Summary
Type 1 diabetes (T1D) results from the specific immune-mediated destruction of the insulin-producing β-cells of the pancreas. In genetically susceptible individuals, a still undetermined initiating ‘hit’ triggers a cascade of events that eventually leads to autoreactive CD8 T cells infiltrating the pancreatic islets and, subsequently, destroying them. There is increasing evidence that viruses, especially enteroviruses, are major environmental candidates; however, despite decades of investigation, we still lack certainty with regard to the causation of T1D. Moreover, studies in animal models of diabetes suggest a protective role of certain enteroviral infections upon diabetes contraction, making the quest for viral involvement in T1D even more difficult. Analyzing the foundation and the results of the most current work in the field, this article gives a brief overview of current knowledge, as well as providing an outlook for future directions.
There is robust evidence for the genetic basis of Type 1 diabetes (T1D), especially with regard to permissive HLA genotypes. However, the frequency of autoimmune diseases has increased considerably in recent decades, the annual increase in T1D incidence is currently estimated to be 3% [1]. This rising incidence, corroborated with a strong inhomogeneity in its geographical distribution, has to be attributed to environmental changes [2,3]. Moreover, the distribution of many autoimmune diseases forms a gradient between the northern and southern hemispheres [4], and the first generation of offspring from immigrants adopt the incidence rate of their new home country [2,5].
The hypothesis that viruses, mainly enteroviruses (EV), might be involved in T1D pathogenesis has a long history; however, we are still lacking a strong causal association between virus infection and T1D. Recent developments in analysis techniques, as well our access to organ libraries such as the Network of Pancreatic Organ Donors (nPOD) in the USA or the collection of Foulis in the UK offer unprecedented opportunities to study these correlations. In this article, we give a brief overview of current studies in the field, as well as providing an outlook for possible future research.
Methods
The information contained herein was gathered by means of a meta-analysis performed using the National Library of Medicine database. The search engines were Google, as well as the National Library of Medicine proprietary search engine PubMed. Furthermore, the abstracts from the Scientific Sessions of the American Diabetes Association, as well as abstracts from the European Association for the Study of Diabetes conference 2012 were used, where applicable.
Brief history
The first expression of the paradigm of EV causes of T1D was formulated in 1974 by Nerup and colleagues who suggested that, in genetically determined hosts, the immune response fails to eliminate an infecting virus (coxsackie virus B [CVB] 4) that, in turn, might infect the pancreatic β-cells and trigger an autoimmune response [6].
Almost four decades later, Dotta and colleagues demonstrated, using electron microscopy, the presence of VP1 capsid protein in β-cell specimens from three out of six T1D organ donors [7]. Richardson and colleagues demonstrated that VP1-immunopositive cells were detected in multiple islets of 44 out of 72 young recent-onset T1D patients, compared with a total of only three islets in three out of 50 neonatal and pediatric normal controls [8]. More recently, the same group found islets positive for VP1 in some β-cells in more than 60% of patients with recent-onset T1D, but in very few age-matched controls within the Foulis pancreas tissue collection [9]. In addition, unpublished studies from the nPOD-virus consortium [Oikarinen M & Hyöty H, Unpublished Data] suggest that a high proportion of the nPOD T1D collection, in which residual β-cells persist, also display evidence of EV infection (as judged by immunostaining for viral protein). This is not the case for age-matched controls.
The epidemiological studies have not been as conclusive thus far. In a recent meta-analysis, Yeung and colleagues found a strong association between EV infection (based on detection with molecular methods) and T1D [10], in contrast with older analyses that suggested the opposite [11]. However, a marked heterogeneity in study design and methods renders these results, while suggestive, insufficient to prove the causal role of EV infection in T1D. For instance, MIDIA and babydiet reported no significant association between EV traces in stool samples and islet autoimmunity [12,13], and the DAISY study found no significant association with islet autoimmunity neither with RNA analyses in serum, saliva or rectal swab samples [14] nor serologically [Rewers M, Unpublished Data]. Furthermore, in a recent study, Yeung and colleagues showed that children with islet autoimmunity have higher levels of multiple cytokines, consistent with an active inflammatory process in the prediabetic state, yet these findings were unrelated to coincident EV infection [15]. However, the DIPP study found a close temporal association between the appearance of the first diabetes-associated autoantibodies and EV infections [16,17], and similar results were seen in the TRIGR study [18].
