Abstract
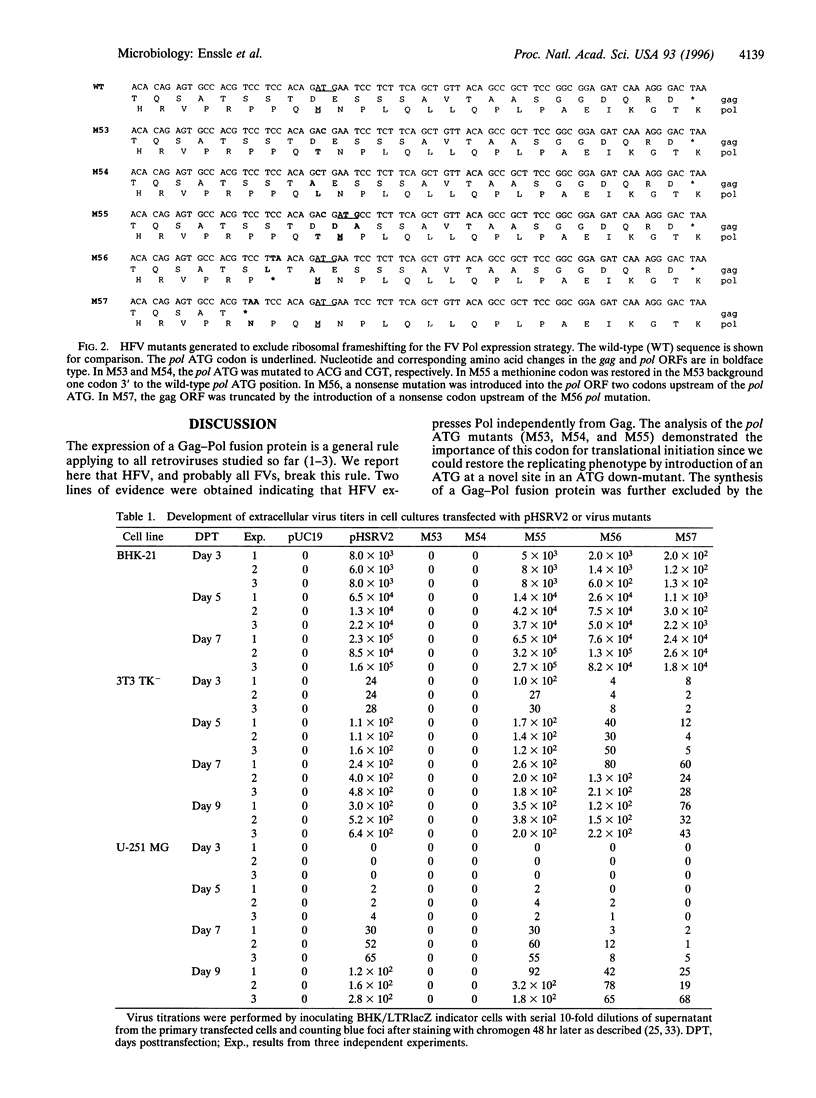
In the foamy virus (FV) subgroup of retroviruses the pol genes are located in the +1 reading frame relative to the gag genes and possess potential ATG initiation codons in their 5' regions. This genome organization suggests either a + 1 ribosomal frameshift to generate a Gag-Pol fusion protein, similar to all other retroviruses studied so far, or new initiation of Pol translation, as used by pararetroviruses, to express the Pol protein. By using a genetic approach we have ruled out the former possibility and provide evidence for the latter. Two down-mutations (M53 and M54) of the pol ATG codon were found to abolish replication and Pol protein expression of the human FV isolate. The introduction of a new ATG in mutation M55, 3' to the down-mutated ATG of mutation M53, restored replication competence, indicating that the pol ATG functions as a translational initiation codon. Two nonsense mutants (M56 and M57), which functionally separated gag and pol with respect to potential frame-shifting sites, were also replication-competent, providing further genetic evidence that FVs express the Pol protein independently from Gag. Our results show that during a particular step of the replication cycle, FVs differ fundamentally from all other retroviruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguzzi A., Wagner E. F., Netzer K. O., Bothe K., Anhauser I., Rethwilm A. Human foamy virus proteins accumulate in neurons and induce multinucleated giant cells in the brain of transgenic mice. Am J Pathol. 1993 Apr;142(4):1061–1071. [PMC free article] [PubMed] [Google Scholar]
- Bieniasz P. D., Rethwilm A., Pitman R., Daniel M. D., Chrystie I., McClure M. O. A comparative study of higher primate foamy viruses, including a new virus from a gorilla. Virology. 1995 Feb 20;207(1):217–228. doi: 10.1006/viro.1995.1068. [DOI] [PubMed] [Google Scholar]
- Chang L. J., Pryciak P., Ganem D., Varmus H. E. Biosynthesis of the reverse transcriptase of hepatitis B viruses involves de novo translational initiation not ribosomal frameshifting. Nature. 1989 Jan 26;337(6205):364–368. doi: 10.1038/337364a0. [DOI] [PubMed] [Google Scholar]
- Clare J. J., Belcourt M., Farabaugh P. J. Efficient translational frameshifting occurs within a conserved sequence of the overlap between the two genes of a yeast Ty1 transposon. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6816–6820. doi: 10.1073/pnas.85.18.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Human immunodeficiency virus as a prototypic complex retrovirus. J Virol. 1991 Mar;65(3):1053–1056. doi: 10.1128/jvi.65.3.1053-1056.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hahn H., Baunach G., Bräutigam S., Mergia A., Neumann-Haefelin D., Daniel M. D., McClure M. O., Rethwilm A. Reactivity of primate sera to foamy virus Gag and Bet proteins. J Gen Virol. 1994 Oct;75(Pt 10):2635–2644. doi: 10.1099/0022-1317-75-10-2635. [DOI] [PubMed] [Google Scholar]
- Herchenröder O., Renne R., Loncar D., Cobb E. K., Murthy K. K., Schneider J., Mergia A., Luciw P. A. Isolation, cloning, and sequencing of simian foamy viruses from chimpanzees (SFVcpz): high homology to human foamy virus (HFV). Virology. 1994 Jun;201(2):187–199. doi: 10.1006/viro.1994.1285. [DOI] [PubMed] [Google Scholar]
- Hirsch R. C., Lavine J. E., Chang L. J., Varmus H. E., Ganem D. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as wel as for reverse transcription. Nature. 1990 Apr 5;344(6266):552–555. doi: 10.1038/344552a0. [DOI] [PubMed] [Google Scholar]
