Abstract
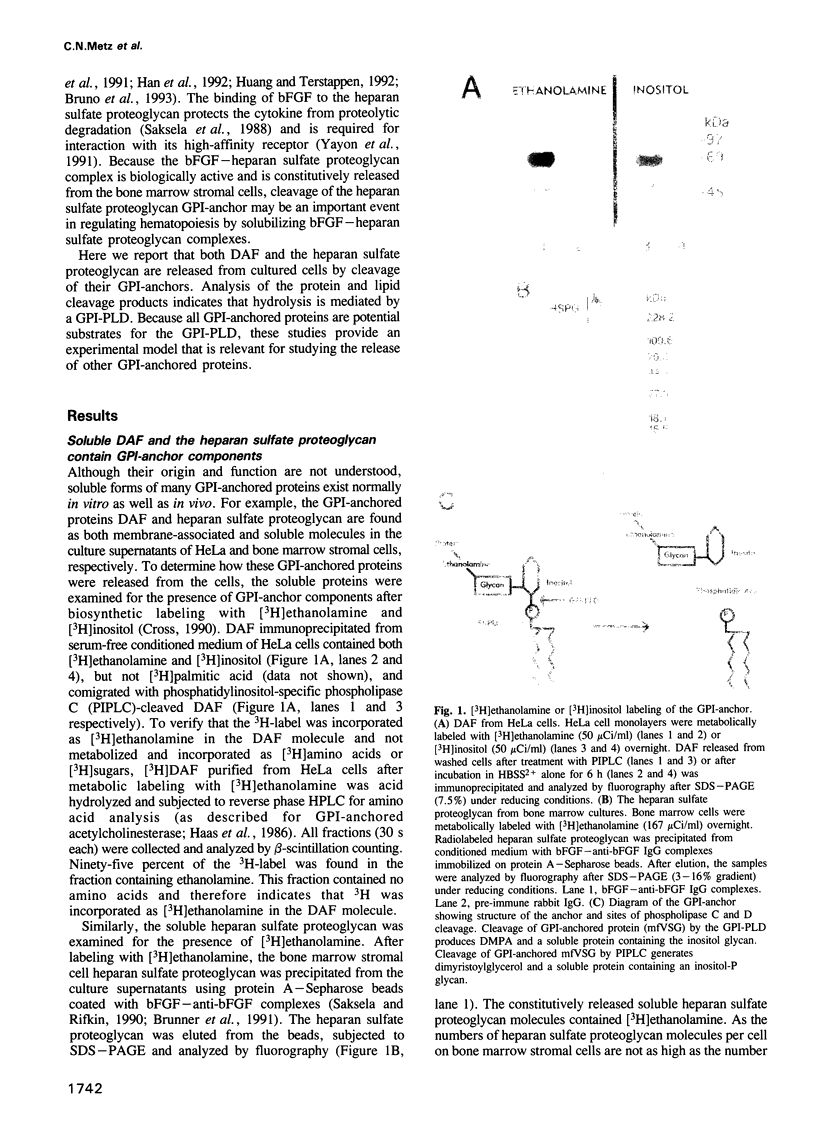
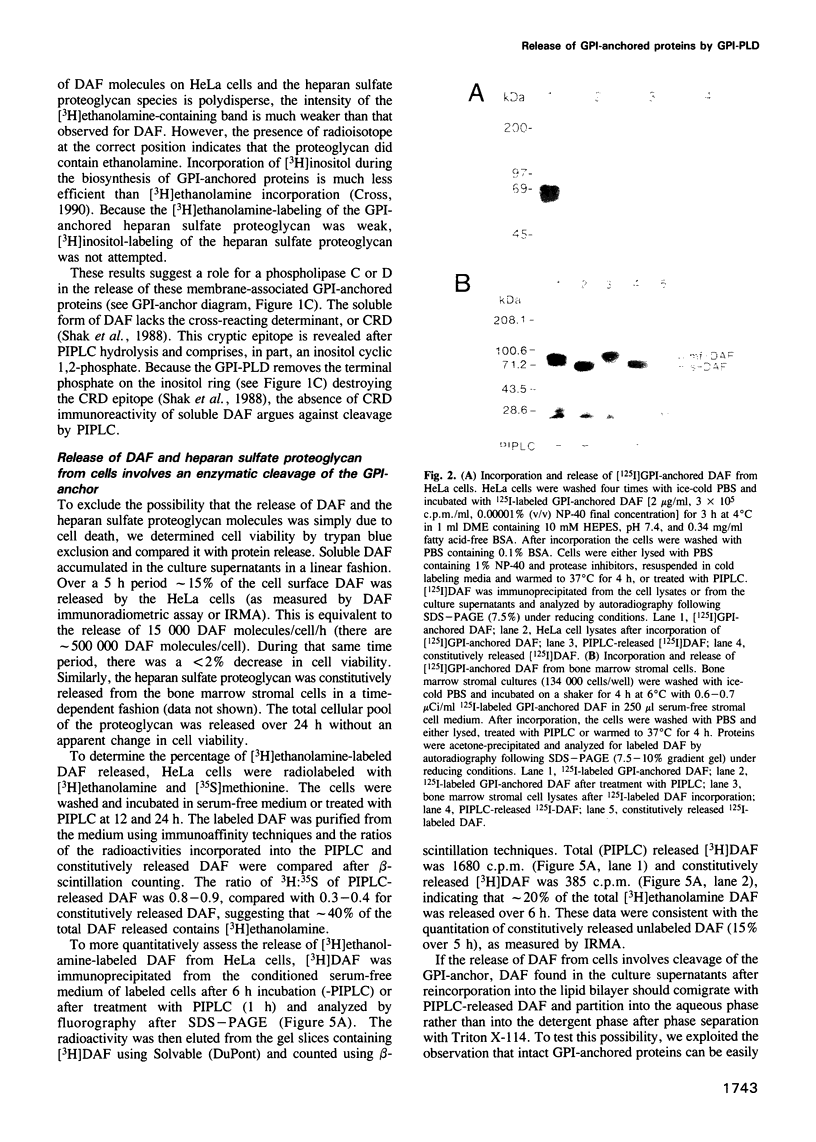
Although many glycosylphosphatidylinositol (GPI)-anchored proteins have been observed as soluble forms, the mechanisms by which they are released from the cell surface have not been demonstrated. We show here that a cell-associated GPI-specific phospholipase D (GPI-PLD) releases the GPI-anchored, complement regulatory protein decay-accelerating factor (DAF) from HeLa cells, as well as the basic fibroblast growth factor-binding heparan sulfate proteoglycan from bone marrow stromal cells. DAF found in the HeLa cell culture supernatants contained both [3H]ethanolamine and [3H]inositol, but not [3H]palmitic acid, whereas the soluble heparan sulfate proteoglycan present in bone marrow stromal cell culture supernatants contained [3H]ethanolamine. 125I-labeled GPI-DAF incorporated into the plasma membranes of these two cell types was released in a soluble form lacking the fatty acid GPI-anchor component. GPI-PLD activity was detected in lysates of both HeLa and bone marrow stromal cells. Treatment of HeLa cells with 1,10-phenanthroline, an inhibitor of GPI-PLD, reduced the release of [3H]ethanolamine-DAF by 70%. The hydrolysis of these GPI-anchored molecules is likely to be mediated by an endogenous GPI-PLD because [3H]ethanolamine DAF is constitutively released from HeLa cells maintained in serum-free medium. Furthermore, using PCR, a GPI-PLD mRNA has been identified in cDNA libraries prepared from both cell types. These studies are the first demonstration of the physiologically relevant release of GPI-anchored proteins from cells by a GPI-PLD.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almqvist P., Carlsson S. R. Characterization of a hydrophilic form of Thy-1 purified from human cerebrospinal fluid. J Biol Chem. 1988 Sep 5;263(25):12709–12715. [PubMed] [Google Scholar]
- Baron M. D., Luzio J. P. The synthesis and turnover of 5'-nucleotidase in primary cultured hepatocytes. Biochim Biophys Acta. 1987 Jan 19;927(1):81–85. doi: 10.1016/0167-4889(87)90068-1. [DOI] [PubMed] [Google Scholar]
- Bazil V., Strominger J. L. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991 Sep 1;147(5):1567–1574. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brunner G., Gabrilove J., Rifkin D. B., Wilson E. L. Phospholipase C release of basic fibroblast growth factor from human bone marrow cultures as a biologically active complex with a phosphatidylinositol-anchored heparan sulfate proteoglycan. J Cell Biol. 1991 Sep;114(6):1275–1283. doi: 10.1083/jcb.114.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner G., Nguyen H., Gabrilove J., Rifkin D. B., Wilson E. L. Basic fibroblast growth factor expression in human bone marrow and peripheral blood cells. Blood. 1993 Feb 1;81(3):631–638. [PubMed] [Google Scholar]