Recently, Oikarinen and colleagues reported small, yet significant, differences in the frequency of serum EV RNA during follow-up of 38 cases who progressed from islet autoimmunity to T1D versus matched controls [19]. The difference in the frequency of enterovirus RNA between case children and control children was highest during the 6-month period before the appearance of the first autoantibody. An accelerating effect of EV infections on progression from autoantibody positivity towards T1D was also seen in the older DiMe study [20,21] as well as in the more recent DAISY follow-up study [22].
Notably, a common finding in almost all longitudinal studies was that in the same individual, EV RNA can rarely be found continuously in stool samples for more than approximately 3 months, and for a much shorter time in serum samples [16,14]. It is estimated that EV RNA can be found in serum for 2 weeks at most; however, there is no conclusive evidence for this. This is an important confounding factor underlining that negativity for virus at diagnosis does not mean missing viral etiology but strengthens the hypothesis of a ‘hit and run’ scenario with multiple viral infections leading cumulatively to pathological effects upon β-cells. Another confounding factor is the seasonality of EV infections. In humans, data from prospective studies suggest a seasonal pattern in the appearance of autoantibodies that resembles the seasonality of EV infections [23]. However, the seasonal pattern observed in the onset of clinical T1D is rather modest and, on another note, EV herd immunity is low in countries with the highest incidence of T1D [24]. Furthermore, the increase in T1D incidence has been rather accompanied by the decrease in EV infections during recent decades [25–27]. One explanation could be that, in those countries, low EV herd immunity leads to children getting their first EV infection at a later age when protection by maternal antibodies have already ceased, therefore, the outcome of an infection can be more severe (i.e., spreading to the pancreas).
Notably, it has recently been demonstrated that gestational EV infections are associated with an increased risk for T1D in the offspring: EV-IgM in early pregnancy increased the risk for islet autoantibodies at delivery in nondiabetic mothers with HLA-DQ 2/2 or 2/X T1D risk genotypes [28].
Hallmarks of T1D pathogenesis with reference to viral infections
In recent years, the authors and others have performed extensive studies on the histopathological features of T1D within the nPOD cohort and, in spite of still restricted concludent information about the prediabetic period, a vast amount of histopathological features of T1D have been found that can be explained by viral infection.For instance:
Insulitis, which is considered to be a hallmark of early T1D pathogenesis, was only found in two out of the three cases that presented at least three autoantibodies, but in none of the other 59 antibody-positive subjects or 62 matched controls [29]. Thus, if autoantibodies are present and insulitis is only observed in a few cases, we have to think of T1D as a relapsing–remitting disease. One of the possible explanations for this scenario is recurrent viral infection: either de novo infection or flare-up of a chronic infection, or viruses, which persist but do not permanently reside in the pancreas (e.g., Herpesviridae);
At the time of diagnosis, the pattern of insulitis is not homogenous, as would be expected in the case of a stochastic development, but is lobular [30,31]. Again, one of the explanations for this phenomenon could be viral infections, as well as vascular or neuronal factors;