- Jacks T. Translational suppression in gene expression in retroviruses and retrotransposons. Curr Top Microbiol Immunol. 1990;157:93–124. doi: 10.1007/978-3-642-75218-6_4. [DOI] [PubMed] [Google Scholar]
- Jacks T., Varmus H. E. Expression of the Rous sarcoma virus pol gene by ribosomal frameshifting. Science. 1985 Dec 13;230(4731):1237–1242. doi: 10.1126/science.2416054. [DOI] [PubMed] [Google Scholar]
- Konvalinka J., Löchelt M., Zentgraf H., Flügel R. M., Kräusslich H. G. Active foamy virus proteinase is essential for virus infectivity but not for formation of a Pol polyprotein. J Virol. 1995 Nov;69(11):7264–7268. doi: 10.1128/jvi.69.11.7264-7268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupiec J. J., Kay A., Hayat M., Ravier R., Périès J., Galibert F. Sequence analysis of the simian foamy virus type 1 genome. Gene. 1991 May 30;101(2):185–194. doi: 10.1016/0378-1119(91)90410-d. [DOI] [PubMed] [Google Scholar]
- Nassal M., Schaller H. Hepatitis B virus replication. Trends Microbiol. 1993 Sep;1(6):221–228. doi: 10.1016/0966-842x(93)90136-f. [DOI] [PubMed] [Google Scholar]
- Netzer K. O., Rethwilm A., Maurer B., ter Meulen V. Identification of the major immunogenic structural proteins of human foamy virus. J Gen Virol. 1990 May;71(Pt 5):1237–1241. doi: 10.1099/0022-1317-71-5-1237. [DOI] [PubMed] [Google Scholar]
- Netzer K. O., Schliephake A., Maurer B., Watanabe R., Aguzzi A., Rethwilm A. Identification of pol-related gene products of human foamy virus. Virology. 1993 Jan;192(1):336–338. doi: 10.1006/viro.1993.1039. [DOI] [PubMed] [Google Scholar]
- Renne R., Friedl E., Schweizer M., Fleps U., Turek R., Neumann-Haefelin D. Genomic organization and expression of simian foamy virus type 3 (SFV-3). Virology. 1992 Feb;186(2):597–608. doi: 10.1016/0042-6822(92)90026-l. [DOI] [PubMed] [Google Scholar]
- Rethwilm A. Regulation of foamy virus gene expression. Curr Top Microbiol Immunol. 1995;193:1–24. doi: 10.1007/978-3-642-78929-8_1. [DOI] [PubMed] [Google Scholar]
- Roychoudhury S., Shih C. cis rescue of a mutated reverse transcriptase gene of human hepatitis B virus by creation of an internal ATG. J Virol. 1990 Mar;64(3):1063–1069. doi: 10.1128/jvi.64.3.1063-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Radziwill G., Schaller H. Synthesis and encapsidation of duck hepatitis B virus reverse transcriptase do not require formation of core-polymerase fusion proteins. Cell. 1989 Jan 13;56(1):85–92. doi: 10.1016/0092-8674(89)90986-0. [DOI] [PubMed] [Google Scholar]
- Schliephake A. W., Rethwilm A. Nuclear localization of foamy virus Gag precursor protein. J Virol. 1994 Aug;68(8):4946–4954. doi: 10.1128/jvi.68.8.4946-4954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Rethwilm A. Replicating foamy virus-based vectors directing high level expression of foreign genes. Virology. 1995 Jun 20;210(1):167–178. doi: 10.1006/viro.1995.1328. [DOI] [PubMed] [Google Scholar]
- Schultze M., Hohn T., Jiricny J. The reverse transcriptase gene of cauliflower mosaic virus is translated separately from the capsid gene. EMBO J. 1990 Apr;9(4):1177–1185. doi: 10.1002/j.1460-2075.1990.tb08225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1618–1622. doi: 10.1073/pnas.82.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. F., Linial M. L. Analysis of the role of the bel and bet open reading frames of human foamy virus by using a new quantitative assay. J Virol. 1993 Nov;67(11):6618–6624. doi: 10.1128/jvi.67.11.6618-6624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]