- Bruno E., Cooper R. J., Wilson E. L., Gabrilove J. L., Hoffman R. Basic fibroblast growth factor promotes the proliferation of human megakaryocyte progenitor cells. Blood. 1993 Jul 15;82(2):430–435. [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Davitz M. A. Decay-accelerating factor (DAF): a review of its function and structure. Acta Med Scand Suppl. 1987;715:111–121. doi: 10.1111/j.0954-6820.1987.tb09911.x. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Hereld D., Shak S., Krakow J., Englund P. T., Nussenzweig V. A glycan-phosphatidylinositol-specific phospholipase D in human serum. Science. 1987 Oct 2;238(4823):81–84. doi: 10.1126/science.2443973. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Hom J., Schenkman S. Purification of a glycosyl-phosphatidylinositol-specific phospholipase D from human plasma. J Biol Chem. 1989 Aug 15;264(23):13760–13764. [PubMed] [Google Scholar]
- Davitz M. A., Schlesinger D., Nussenzweig V. Isolation of decay accelerating factor (DAF) by a two-step procedure and determination of its N-terminal sequence. J Immunol Methods. 1987 Feb 26;97(1):71–76. doi: 10.1016/0022-1759(87)90107-4. [DOI] [PubMed] [Google Scholar]
- Dexter T. M. Stromal cell associated haemopoiesis. J Cell Physiol Suppl. 1982;1:87–94. doi: 10.1002/jcp.1041130414. [DOI] [PubMed] [Google Scholar]
- Durbec P., Gennarini G., Goridis C., Rougon G. A soluble form of the F3 neuronal cell adhesion molecule promotes neurite outgrowth. J Cell Biol. 1992 May;117(4):877–887. doi: 10.1083/jcb.117.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood P. C., Deutsch J. C., Kolhouse J. F. The conversion of the human membrane-associated folate binding protein (folate receptor) to the soluble folate binding protein by a membrane-associated metalloprotease. J Biol Chem. 1991 Feb 5;266(4):2346–2353. [PubMed] [Google Scholar]
- Ferguson M. A. Colworth Medal Lecture. Glycosyl-phosphatidylinositol membrane anchors: the tale of a tail. Biochem Soc Trans. 1992 May;20(2):243–256. doi: 10.1042/bst0200243. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fukami K., Takenawa T. Phosphatidic acid that accumulates in platelet-derived growth factor-stimulated Balb/c 3T3 cells is a potential mitogenic signal. J Biol Chem. 1992 Jun 5;267(16):10988–10993. [PubMed] [Google Scholar]
- Furley A. J., Morton S. B., Manalo D., Karagogeos D., Dodd J., Jessell T. M. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990 Apr 6;61(1):157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Gabbianelli M., Sargiacomo M., Pelosi E., Testa U., Isacchi G., Peschle C. "Pure" human hematopoietic progenitors: permissive action of basic fibroblast growth factor. Science. 1990 Sep 28;249(4976):1561–1564. doi: 10.1126/science.2218497. [DOI] [PubMed] [Google Scholar]