The upregulation of MHC I on most islets, a phenomenon previously described by Foulis et al. [32], that can persist for years [31] and was also found to occur without a concurrent inflammatory infiltrate; defined as the presence of inflammatory cells [7,33]. One hypothesis able to concatenate these findings can be the virus-induced secretion of interferons [34,35]. The presence of IFN-α and MHC I upregulation are concomitant events in β-cells [32] and the ability of both IFN-α [32] and IFN-γ [34,36] to induce MHC I upregulation is well known. The persistence of EV in islet cells is associated with the chronic synthesis of IFN-α [35,37] and other cytokines [37] in human islets inoculated with CVB3 virus. Furthermore, CVB4, in the presence of antibodies and through the specific viral receptor coxsackie adenovirus receptor, was shown to infect monocytes resulting in IFN-α synthesis [38]. In addition, in a mouse model of virus-induced diabetes [34], as well as in the nonobese diabetic (NOD) mouse model [39], the presence of IFN-γ was essential for the contraction of diabetes. However, despite strong evidence for the presence of viral protein within islets [7], we still lack conclusive proof of viral genomes in islets or β-cells;
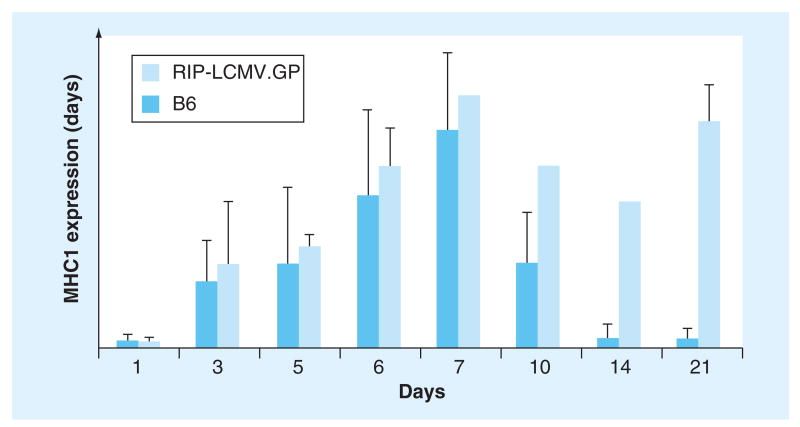
Viral infections can upregulate MHC I (Figure 1), therefore, creating a fertile, inflammatory field that eventually leads to the unmasking of β-cell antigens with subsequent infiltration with CD8+ cells and the killing of the β-cells through direct and indirect mechanisms [40];
Figure 1. Time course of MHC class I induction in nontransgenic and rat insulin promoter-linked lymphocytic choriomeningitis virus glycoprotein-expressing transgenic mice after lymphocytic choriomeningitis virus infection.
Upregulation of MHC I occurs as early as 2 days postinfection (not shown) and reaches baseline levels in nontransgenic mice at approximately day 21 (no islet infiltration), whereas RIP-LCMV.GP transgenic mice continue to exhibit elevated MHC class I levels reflecting ongoing islet infiltration and destruction. At later timepoints, only very few islets were available in RIP-LCMV.GP mice, reflecting onset of Type 1 diabetes.
B6: Nontransgenic; RIP-LCMV.GP: Rat insulin promoter-linked lymphocytic choriomeningitis virus glycoprotein.
Reproduced with permission from [20].
EVs have especially been shown to have a strong pancreotropism: severe islet damage was demonstrated in fatal CVB infection cases [41], islets demonstrate strong expression of the coxsackie adenovirus receptor [35,42] and β-cells are permissive for EV in vitro [43].
Taken together, these observations suggest that T1D might evolve through a series of inflammatory ‘hits’ affecting certain areas of the pancreas in a relapsing–remitting fashion, and that viruses may well fit into this scenario.
Direct pathogenetic associations between EV infections & T1D
In spite of the fact that a direct causal link between EV infection and T1D contraction is still lacking in humans (except in rare cases of fulminant T1D where an association with EV infection was discussed in case reports [44]), there are several examples of EV-induced diabetes in animals: we have seen diabetes development upon infection with CVB [45] and encephalomyocarditis virus [46] in mice; with the Lyungan virus in voles [47]; Kilham virus in rats [48]; and with the bovine viral diarrhea virus in cattle [49].
