Abstract
Objective
The anterior approach for multilevel CSM has been developed and obtained favorable outcomes. However, the operation difficulty, invasiveness and operative risks increase when multi-level involved. This study was to assess surgical parameters, complications, clinical and radiological outcomes in the treatment of 2-, 3- and 4-level CSM.
Methods
A total of 248 patients with 2-, 3- or 4-level CSM who underwent anterior decompression and fusion procedures between October 2005 and June 2011 were divided into three groups, the 2-level group (106 patients), the 3-level group (98 patients) and the 4-level group (44 patients). The clinical and Radiographic outcomes including Japanese Orthopedic Association (JOA) score, Neck Disability Index (NDI) score, Odom's Scale, hospital stay, blood loss, operation time, fusion rate, cervical lordosis, cervical range of motion (ROM), and complications were compared.
Results
At a minimum of 2-year follow-up, no statistical differences in JOA score, NDI score, Odom's Scale, hospital stay, fusion rate and cervical lordosis were found among the 3 groups. However, the mean postoperative NDI score of the 4-level group was significantly higher than that in the other two groups (P<0.05), and in terms of postoperative total ROM, the 3-level group was superior to the 4-level group and inferior to 2-level group (P<0.05). The decrease rate of ROM in the 3-level group was significantly higher than that in the 2-level group, and lower than that in the 4-level group (P<0.05).
Conclusions
As the number of involved levels increased, surgical results become worse in terms of operative time, blood loss, NDI score, cervical ROM and complication rates postoperatively. An appropriate surgical procedure for multilevel CSM should be chosen according to comprehensive clinical evaluation before operation, thus reducing fusion and decompression levels if possible.
Introduction
Cervical spondylotic myelopathy (CSM) is a common spinal cord disorder that develops in elderly individuals. Anterior cervical decompression and fusion is an effective and reliable procedure for the treatment of CSM [1]. However, multilevel CSM is a challenging clinical problem. The optimal surgical approach for multilevel CSM remains controversial. Anterior, posterior and combined anterior and posterior surgical approaches for patients with multilevel CSM all have been advocated [2]–[5]. Although laminectomy and laminoplasty have been effective for the treatment of multilevel cervical spondylotic myelopathy, progressive cervical kyphosis, C5 nerve root palsy, and axial neck pain are major disadvantages of these techniques [6]–[8]. The ventral aspect of the spinal cord and nerve roots is most often compressed [9]. Anterior cervical corpectomy and fusion better restores cervical lordosis and directly decompresses the spinal cord by removing the offending soft or hard discs [10]. This surgical procedure is widely used for the treatment of multilevel CSM [11]. Recent studies showed that multilevel anterior cervical discectomy and fusion (ACDF) and anterior cervical hybrid decompression and fusion (ACHDF) could obtain satisfying clinical efficacy in the management of multilevel CSM for appropriate patients [12]–[14]. In addition, discontinuous corpectomy and fusion (DCF) with reservation of the middle vertebra has been proved to be a safe and effective surgical treatment for multilevel CSM in our previous study [15].
The anterior approach treatment of 2-level CSM had been developed and obtained favorable outcomes [16], [17]. Some studies also exist regarding procedures involving 3 and 4-level segments [13]–[15], [18]. However, as number of fused and decompressed levels increased, the operation difficulty, invasiveness and operative risks are higher. Thus we decided to conduct this retrospective study to assess surgical parameters, complications, clinical and radiological outcomes in the treatment of 2-, 3- and 4-level CSM.
Materials and Methods
Patient Population
Two hundred and sixty patients who underwent surgery for 2-, 3- or 4-level CSM between October 2005 and June 2011 were included in the study. Twelve patients were excluded because they did not complete 2-year follow-up. The study group comprised 100 women and 148 men of median age 60.2 years (age range, 39–82 y). Radiological diagnoses were established in each patient via routine preoperative cervical anteroposterior, lateral, flexion-extension radiographs and cervical magnetic resonance imaging (MRI) or computed tomography (CT) scans. All patients had symptoms and signs of neural compression that were refractory to conservative treatment. The indication for number of surgical levels depended on several factors: extent of spinal cord compression, extent of the signal alteration of the spinal cord in the MRI, segmental and cervical alignment. The exclusion criteria were as follows: we excluded patients whose primary symptoms were axial pain or radicular symptoms, and not myelopathy symptoms. Severe stenosis patients were excluded (Pavlov ratio less than 0.70). Patients with severe ischemic heart disease, Lung disorders and blood dyscrasias were excluded. Patients who underwent prior cervical spine surgery or underwent surgery for fractures, tumors, or infection were also excluded. According to the segments involvement, these patients were divided into three groups, the 2-level group (106 patients), the 3-level group (98 patients) and the 4-level group (44 patients).
