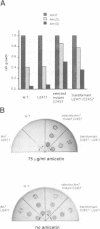
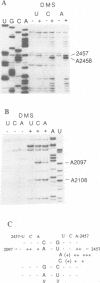
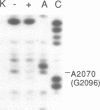
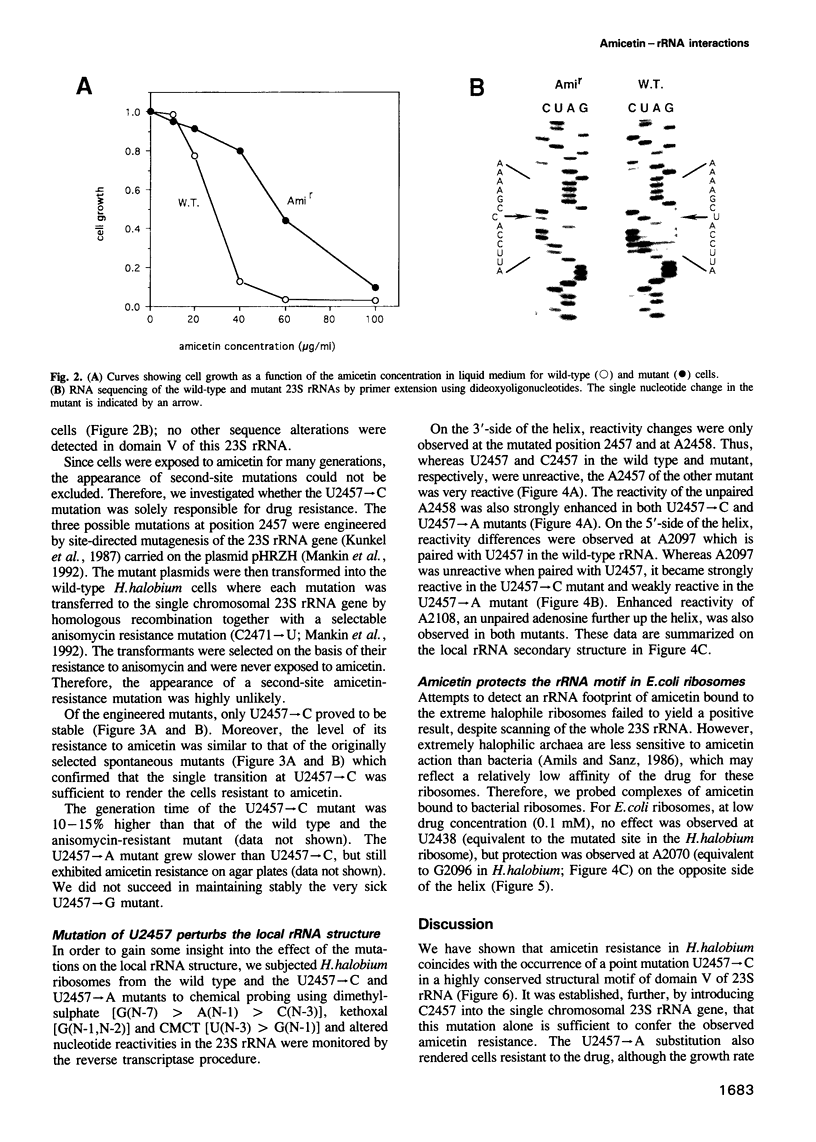
Abstract
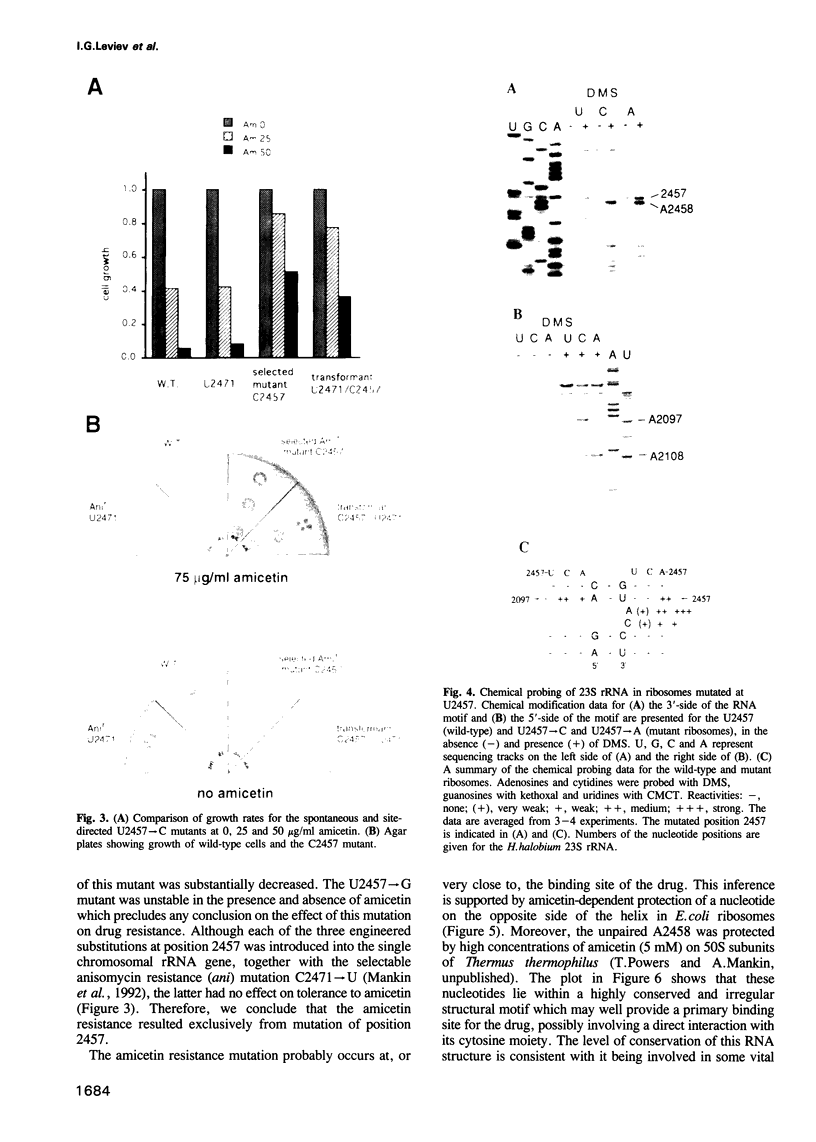
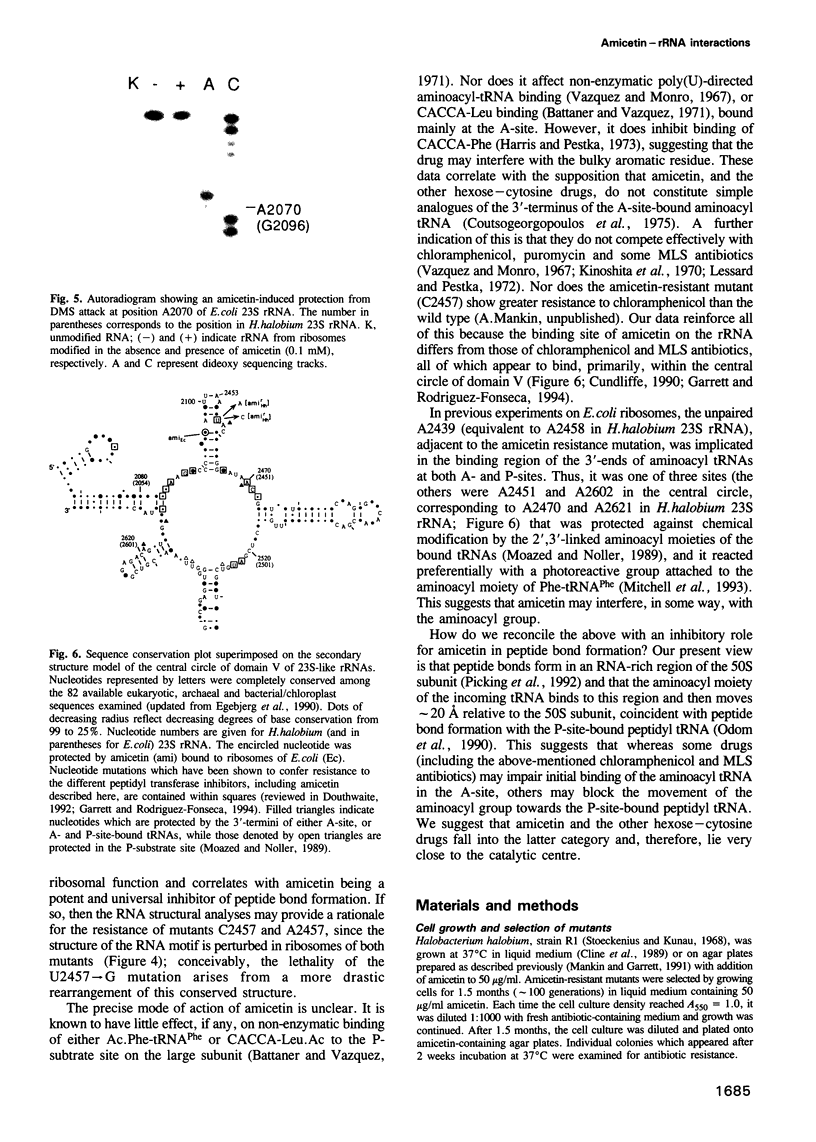
The binding site and probable site of action have been determined for the universal antibiotic amicetin which inhibits peptide bond formation. Evidence from in vivo mutants, site-directed mutations and chemical footprinting all implicate a highly conserved motif in the secondary structure of the 23S-like rRNA close to the central circle of domain V. We infer that this motif lies at, or close to, the catalytic site in the peptidyl transfer centre. The binding site of amicetin is the first of a group of functionally related hexose-cytosine inhibitors to be localized on the ribosome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battaner E., Vazquez D. Inhibitors of protein synthesis by ribosomes of the 80-S type. Biochim Biophys Acta. 1971 Dec 16;254(2):316–330. doi: 10.1016/0005-2787(71)90840-9. [DOI] [PubMed] [Google Scholar]
- Cline S. W., Lam W. L., Charlebois R. L., Schalkwyk L. C., Doolittle W. F. Transformation methods for halophilic archaebacteria. Can J Microbiol. 1989 Jan;35(1):148–152. doi: 10.1139/m89-022. [DOI] [PubMed] [Google Scholar]
- Coutsogeorgopoulos C., Bloch A., Watanabe K. A., Fox J. J. Inhibitors of protein synthesis. 4. Studies on the structure-activity relationship of gougerotin and some of its analogs. J Med Chem. 1975 Aug;18(8):771–776. doi: 10.1021/jm00242a001. [DOI] [PubMed] [Google Scholar]