Viral infection can protect from diabetes
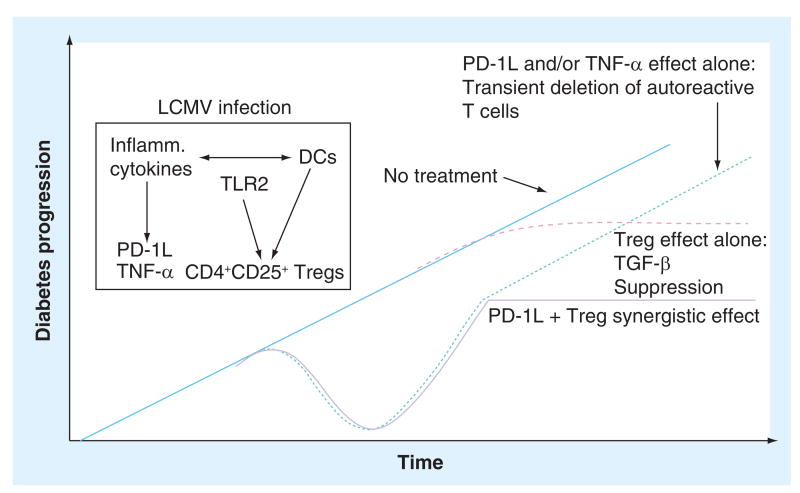
Under certain circumstances, infection with CVB can either accelerate (in the lymphocytic choriomeningitis virus diabetes mouse model) or abrogate (in the NOD mouse model, when induced at an early timepoint) the development of T1D [50]. The author's studies suggest that the timing of the infection is, in the latter case, very important. Mechanistical explanations comprise the TGF-β augmentation of a bystander Treg cell population, as well as reduction in T-effector activity through virally induced PD-1L and TNF-α (Figure 2) [51,52]. When regarded from an evolutionary point of view, this hypothesis makes sense: the body tries to limit the damage provoked by its own immune system while fighting a viral infection by enhancing the Treg response. Transferring a small number of Treg cells, which would not normally suffice to provide protection from a NOD mouse that has previously been infected with CVB3 to an unmolested NOD mouse, will protect the latter from developing T1D – thus, the viral infection positively stimulates the Treg compartment [53]. These enhancing effects upon polyclonal Tregs are mainly elicited through TLR2 [53]. These observations could help explain the proverbial hygiene hypothesis or, to a lesser extent, the suggested protection from T1D development in mice by infection with certain helminthes [54].
Figure 2. How viral infections stop Type 1 diabetes; TGF-β-mediated invigoration of a bystander Treg population, as well as downregulation of autoaggressive T cells through virally mediated induction of PD-1L and TNF-α.
DC: Dendritic cell; LCMV: Lymphocytic choriomeningitis virus.
Reproduced with permission from [43].
Conclusion
Novel insights in the histopathology of T1D make a causal link to viral infections very probable. Viruses can initiate autoimmunity, promote it, and precipitate and abrogate the onset of disease; however, all these roles have yet to be confirmed in larger prospective studies.
There have been many discussions regarding whether there is a rationale for developing a vaccine for CVB. A separate analysis of probes from the Finnish DIPP study has shown a higher prevalence of viral CVB RNA in stool probes from children who developed T1D autoantibodies and, within these results, a higher occurrence of the subtype CVB1 [Hyöti H, Unpublished Data]. However, given the fact that there are more than 100 EV serotypes, more systematic studies are required with more uniform assays to prove a pathogenetic causality of such a strength that the development of a vaccine can ethically and financially be considered. In conclusion, prospective studies with high case numbers, a much more frequent sampling of various specimens and a highly standardized methodology are necessary to prove statistically significant associations between EV infections and the risk of islet autoimmunity or T1D. These analyses can only be performed via a concise collaborative effort of scientists from various fields. A very promising approach in this regard is the recently founded nPOD-V consortium [101].