Surgical Technique
All operations were performed by an experienced spine surgeon (H.T.) with more than 30 years of clinical experience in cervical spine surgery. The choice of the operation was dependent on the characteristics of cord compression. Large osteophyte and disc complexes extending posterior to the vertebral body were decompressed by corpectomy. If the compression is caused by the anterior degenerative discs, discectomy is performed. Anterior cervical corpectomy and discectomy were performed as described previously [13], [19], [20]. The operative procedure for DCF has been described by our previous study [15]. The ACHDF procedure included 1-level ACDF and 1-level ACCF. For complete decompression, the posterior longitudinal ligament was also removed to expose the dura throughout the length of the corpectomy and discectomy. For the corpectomy procedures, cartilaginous end plates were removed from the adjoining vertebral bodies. The corpectomized bone was harvested, morsellized, and packed into appropriately sized titanium mesh (DePuy Spine, New Brunswick, New Jersey). For discectomy procedure, PEEK interbody cage (DePuy Spine, New Brunswick, New Jersey) was used to fill the space generated by discectomy. Finally, an appropriately sized anterior cervical locking plate was firmly fixed into the vertebrae with screws (DePuy Spine, New Brunswick, New Jersey).
When CSM was 2-level, 2-level ACDF, or 1-level ACCF was performed. When CSM was 3-level, 3-level ACDF, ACHDF or 2-level ACCF was performed. When CSM was 4-level, 4-level ACDF or DCF was performed. In the 2-level group, 42 patients underwent ACCF, 62 patients underwent ACDF. In the 3-level group, 18 patients underwent ACCF, 33 patients underwent ACDF, and 47 patients underwent ACHDF. In the 4-level group, 19 patients underwent ACDF, 25 patients underwent DCF(Table 1). All patients wore Philadelphia collars for an average of 10 weeks postoperatively (Fig. 1).
Table 1. Showing number of different procedures in three groups.
| 2level | 3level | 4level | |
| ACCF | 42 | 18 | 0 |
| ACDF | 64 | 33 | 19 |
| DCF | 0 | 0 | 25 |
| ACHDF | 0 | 47 | 0 |
| total | 106 | 98 | 44 |
ACCF: anterior cervical corpectomy and fusion;
ACDF: anterior cervical discectomy and fusion;
DCF: Discontinuous corpectomy and fusion (DCF) with reservation of the middle vertebra;
ACHDF: anterior cervical hybrid decompression and fusion.
Figure 1. Postoperative lateral radiographs of four surgical techniques.
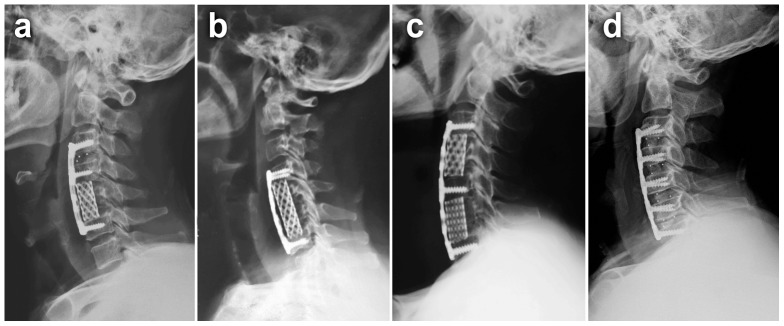
a Anterior cervical hybrid decompression and fusion (ACHDF). b Anterior cervical corpectomy and fusion (ACCF). c Discontinuous corpectomy and fusion (DCF) with reservation of the middle vertebra. d Anterior cervical discectomy and fusion (ACDF).
Ethics Statement
The clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki. All of the patients signed the informed consent form before their information was stored in the hospital database and used for research purposes. The study was approved by the Ethical Committee of the Tenth People's Hospital of Tongji University.
Outcome Measures
Neurological function was assessed using the Japanese Orthopedic Association (JOA) scores. Postoperative patients' satisfaction was based on Odom's criteria. The Neck Disability Index (NDI) score was also recorded for the evaluation of neck-shoulder pain levels. The degrees of preoperative and final follow-up fusion segmental lordosis of C2–C7 was measured using the Cobb method described in previous study [5] (Fig. 2). Lateral radiographs in flexion and extension were assessed before and after surgery. Total cervical range of motion (ROM) was defined as the angle formed between the lower endplate of the C2 and the upper endplate of the C7 using Cobb's method on flexion/extension lateral radiographs [21]. The decrease rate of ROM after the operation was calculated by [(pepreoperative ROM – postoperative ROM)/preoperative ROM] ×100%. Bone fusion was judged by the absence of motion more than 2° between the spinous processes on flexion–extension lateral radiographs, the absence of radiolucent gap between the graft and end plate, and the presence of continuous bridging bony trabeculae at the graft-endplate interface. Movement of ≥2° on flexion/extension radiographs was regarded as a pseudarthrosis [22], [23]. Graft dislodgement was defined as graft beyond the leading edge of the upper and lower vertebral connection 2–4 mm on lateral radiographs. The graft subsidence was defined as loss of height of the fusion segments on lateral radiographs at day 1 after the surgery and at bony fusion. The incidence of dysphagia was defined as that solid or dry food gets stuck in the process of swallowing.