- Crick F. H. The origin of the genetic code. J Mol Biol. 1968 Dec;38(3):367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Davies J. What are antibiotics? Archaic functions for modern activities. Mol Microbiol. 1990 Aug;4(8):1227–1232. doi: 10.1111/j.1365-2958.1990.tb00701.x. [DOI] [PubMed] [Google Scholar]
- Douthwaite S. Functional interactions within 23S rRNA involving the peptidyltransferase center. J Bacteriol. 1992 Feb;174(4):1333–1338. doi: 10.1128/jb.174.4.1333-1338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthwaite S., Powers T., Lee J. Y., Noller H. F. Defining the structural requirements for a helix in 23 S ribosomal RNA that confers erythromycin resistance. J Mol Biol. 1989 Oct 20;209(4):655–665. doi: 10.1016/0022-2836(89)93000-3. [DOI] [PubMed] [Google Scholar]
- Harris R., Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. XXIV. Effects of antibiotics on binding of aminoacyl-oligonucleotides to ribosomes. J Biol Chem. 1973 Feb 25;248(4):1168–1174. [PubMed] [Google Scholar]
- Kinoshita T., Tanaka N., Umezawa H. Binding of blasticidin S to ribosomes. J Antibiot (Tokyo) 1970 Jun;23(6):288–290. doi: 10.7164/antibiotics.23.288. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lessard J. L., Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. 23. Chloramphenicol, aminoacyl-oligonucleotides, and Escherichia coli ribosomes. J Biol Chem. 1972 Nov 10;247(21):6909–6912. [PubMed] [Google Scholar]
- Mankin A. S., Garrett R. A. Chloramphenicol resistance mutations in the single 23S rRNA gene of the archaeon Halobacterium halobium. J Bacteriol. 1991 Jun;173(11):3559–3563. doi: 10.1128/jb.173.11.3559-3563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin A. S., Zyrianova I. M., Kagramanova V. K., Garrett R. A. Introducing mutations into the single-copy chromosomal 23S rRNA gene of the archaeon Halobacterium halobium by using an rRNA operon-based transformation system. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6535–6539. doi: 10.1073/pnas.89.14.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Stade K., Osswald M., Brimacombe R. Site-directed cross-linking studies on the E. coli tRNA-ribosome complex: determination of sites labelled with an aromatic azide attached to the variable loop or aminoacyl group of tRNA. Nucleic Acids Res. 1993 Feb 25;21(4):887–896. doi: 10.1093/nar/21.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989 May 19;57(4):585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Ribosomal RNA and translation. Annu Rev Biochem. 1991;60:191–227. doi: 10.1146/annurev.bi.60.070191.001203. [DOI] [PubMed] [Google Scholar]
- Odom O. W., Picking W. D., Hardesty B. Movement of tRNA but not the nascent peptide during peptide bond formation on ribosomes. Biochemistry. 1990 Dec 4;29(48):10734–10744. doi: 10.1021/bi00500a004. [DOI] [PubMed] [Google Scholar]
- Picking W. D., Odom O. W., Hardesty B. Evidence for RNA in the peptidyl transferase center of Escherichia coli ribosomes as indicated by fluorescence. Biochemistry. 1992 Dec 22;31(50):12565–12570. doi: 10.1021/bi00165a004. [DOI] [PubMed] [Google Scholar]
- Toeckenius W., Kunau W. H. Further characterization of particulate fractions from lysed cell envelopes of Halobacterium halobium and isolation of gas vacuole membranes. J Cell Biol. 1968 Aug;38(2):337–357. doi: 10.1083/jcb.38.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez D., Monro R. E. Effects of some inhibitors of protein synthesis on the binding of aminoacyl tRNA to ribosomal subunits. Biochim Biophys Acta. 1967 Jun 20;142(1):155–173. doi: 10.1016/0005-2787(67)90524-2. [DOI] [PubMed] [Google Scholar]