Future perspective
Access to more tissue specimens, the fast development of novel techniques and a new, collaborative effort will open new avenues in the research of the viral causes of T1D. However, we should also start thinking outside of the box and consider other viral pathogens than EV, which might be a better fit for the relapsing–remitting scenario and for the lobular fashion of the disease. One idea could be that the virus does not infect the endocrine pancreas, but instead infects other adjacent structures. Elegant studies by Dosch and colleagues have shown that the first structures to be involved in T1D pathogenesis might be sensoric neurons innervating the islet [55]. Herpesviridae are known to persist in neuronal structures and to occasionally descend to their target tissue; however, they can rarely be found there (hit and run theory).
The authors also encourage a closer look towards other environmental factors that affect viral infections, such as the intestinal microbiota. Recently, a small study including DIPP children who eventually progressed to T1D and controls, examined the intestinal microbiome in feces collected either before, at seroconversion or close to the diagnosis of overt T1D [56] and showed that, in comparison to controls, children who developed T1D developed a less diverse microbiome with preponderantly nonbutyrate-producing lactate-utilizing bacteria that prevented optimal mucin synthesis [57]. Since the microbiota are present at the sites used by viruses to gain entry to their host, they can potentially alter the course and outcome of infection.
The causal link between EV and T1D pathogenesis is yet to be found. It probably involves a complex interplay between viruses, β-cells, and the innate and adaptive immune systems in the given genotypical context of an individual. Finding these associations, although challenging, can be decisive in developing new preventive and therapeutic strategies to fight this disease.
Practice Points.
Epidemiological data suggest the increasing importance of environmental factors in Type 1 diabetes (T1D) pathogenesis.
Histopathological features of early T1D suggest viruses to be prime environmental candidates.
The hypothesis of enteroviral involvement in T1D was formulated in 1974 but the first proof of the association between enteroviridae and T1D came 40 years later, when virus protein 1-immunopositive cells were detected in multiple islets of 44 out of 72 young recent-onset T1D patients, compared with three islets from three out of 50 normal controls.
The majority of the histopathological hallmarks of early diabetes pathogenesis could be explained by viral infection: insulitis, MHC I upregulation and interferon production.
Enteroviruses have a strong pancreotropism and islets show strong expression of the coxsackie adenovirus receptor.
Enterovirus can induce diabetes in animal models, but also protect from the development of T1D in other animal models.
More systematic analyses are needed to address the role of enterovirus in T1D.
These analyses can only be performed via a concise collaborative effort of scientists from various fields.
A very promising approach in this regard is the recently founded Network of Pancreatic Organ Donors–Virus consortium.
Footnotes
Financial & competing interests disclosure: MG von Herrath holds a position with NovoNordisk Inc.; however, no therapeutic strategies or products from Novo-Nordisk are discussed herein. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, Laporte R, Tuomilehto J. Incidence of childhood Type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000;23(10):1516–1526. [Google Scholar]
- 2.Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwright R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ. 1992;304(6833):1020–1022. doi: 10.1136/bmj.304.6833.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood Type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364(9446):1699–1700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- 4.Bach JF. Immunotherapy of Type 1 diabetes: lessons for other autoimmune diseases. Arthritis Res. 2002;4(Suppl. 3):S3–S15. doi: 10.1186/ar554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delli AJ, Lindblad B, Carlsson A, et al. Type 1 diabetes patients born to immigrants to Sweden increase their native diabetes risk and differ from Swedish patients in HLA types and islet autoantibodies. Pediatr Diabetes. 2010;11(8):513–520. doi: 10.1111/j.1399-5448.2010.00637.x. [DOI] [PubMed] [Google Scholar]