Figure 2. A 65-year-old male developed numbness in his two hands and weakness in his four extremities for 2 years.
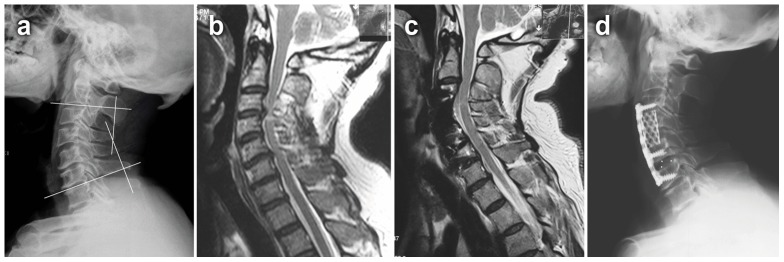
Preoperative imaging studies showed that the spinal cord compressed at C3–C6. He was performed with 3-level ACHDF. After operation, his JOA scores improved from 7 preoperation to 13 postoperation. a Preoperative lateral X-ray. The segmental lordosis of C2–C7 was defined as the angle formed by the lower endplate of C2 vertebral body and the upper endplate of C7 vertebral body. b Preoperative MRI. c 2-year postoperative MRI. d 2-year postoperative lateral X-ray.
The following data were recorded for each patient: history; symptoms at admission; duration of symptoms; physical and neurological findings at presentation; intraoperative spinal observations; preoperative and postoperative neurological function; preoperative and postoperative radiological findings and intra- and postoperative complications. Postoperative follow-up visits were done regularly at 3months, 6 months, 12 months 18 months and 2 years. The subsequent follow-up examinations were performed at every 6-month interval. All the patients were observed for at least 24 months after surgery.
Statistical Analysis
Data were analyzed using Microsoft Excel 2003 (Microsoft, Redmond, Washington) and SPSS version 19.0 software (SPSS, Inc, Chicago, Illinois). The changes in clinical effects and cervical lordosis in each group after surgeries were analyzed by the Wilcoxon rank-sum test. The Kruskal-Wallis H test was used to investigate whether the statistical differences of results exist among the groups postoperatively and post-hoc analysis was performed using the Nemenyi test. The chi-square test was used in the comparisons of the complication among the groups. A P value less than 0.05 was considered statistically significant.
Results
Perioperative Parameters
The overall follow-up period of the patients ranged from 24 to 48 months (average 29.2 months). The duration of symptoms at presentation ranged from 6 months to 4 years (median, 1.6 years). Major symptoms at presentation were arm numbness or paresthesias (n = 194; 78.2%), neck and arm pain (n = 172; 69.4%), motor weakness (n = 155; 62.5%), and gait disorder (n = 89; 35.9%). Most patients showed various degrees of symptom relief postoperatively. The general information was presented in Table 2. No significant intergroup differences were found in terms of age, gender and hospital stay (P>0.05). The mean operative time and blood loss in the 2-level group were significant lower than the 3-level group (P<0.05). The 4-level group required a significantly longest operative time than the other two groups (P<0.05) and had more operative blood loss (P<0.05).
Table 2. Demographic data of patients.
| 2-level group (n = 106) | 3-level group (n = 98) | 4-level group (n = 44) | P | |
| Age (year) | 59.24±9.60 | 60.60±9.84 | 61.50±10.00 | 0.451 |
| Gender (Male/Female) | 63/43 | 60/38 | 25/19 | 0.883 |
| Active smokers(yes/no) | 33/73 | 36/62 | 14/30 | 0.676 |
| Patient with diabetes | 19 | 21 | 5 | 0.354 |
| Patient with hypertension | 22 | 23 | 11 | 0.821 |
| Hospital stay (day) | 11.71±2.67 | 11.59±3.00 | 12.41±2.56 | 0.074 |
| Operative time (min) | 106.51±17.90* | 129.64±16.87* | 166.14±20.65* | 0.000 |
| Blood loss (mL) | 126.42+28.86# | 154.00±30.32# | 194.77±42.34# | 0.000 |
Statistical significance was set at a P<0.05.
The Kruskal-Wallis H test was used to investigate whether the statistical differences exist among the groups.
*Operative time: P = 0.000 (2-level and 3-level groups); P = 0.000(2-level and 4-level groups); P = 0.000 (3-level and 4-level groups) by Nemenyi test.
Blood loss: P = 0.000 (2-level and 3-level groups); P = 0.000(2-level and 4-level groups); P = 0.000 (3-level and 4-level groups) by Nemenyi test.
