow L., Birchler J. A. A dosage-sensitive modifier of retrotransposon-induced alleles of the Drosophila white locus. EMBO J. 1989 Mar;8(3):879–889. doi: 10.1002/j.1460-2075.1989.tb03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D. C., Laski F. A., Rubin G. M. Identification and immunochemical analysis of biologically active Drosophila P element transposase. Cell. 1986 Jan 17;44(1):21–32. doi: 10.1016/0092-8674(86)90481-2. [DOI] [PubMed] [Google Scholar]
- Rio D. C. Regulation of Drosophila P element transposition. Trends Genet. 1991 Sep;7(9):282–287. doi: 10.1016/0168-9525(91)90309-E. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman R. R., Pirrotta V., Geyer P. K. The su(Hw) protein insulates expression of the Drosophila melanogaster white gene from chromosomal position-effects. EMBO J. 1993 Feb;12(2):435–442. doi: 10.1002/j.1460-2075.1993.tb05675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebel C. W., Fresco L. D., Rio D. C. The mechanism of somatic inhibition of Drosophila P-element pre-mRNA splicing: multiprotein complexes at an exon pseudo-5' splice site control U1 snRNP binding. Genes Dev. 1992 Aug;6(8):1386–1401. doi: 10.1101/gad.6.8.1386. [DOI] [PubMed] [Google Scholar]
- Siebel C. W., Rio D. C. Regulated splicing of the Drosophila P transposable element third intron in vitro: somatic repression. Science. 1990 Jun 8;248(4960):1200–1208. doi: 10.1126/science.2161558. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Corces V. G. Drosophila transposable elements: mechanisms of mutagenesis and interactions with the host genome. Adv Genet. 1991;29:229–300. doi: 10.1016/s0065-2660(08)60109-1. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Streck R. D., Macgaffey J. E., Beckendorf S. K. The structure of hobo transposable elements and their insertion sites. EMBO J. 1986 Dec 20;5(13):3615–3623. doi: 10.1002/j.1460-2075.1986.tb04690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff R. C. Transposable DNA elements and life history traits. I. Transposition of P DNA elements in somatic cells reduces the lifespan of Drosophila melanogaster. Genetica. 1992;86(1-3):143–154. doi: 10.1007/BF00133717. [DOI] [PubMed] [Google Scholar]
- Yannopoulos G., Stamatis N., Monastirioti M., Hatzopoulos P., Louis C. hobo is responsible for the induction of hybrid dysgenesis by strains of Drosophila melanogaster bearing the male recombination factor 23.5MRF. Cell. 1987 May 22;49(4):487–495. doi: 10.1016/0092-8674(87)90451-x. [DOI] [PubMed] [Google Scholar]
- Yannopoulos G., Stamatis N., Zacharopoulou A., Pelecanos M. Site-specific breaks induced by the male recombination factor 23.5 MRF in Drosophila melanogaster. Mutat Res. 1983 Mar;108(1-3):185–202. doi: 10.1016/0027-5107(83)90120-3. [DOI] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. Translation of unspliced transcripts after heat shock. Science. 1988 Dec 16;242(4885):1544–1548. doi: 10.1126/science.3201243. [DOI] [PubMed] [Google Scholar]
- Yost H. J., Petersen R. B., Lindquist S. RNA metabolism: strategies for regulation in the heat shock response. Trends Genet. 1990 Jul;6(7):223–227. doi: 10.1016/0168-9525(90)90183-7. [DOI] [PubMed] [Google Scholar]