- 6.Nerup J, Platz P, Andersen OO, et al. HL-A antigens and diabetes mellitus. Lancet. 1974;2(7885):864–866. doi: 10.1016/s0140-6736(74)91201-x. [DOI] [PubMed] [Google Scholar]
- 7.Dotta F, Censini S, Van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset Type 1 diabetic patients. Proc Natl Acad Sci USA. 2007;104(12):5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human Type 1 diabetes. Diabetologia. 2009;52(6):1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 9.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Immunohistochemical analysis of the relationship between islet cell proliferation and the production of the enteroviral capsid protein, VP1, in the islets of patients with recent-onset Type 1 diabetes. Diabetologia. 2011;54(9):2417–2420. doi: 10.1007/s00125-011-2192-7. [DOI] [PubMed] [Google Scholar]
- 10.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and Type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green J, Casabonne D, Newton R. Coxsackie B virus serology and Type 1 diabetes mellitus: a systematic review of published case-control studies. Diabet Med. 2004;21(6):507–514. doi: 10.1111/j.1464-5491.2004.01182.x. [DOI] [PubMed] [Google Scholar]
- 12.Simonen-Tikka Ml, Hiekka AK, Klemola P, et al. Early human enterovirus infections in healthy Swedish children participating in the PRODIA pilot study. J Med Virol. 2012;84(6):923–930. doi: 10.1002/jmv.23284. [DOI] [PubMed] [Google Scholar]
- 13.Tapia G, Cinek O, Rasmussen T, et al. Human enterovirus RNA in monthly fecal samples and islet autoimmunity in Norwegian children with high genetic risk for Type 1 diabetes: the MIDIA study. Diabetes Care. 2010;34(1):151–155. doi: 10.2337/dc10-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graves PM, Rotbart HA, Nix WA, et al. Prospective study of enteroviral infections and development of beta-cell autoimmunity. Diabetes autoimmunity study in the young (DAISY) Diabetes Res Clin Pract. 2003;59(1):51–61. doi: 10.1016/s0168-8227(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 15.Yeung WC, Al-Shabeeb A, Pang CN, et al. Children with islet autoimmunity and enterovirus infection demonstrate a distinct cytokine profile. Diabetes. 2012;61(6):1500–1508. doi: 10.2337/db11-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salminen KK, Vuorinen T, Oikarinen S, et al. Isolation of enterovirus strains from children with preclinical Type 1 diabetes. Diabet Med. 2004;21(2):156–164. doi: 10.1111/j.1464-5491.2004.01097.x. [DOI] [PubMed] [Google Scholar]
- 17.Lonnrot M, Korpela K, Knip M, et al. Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes. 2000;49(8):1314–1318. doi: 10.2337/diabetes.49.8.1314. [DOI] [PubMed] [Google Scholar]
- 18.Sadeharju K, Lonnrot M, Kimpimaki T, et al. Enterovirus antibody levels during the first two years of life in prediabetic autoantibody-positive children. Diabetologia. 2001;44(7):818–823. doi: 10.1007/s001250100560. [DOI] [PubMed] [Google Scholar]
- 19.Oikarinen S, Martiskainen M, Tauriainen S, et al. Enterovirus RNA in blood is linked to the development of Type 1 diabetes. Diabetes. 2010;60(1):276–279. doi: 10.2337/db10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonnrot M, Salminen K, Knip M, et al. Enterovirus RNA in serum is a risk factor for beta-cell autoimmunity and clinical Type 1 diabetes: a prospective study Childhood Diabetes in Finland (DiMe) Study Group. J Med Virol. 2000;61(2):214–220. [PubMed] [Google Scholar]