Clinical and Radiological Outcomes
Table 3 showed the Clinical and Radiological Results. The Japanese Orthopaedic Association scores significantly increased from 8.80±1.05 to 12.84±1.34 in the 2-level group (P<0.05), from 8.52±1.18 to 12.83±1.59 in the 3-level group (P<0.05), and from 8.45±1.15 to 12.41±1.47 in the 4-level group (P<0.05), respectively. There were no significant differences in JOA scores and Odom criteria among the 3 groups (P>0.05). In terms of NDI score, total cervical ROM and cervical lordosis, no significant intergroup difference was found preoperatively (P>0.05). NDI score, total cervical ROM and cervical lordosis were statistically different between preoperation and postoperation in each group (P<0.05). There were no significant differences in cervical lordosis among the 3 groups. However, in terms of NDI score, the mean postoperative NDI score of the 4-level group was significantly higher than that in the other two groups (P<0.05), and in terms of postoperative total ROM, the 3-level group was superior to the 4-level group and inferior to 2-level group (P<0.05). The decrease rate of ROM in the 3-level group was significantly higher than that in the 2-level group, and lower than that in the 4-level group (P<0.05). Postoperative radiographs demonstrated that fusion rates were 89.6% in the 2-level group, 91.2% in the 3-level group and 87.8% in the 4-level group at 3 months postoperatively. The fusion rates were 96.2% in the 2-level group, 96.9% in the 3-level group and 97.2% in the 4-level group at 24 months postoperatively.
Table 3. Comparisons of Clinical and Radiographic Outcomes.
| 2-level group (n = 106) | 3-level group (n = 98) | 4-level group (n = 44) | P | |
| Preoperative JOA scores | 8.80±1.05 | 8.52±1.18 | 8.45±1.15 | 0.108 |
| JOA scores at the final follow-up | 12.84±1.34 | 12.83±1.59 | 12.41±1.47 | 0.286 |
| Preoperative NDI scores | 21.77±3.97 | 21.58±4.20 | 22.20±3.51 | 0.708 |
| NDI scores at the final follow-up | 11.63±1.62 | 12.12±2.22 | 13.27±2.17* | 0.000 |
| Odom's Scale (Excellent/good/fair/bad) | 14/39/42/11 | 18/36/39/5 | 4/16/20/4 | 0.443 |
| Preoperative Segmental lordosis (degree) | 12.40±1.96 | 12.16±3.23 | 11.61±4.21 | 0.780 |
| Segmental lordosis at the final follow-up (degree) | 20.44±2.27 | 20.32±2.46 | 19.84±2.88 | 0.685 |
| Preoperative ROM (degree) | 43.31±3.61 | 43.08±4.11 | 42.55±3.43 | 0.646 |
| ROM at the final follow-up (degree) | 36.06±3.37# | 34.65±4.20# | 29.80±2.33# | 0.000 |
| Decrease rate of ROM (%) | 16.72±3.71▴ | 19.67±4.51▴ | 29.71±6.04▴ | 0.000 |
JOA Japanese orthopedic association; NDI neck disability index; ROM, range of motion.
Statistical significance was set at a P<0.05.
The Kruskal-Wallis H test was used to investigate whether the statistical differences exist among the groups.
*NDI scores at the final follow-up: P = 0.078 (2-level and 3-level groups); P = 0.000(2-level and 4-level groups); P = 0.002 (3-level and 4-level groups) by Nemenyi test.
ROM at the final follow-up: P = 0.005 (2-level and 3-level groups); P = 0.000(2-level and 4-level groups); P = 0.000 (3-level and 4-level groups) by Nemenyi test.
Decrease rate of ROM: P = 0.005 (2-level and 3-level groups); P = 0.000(2-level and 4-level groups); P = 0.000 (3-level and 4-level groups) by Nemenyi test.
Complications
In the 2-level group, a total of 12 (11.3%) patients developed postoperative complications including dysphagia (3 cases), dysphonia (2 cases), C5 palsy (0 cases), cerebral fluid leakage (2 case), pseudarthrosis (2 case), graft displacement (1 case), and subsidence (2 cases). In the 3-level group, a total of 18 (18.4%) patients developed postoperative complications including dysphagia (6 cases), dysphonia (3 cases), C5 palsy (1 cases), cerebral fluid leakage (2 case), pseudarthrosis (2 case), graft displacement (1 case), and subsidence (3 cases). In the 4-level group, a total of 17 (38.6%) patients developed postoperative complications including dysphagia((8 cases), dysphonia (2 cases), C5 palsy (1 case), cerebral fluid leakage (2 case), pseudarthrosis (1case), and subsidence (3 cases). The patient with pseudarthrosis was asymptomatic and did not receive second surgery. Cerebrospinal fluid leakage usually stopped after 3 to 5 days of conservative treatment with local pressure. The graft displacement and subsidence complications all occurred in patients with titanium mesh (ACCF, ACHDF and DCF). Statistical analysis showed that the 3-level group had higher incidence of postoperative complications than the 2-level groups, and the postoperative complications incidence of the 4-level group is highest (P<0.05) (Table 4).
Table 4. Complications.
| 2-level group (n = 106) | 3-level group (n = 98) | 4-level group (n = 44) | |
| Dysphagia | 3(2.8%) | 6(6.1%) | 8(18.2%) |
| Dysphonia | 2(1.9%) | 3(3.1%) | 2(4.5%) |
| C5 palsy | 0 | 1(1.0%) | 1(2.3%) |
| CSF leakage | 2(1.9%) | 3(3.1%) | 2(4.5%) |
| Pseudarthrosis | 2(1.9%) | 1(1.0%) | 1(2.3%) |
| Graft displacement | 1(1.0%) | 1(1.0%) | 0 |
| Subsidence | 2(1.9%) | 3(3.1%) | 3(6.8%) |
| Total | 12(11.3%)* | 18(18.4%)* | 17(38.6%)* |
Statistical significance was set at a P<0.05.
The chi-square test was used in the comparisons of the complication among the groups.
*Total: P = 0.038 (2-level and 3-level groups); P = 0.010(2-level and 4-level groups); P = 0.000 (3-level and 4-level groups).
Discussion
Anterior cervical decompression and fusion was first reported by Robinson and Smith [24] and popularized by Cloward [25] in the 1950s. The advantages of anterior decompression are the direct decompression and resection of the object causing pressure on the spinal cord in front, including soft disc herniations backwards, osteophytic proliferation, and ossification of the posterior longitudinal ligament. Although the surgical treatment of multilevel CSM is associated with less predictable outcomes and a higher frequency of complications, anterior approach in multilevel procedures is beneficial [13].
According to our retrospective review of 248 patients, all the three groups demonstrated a significant increase in JOA scores that were maintained at the finally follow-up. The clinical outcomes showed no significant differences among the three groups. The differences of Odom criteria and cervical lordosis were not statistically significant either. However, significant differences were observed in NDI score and total cervical ROM. The NDI score was significantly higher in the 4-level group than that in the other two groups. Wu et al. [26] found that patients might not experience great difficulties in performing daily activities. However, in our study, as number of fused levels increased, a small part of patients still complained of different degrees of neck pain and stiffness during follow up. We believed that this difference may be due to vertebral excessive distraction during operation [27]. Disc degeneration is usually more serious in patients with multi-level CSM. Intervertebral disc space is too narrow because of subsidence. Surgeons might habitually turn the lever of the Caspar retractor without considering how much excessive force is being applied to the vertebra for the purpose of physiological lordosis restoration and intervertebral disc space exposure. Some studies have proved that the mechanical load on the facet joints caused neck pain [28], [29]. Overdistraction could cause posterior neck pain due to stretching of the facet joint [29], [30]. Therefore, we hypothesized that vertebral excessive distraction might be related to subsequent neck pain. In addition, the total cervical ROM was significantly decreased when more levels had been fused. we found that the loss of neck motion after fusion also made patients feel depressed and anxious subjectively during follow up, especially the patients with 4-level fusion.
No surgeon can afford to neglect the complications of cervical procedures. As involved segments increased, the incidence of operative complications increased, too [31]–[36]. Kang et al. [37] reported the risk of dysphagia was greater in the group who underwent multilevel rather than single level surgery. Danto et al. [32] also showed that the risk of developing dysphagia and/or dysphonia increases with the number of surgical levels. In our study, we also found the 3-level and 4-level group had higher incidence of postoperative complications than the 2-level groups. A right-sided, 5 cm transverse straight incision was used in our surgical procedure so as to reduce the exposure area and retractor pressure on the esophagus. Tracheal/esophageal traction exercise (TTE) treatment [38] was also used preoperatively. However, there were still 14 patients feeling dysphagia and 5 patients feeling dysphonia in various degrees after surgery in the 3-level and 4-level group, and only 3 patients feeling dysphagia and 2 patients feeling dysphonia after surgery in the 2-level group. This kind of complication seems cannot be easily prevented when the number of fused levels increased. Meanwhile, there was a trend toward higher rate of dysphagia and dysphonia in 4-level group than 3-level group.
Bone- and/or plate-related complications after multilevel corpectomy have been reported in high rates, even when internal fixation was used [39]. Failure rates increase with 3 or more levels of corpectomy [33]. In the present study, there were 1 case of graft displacement and 2 cases of subsidence in the 2-level group, 1 case of graft displacement and 3 cases of subsidence in the 3-level group, and 3 cases of subsidence in the 4-level group. All these patients had undergone corpectomy (ACCF, ACHDF and DCF), and none of patients with ACDF developed this kind of complications (Fig. 3). Liu et al. [5] reported that multilevel discectomy and fusion offer more fixation points to hold the construct rigidly in place. The failure rate is lower than corpectomy, especially in terms of graft dislodgement and subsidence. The choice of the operation procedure was dependent on the characteristics of cord compression in our study. Because large osteophyte and disc complexes extending posterior to the vertebral body may not be easily removed by discectomy, corpectomy is more suitable in such cases. Over all, if the compressive pathology could be resolved by discectomy, ACDF should be the treatment of choice. If subsidence of the intervertebral space is serious and the compression is mainly caused by the osteophyte or nucleus pulposus bulges out beyond the damaged posterior longitudinal ligament and extending posterior to the vertebral body. We usually select the ACHDF or DCF procedures. A long corpectomy was the last choice considered.