- 21.Hyoty H, Hiltunen M, Knip M, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995;44(6):652–657. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 22.Stene Lc, Oikarinen S, Hyoty H, et al. Enterovirus infection and progression from islet autoimmunity to Type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY) Diabetes. 2010;59(12):3174–3180. doi: 10.2337/db10-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimpimaki T, Kupila A, Hamalainen AM, et al. The first signs of beta-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab. 2001;86(10):4782–4788. doi: 10.1210/jcem.86.10.7907. [DOI] [PubMed] [Google Scholar]
- 24.Knip M. Pathogenesis of Type 1 diabetes: implications for incidence trends. Horm Res Paediatr. 2011;76(Suppl. 1):57–64. doi: 10.1159/000329169. [DOI] [PubMed] [Google Scholar]
- 25.Viskari HR, Koskela P, Lonnrot M, et al. Can enterovirus infections explain the increasing incidence of Type 1 diabetes. Diabetes Care. 2000;23(3):414–416. doi: 10.2337/diacare.23.3.414. [DOI] [PubMed] [Google Scholar]
- 26.Viskari H, Ludvigsson J, Uibo R, et al. Relationship between the incidence of Type 1 diabetes and enterovirus infections in different European populations: results from the EPIVIR project. J Med Virol. 2004;72(4):610–617. doi: 10.1002/jmv.20033. [DOI] [PubMed] [Google Scholar]
- 27.Viskari H, Ludvigsson J, Uibo R, et al. Relationship between the incidence of Type 1 diabetes and maternal enterovirus antibodies: time trends and geographical variation. Diabetologia. 2005;48(7):1280–1287. doi: 10.1007/s00125-005-1780-9. [DOI] [PubMed] [Google Scholar]
- 28.Resic Lindehammer S, Honkanen H, Nix WA, et al. Seroconversion to islet autoantibodies after enterovirus infection in early pregnancy. Viral Immunol. 2012;25(4):254–261. doi: 10.1089/vim.2012.0022. [DOI] [PubMed] [Google Scholar]
- 29.In't Veld P, Lievens D, De Grijse J, et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56(9):2400–2404. doi: 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- 30.Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK. Immunopathology of the human pancreas in Type-I diabetes. Semin Immunopathol. 2010;33(1):9–21. doi: 10.1007/s00281-010-0205-0. [DOI] [PubMed] [Google Scholar]
- 31.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term Type 1 diabetes patients. J Exp Med. 2012;209(1):51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foulis AK, Farquharson MA, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in Type 1 diabetes mellitus. Lancet. 1987;2(8573):1423–1427. doi: 10.1016/s0140-6736(87)91128-7. [DOI] [PubMed] [Google Scholar]
- 33.Foulis AK, Farquharson MA, Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in Type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987;30(5):333–343. doi: 10.1007/BF00299027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seewaldt S, Thomas HE, Ejrnaes M, et al. Virus-induced autoimmune diabetes: most beta-cells die through inflammatory cytokines and not perforin from autoreactive (anti-viral) cytotoxic T-lymphocytes. Diabetes. 2000;49(11):1801–1809. doi: 10.2337/diabetes.49.11.1801. [DOI] [PubMed] [Google Scholar]
- 35.Chehadeh W, Kerr-Conte J, Pattou F, et al. Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in beta cells. J Virol. 2000;74(21):10153–10164. doi: 10.1128/jvi.74.21.10153-10164.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pujol-Borrell R, Todd I, Doshi M, et al. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987;326(6110):304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- 37.Schulte BM, Lanke KH, Piganelli JD, et al. Cytokine and chemokine production by human pancreatic islets upon enterovirus infection. Diabetes. 2012;61(8):2030–2036. doi: 10.2337/db11-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goffard A, Alidjinou EK, Sane F, et al. Antibodies enhance the infection of phorbolester-differentiated human monocyte-like cells with coxsackievirus B4. Microbes Infect. 2013;15(1):18–27. doi: 10.1016/j.micinf.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Campbell IL, Kay TW, Oxbrow L, Harrison LC. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest. 1991;87(2):739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppieters K, Amirian N, von Herrath M. Intravital imaging of CTLs killing islet cells in diabetic mice. J Clin Invest. 2012;122(1):119–131. doi: 10.1172/JCI59285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ylipaasto P, Klingel K, Lindberg AM, et al. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia. 2004;47(2):225–239. doi: 10.1007/s00125-003-1297-z. [DOI] [PubMed] [Google Scholar]