Figure 3. A 62-year-old female patient with cervical spondylotic myelopathy at the C3–C6 level.
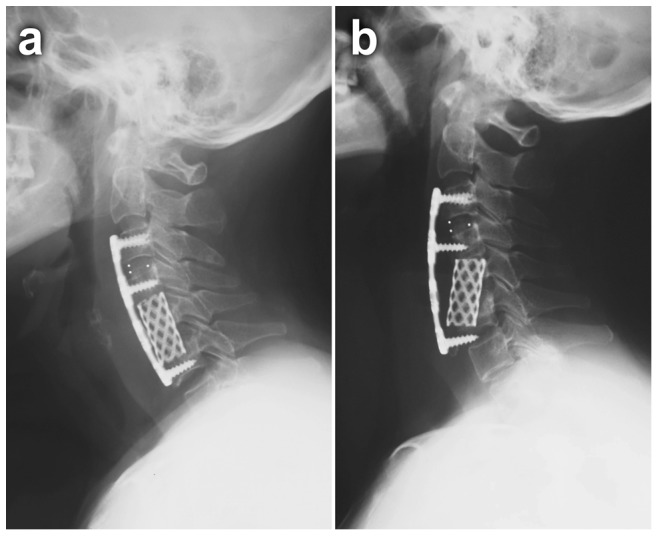
She was performed with 3-level ACHDF. The postoperative lateral view of this patient showed displacement of the titanium mesh cage and screw loosening at the three months follow-up. a Immediate postoperative lateral X-ray. b 3-month postoperative lateral X-ray.
Furthermore, the controversy of multi-level CSM is not only the surgical approach, but also the selection of fused and decompressed levels. The choice of surgical procedure for treatment should be dictated by several factors, including the location of compression, extent of the pathology, the patient's chief complaint and medical condition. The complete cervical spine radiography, CT and MRI were preformed in all patients before surgeries. However, it is difficult to confirm if all the herniated discs are related to the symptoms of the patients at times. The operated segments might be chosen unilaterally according to the imaging examination. In our study, we also observed that operation time and blood loss both increased when more segements were fused, and the operation difficulty, invasiveness and operative risks were higher. Kou et al. [40] reported that multilevel surgical procedure was established a significant risk factor for epidural hematoma after operation. Grabowski et al reported that complex anterior cervical surgery had higher risk of esophageal and vertebral artery injuries [41]. Sagi et al. [42] also found that prolonged procedures exposing more than three vertebral levels that include C2, C3, or C4 with more than 300-mL blood loss should be watched carefully for respiratory insufficiency. In our study, we mainly focused on surgical results of patients with different number of operated levels. An appropriate surgical procedure for multi-level CSM should be chosen according to comprehensive clinical evaluation. Good decompression is necessary for optimal surgical outcome. Furthermore, reduce fusion and decompression segments so as to minimize operation trauma and surgical risks.
This study had some limitations. First, the investigation was retrospective study. The patient's number in 4-level group was relative small. Second, different surgical procedures performed in the same group might have influence on the fusion rate and Instrumentation and graft related-complications. Finally, the incidence of adjacent segment disease cannot be followed adequately because the follow-up period was a minimum of 2 year. Therefore, a longer randomized controlled trial study is needed.
Conclusion
On the basis of our findings, we can conclude that surgical results become worse in terms of operative time, blood loss, postoperative NDI score, postoperative cervical ROM and complication rates when the number of involved levels increased. An appropriate surgical procedure for multi-level CSM should be chosen according to comprehensive clinical evaluation before operation, thus reducing fusion and decompression levels if possible.
Acknowledgments
The authors thank their patients for their permission to publish this article.
Funding Statement
The authors have no support or funding to report.