- 42.Oikarinen M, Tauriainen S, Honkanen T, et al. Analysis of pancreas tissue in a child positive for islet cell antibodies. Diabetologia. 2008;51(10):1796–1802. doi: 10.1007/s00125-008-1107-8. [DOI] [PubMed] [Google Scholar]
- 43.Skog O, Korsgren O, Frisk G. Modulation of innate immunity in human pancreatic islets infected with enterovirus in vitro. J Med Virol. 2011;83(4):658–664. doi: 10.1002/jmv.21924. [DOI] [PubMed] [Google Scholar]
- 44.Akatsuka H, Yano Y, Gabazza EC, et al. A case of fulminant Type 1 diabetes with coxsackie B4 virus infection diagnosed by elevated serum levels of neutralizing antibody. Diabetes Res Clin Pract. 2009;84(3):e50–e52. doi: 10.1016/j.diabres.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4(7):781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 46.Hirasawa K, Jun HS, Han HS, Zhang ML, Hollenberg MD, Yoon JW. Prevention of encephalomyocarditis virus-induced diabetes in mice by inhibition of the tyrosine kinase signalling pathway and subsequent suppression of nitric oxide production in macrophages. J Virol. 1999;73(10):8541–8548. doi: 10.1128/jvi.73.10.8541-8548.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niklasson B, Heller KE, Schonecker B, et al. Development of Type 1 diabetes in wild bank voles associated with islet autoantibodies and the novel ljungan virus. Int J Exp Diabesity Res. 2003;4(1):35–44. doi: 10.1080/15438600303733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellerman KE, Richards CA, Guberski DL, Shek WR, Like AA. Kilham rat triggers T-cell-dependent autoimmune diabetes in multiple strains of rat. Diabetes. 1996;45(5):557–562. doi: 10.2337/diab.45.5.557. [DOI] [PubMed] [Google Scholar]
- 49.Tajima M, Yazawa T, Hagiwara K, Kurosawa T, Takahashi K. Diabetes mellitus in cattle infected with bovine viral diarrhea mucosal disease virus. Zentralbl Veterinarmed A. 1992;39(8):616–620. doi: 10.1111/j.1439-0442.1992.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 50.Filippi CM, Estes EA, Oldham JE, von Herrath MG. Immunoregulatory mechanisms triggered by viral infections protect from Type 1 diabetes in mice. J Clin Invest. 2009;119(6):1515–1523. doi: 10.1172/JCI38503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christen U, Benke D, Wolfe T, et al. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest. 2004;113(1):74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Herrath M. Can we learn from viruses how to prevent Type 1 diabetes? The role of viral infections in the pathogenesis of Type 1 diabetes and the development of novel combination therapies. Diabetes. 2009;58(1):2–11. doi: 10.2337/db08-9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filippi CM, Ehrhardt K, Estes EA, Larsson P, Oldham JE, von Herrath MG. TLR2 signaling improves immunoregulation to prevent Type 1 diabetes. Eur J Immunol. 2011;41(5):1399–1409. doi: 10.1002/eji.200939841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hubner MP, Shi Y, Torrero MN, et al. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a Type 2 immune shift and requires TGF-beta. J Immunol. 2012;188(2):559–568. doi: 10.4049/jimmunol.1100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Razavi R, Chan Y, Afifiyan FN, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127(6):1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 56.Giongo A, Gano KA, Crabb DB, et al. Toward defining the autoimmune microbiome for Type 1 diabetes. ISME J. 2010;5(1):82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown CT, Davis-Richardson AG, Giongo A, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for Type 1 diabetes. PLoS One. 2011;6(10):e25792. doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 101.Juvenile Diabetes Research Foundation. The JDRF nPOD Viral Work group: nPOD-V. 2012 http://onlineapps.jdfcure.org/AbstractReport.cfm?grant_id=38506&abs_type=LAY.