References
- 1. Reitman CA, Hipp JA, Nguyen L, Esses SI (2004) Changes in segmental intervertebral motion adjacent to cervical arthrodesis: a prospective study. Spine (Phila Pa 1976) 29(11): E221–E226. [DOI] [PubMed] [Google Scholar]
- 2. Konya D, Ozgen S, Gercek A, Pamir MN (2009) Outcomes for combined anterior and posterior surgical approaches for patients with multisegmental cervical spondylotic myelopathy. J Clin Neurosci 16: 404–409. [DOI] [PubMed] [Google Scholar]
- 3. Jiang JL, Li XL, Zhou XG, Lin H, Dong J (2012) Plate-only open-door laminoplasty with fusion for treatment of multilevel degenerative cervical disease. J Clin Neurosci 19(6): 804–809. [DOI] [PubMed] [Google Scholar]
- 4. Manzano GR, Casella G, Wang MY, Vanni S, Levi AD (2012) A prospective, randomized trial comparing expansile cervical laminoplasty and cervical laminectomy and fusion for multilevel cervical myelopathy. Neurosurgery 70(2): 264–277. [DOI] [PubMed] [Google Scholar]
- 5. Lin Q, Zhou X, Wang X, Cao P, Tsai N, et al. (2012) A comparison of anterior cervical discectomy and corpectomy in patients with multilevel cervical spondylotic myelopathy. Eur Spine J 21(3): 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Herkowitz HN (1988) A comparison of anterior cervical fusion, cervical laminectomy, and cervical laminoplasty for the surgical management of multiple level spondylotic radiculopathy. Spine (Phila Pa 1976) 13(7): 774–780. [DOI] [PubMed] [Google Scholar]
- 7. Yonenobu K, Hosono N, Iwasaki M, Asano M, Ono K (1992) Laminoplasty versus subtotal corpectomy. A comparative study of results in multisegmental cervical spondylotic myelopathy. Spine (Phila Pa 1976) 17(11): 1281–1284. [PubMed] [Google Scholar]
- 8. Sakaura H, Hosono N, Mukai Y, Ishii T, Yoshikawa H (2003) C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976) 28(21): 2447–2451. [DOI] [PubMed] [Google Scholar]
- 9. Lebl DR, Hughes A, Cammisa FP Jr, O'Leary PF (2011) Cervical spondylotic myelopathy: pathophysiology, clinical presentation, and treatment. HSS J 7(2): 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu T, Xu W, Cheng T, Yang HL (2011) Anterior versus posterior surgery for multilevel cervical myelopathy, which one is better? A systematic review. Eur Spine J 20(2): 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sevki K, Mehmet T, Ufuk T, Azmi H, Mercan S, et al. (2004) Results of surgical treatment for degenerative cervical myelopathy: anterior cervical corpectomy and stabilization. Spine 29(22): 2493–2500. [DOI] [PubMed] [Google Scholar]
- 12. Liu Y, Yu KY, Hu JH (2009) Hybrid decompression technique and two-level corpectomy are effective treatments for three-level cervical spondylotic myelopathy. J Zhejiang Univ Sci B 10(9): 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang SW, Kakarla UK, Maughan PH, DeSanto J, Fox D, et al. (2010) Four-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Neurosurgery 66(4): 639–646 discussion 646–647. [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, Hou Y, Yang L (2012) Comparison of 3 reconstructive techniques in the surgical management of multilevel cervical spondylotic myelopathy. Spine (Phila Pa 1976) 37(23): e1450–e1458. [DOI] [PubMed] [Google Scholar]
- 15. Shunzhi Y, Zhonghai L, Fengning L, Zhi C, Tiesheng H (2013) Surgical Management of 4-level Cervical Spondylotic Myelopathy. Orthopedics 36(5): e613–e620. [DOI] [PubMed] [Google Scholar]
- 16. Park Y, Maeda T, Cho W, Riew KD (2010) Comparison of anterior cervical fusion after two-level discectomy or single-level corpectomy: sagittal alignment, cervical lordosis, graft collapse, and adjacent-level ossification. Spine J 10(3): 193–199. [DOI] [PubMed] [Google Scholar]
- 17. Oh MC, Zhang HY, Park JY, Kim KS (2009) Two-level anterior cervical discectomy versus one-level corpectomy in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 34(7): 692–696. [DOI] [PubMed] [Google Scholar]
- 18. Guo Q, Bi X, Ni B, Lu X, Chen J, et al. (2011) Outcomes of three anterior decompression and fusion techniques in the treatment of three-level cervical spondylosis. Eur Spine J 20(9): 1539–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stewart TJ, Schlenk RP, Benzel EC (2007) Multiple level discectomy and fusion. Neurosurgery 60(1): S143–S148. [DOI] [PubMed] [Google Scholar]
- 20. Majd ME, Vadhva M, Holt RT (1999) Anterior cervical reconstruction using titanium cages with anterior plating. Spine (Phila Pa 1976) 24(15): 1604–1610. [DOI] [PubMed] [Google Scholar]
- 21. Penning L (1978) Normal movements of the cervical spine. Am J Roentogenol 130(2): 317–326. [DOI] [PubMed] [Google Scholar]
- 22. Hacker RJ, Cauthen JC, Gilbert TJ, Griffith SL (2000) A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine (Phila Pa 1976) 25(20): 2646–2654. [DOI] [PubMed] [Google Scholar]
- 23. Hacker RJ, Robert J (2000) A randomized prospective study of an anterior cervicalinterbody fusion device with a minimum of 2 years of follow-up results. Journal of Neurosurgery Spine 93(2): 222–226. [DOI] [PubMed] [Google Scholar]
- 24. Robinson RA, Smith GW (1955) Anterolateral cervical disc removal and interbody fusion for the cervical disc syndrome. Bull John Hopkins Hosp 96: 223–224. [Google Scholar]
- 25. Cloward RB (1958) The anterior approach for removal of ruptured cervical disks. J Neurosurg 15(6): 602–617. [DOI] [PubMed] [Google Scholar]
- 26. Wu XD, Wang XW, Yuan W, Liu Y, Tsai N, et al. (2012) The effect of multilevel anterior cervical fusion on neck motion. Eur Spine J 21(7): 1368–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ha SM, Kim JH, Oh SH, Song JH, Kim HI, et al. (2013) Vertebral Distraction during Anterior Cervical Discectomy and Fusion Causes Postoperative Neck Pain. J Korean Neurosurg Soc 53(5): 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manchikanti L, Singh V, Falco FJ, Cash KM, Fellows B (2008) Cervical medial branch blocks for chronic cervical facet joint pain: a randomized, double-blind, controlled trial with one-year follow-up. Spine (Phila Pa 1976) 33(17): 1813–1820. [DOI] [PubMed] [Google Scholar]
- 29. Winkelstein BA, Santos DG (2008) An intact facet capsular ligament modulates behavioral sensitivity and spinal glial activation produced by cervical facet joint tension. Spine (Phila Pa 1976) 33(8): 856–862. [DOI] [PubMed] [Google Scholar]
- 30. Lee KE, Thinnes JH, Gokhin DS, Winkelstein BA (2004) A novel rodent neck pain model of facet-mediated behavioral hypersensitivity: implications for persistent pain and whiplash injury. J Neurosci Methods 137(2): 151–159. [DOI] [PubMed] [Google Scholar]
- 31. Zeng JH, Zhong ZM, Chen JT (2013) Early dysphagia complicating anterior cervical spine surgery: incidence and risk factors. Arch Orthop Trauma Surg 133(8): 1067–1071. [DOI] [PubMed] [Google Scholar]
- 32. Danto J, DiCapua J, Nardi D, Pekmezaris R, Moise G, et al. (2012) Multiple cervical levels: increased risk of dysphagia and dysphonia during anterior cervical discectomy. J Neurosurg Anesthesiol 24(4): 350–355. [DOI] [PubMed] [Google Scholar]
- 33. Vaccaro AR, Falatyn SP, Scuderi GJ, Eismont FJ, McGuire RA, et al. (1998) Early failure of long segment anterior cervical plate fixation. J Spinal Disord 11(5): 410–415. [PubMed] [Google Scholar]
- 34. David KS, Rao RD (2006) Bilateral C5 motor paralysis following anterior cervical surgery—a case report. Clin Neurol Neurosurg 108(7): 675–681. [DOI] [PubMed] [Google Scholar]
- 35. Panjabi MM, Isomi T, Wang JL (1999) Loosening at the screw-vertebra junction in multilevel anterior cervical plate constructs. Spine (Phila Pa 1976) 24(22): 2383–2388. [DOI] [PubMed] [Google Scholar]
- 36. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV (2003) Early reconstruction failures after multilevel cervical corpectomy. Spine (Phila Pa 1976) 28(2): 140–142. [DOI] [PubMed] [Google Scholar]
- 37. Kang SH, Kim DK, Seo KM, Kim KT, Kim YB (2011) Multi-level spinal fusion and postoperative prevertebral thickness increase the risk of dysphagia after anterior cervical spine surgery. J Clin Neurosci 18(10): 1369–1373. [DOI] [PubMed] [Google Scholar]
- 38. Chen Z, Wei X, Li F, He P, Huang X, et al. (2012) Tracheal traction exercise reduces the occurrence of postoperative Dysphagia after anterior cervical spine surgery. Spine (Phila Pa 1976) 37(15): 1292–1296. [DOI] [PubMed] [Google Scholar]
- 39. Yalamanchili PK, Vives MJ, Chaudhary SB (2012) Cervical spondylotic myelopathy: factors in choosing the surgical approach. Adv Orthop 2012: 783762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kou J, Fischgrund J, Biddinger A, Herkowitz H (2002) Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976) 27(15): 1670–1673. [DOI] [PubMed] [Google Scholar]
- 41. Grabowski G, Cornett CA, Kang JD (2012) Esophageal and vertebral artery injuries during complex cervical spine surgery—avoidance and management. Orthop Clin North Am 43(1): 63–74. [DOI] [PubMed] [Google Scholar]
- 42. Sagi HC, Beutler W, Carroll E, Connolly PJ (2002) Airway complications associated with surgery on the anterior cervical spine. Spine (Phila Pa 1976) 27(9): 949–953. [DOI] [PubMed] [Google Scholar]