- Gallicchio V. S., Hughes N. K., Hulette B. C., DellaPuca R., Noblitt L. Basic fibroblast growth factor (B-FGF) induces early- (CFU-s) and late-stage hematopoietic progenitor cell colony formation (CFU-gm, CFU-meg, and BFU-e) by synergizing with GM-CSF, Meg-CSF, and erythropoietin, and is a radioprotective agent in vitro. Int J Cell Cloning. 1991 May;9(3):220–232. doi: 10.1002/stem.5530090306. [DOI] [PubMed] [Google Scholar]
- Haas R., Brandt P. T., Knight J., Rosenberry T. L. Identification of amine components in a glycolipid membrane-binding domain at the C-terminus of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3098–3105. doi: 10.1021/bi00359a005. [DOI] [PubMed] [Google Scholar]
- Han Z. C., Bellucci S., Wan H. Y., Caen J. P. New insights into the regulation of megakaryocytopoiesis by haematopoietic and fibroblastic growth factors and transforming growth factor beta 1. Br J Haematol. 1992 May;81(1):1–5. doi: 10.1111/j.1365-2141.1992.tb08161.x. [DOI] [PubMed] [Google Scholar]
- He H. T., Finne J., Goridis C. Biosynthesis, membrane association, and release of N-CAM-120, a phosphatidylinositol-linked form of the neural cell adhesion molecule. J Cell Biol. 1987 Dec;105(6 Pt 1):2489–2500. doi: 10.1083/jcb.105.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereld D., Krakow J. L., Bangs J. D., Hart G. W., Englund P. T. A phospholipase C from Trypanosoma brucei which selectively cleaves the glycolipid on the variant surface glycoprotein. J Biol Chem. 1986 Oct 15;261(29):13813–13819. [PubMed] [Google Scholar]
- Hoener M. C., Brodbeck U. Phosphatidylinositol-glycan-specific phospholipase D is an amphiphilic glycoprotein that in serum is associated with high-density lipoproteins. Eur J Biochem. 1992 Jun 15;206(3):747–757. doi: 10.1111/j.1432-1033.1992.tb16981.x. [DOI] [PubMed] [Google Scholar]
- Hortsch M., Goodman C. S. Drosophila fasciclin I, a neural cell adhesion molecule, has a phosphatidylinositol lipid membrane anchor that is developmentally regulated. J Biol Chem. 1990 Sep 5;265(25):15104–15109. [PubMed] [Google Scholar]
- Huang K. S., Li S., Fung W. J., Hulmes J. D., Reik L., Pan Y. C., Low M. G. Purification and characterization of glycosyl-phosphatidylinositol-specific phospholipase D. J Biol Chem. 1990 Oct 15;265(29):17738–17745. [PubMed] [Google Scholar]
- Huang S., Terstappen L. W. Formation of haematopoietic microenvironment and haematopoietic stem cells from single human bone marrow stem cells. Nature. 1992 Dec 24;360(6406):745–749. doi: 10.1038/360745a0. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Nussenzweig V. Endogenous association of decay-accelerating factor (DAF) with C4b and C3b on cell membranes. J Immunol. 1986 May 1;136(9):3390–3395. [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemens M. R., Sherman W. R., Holmberg N. J., Ruedi J. M., Low M. G., Thompson L. F. Characterization of soluble vs membrane-bound human placental 5'-nucleotidase. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1371–1377. doi: 10.1016/0006-291x(90)91601-n. [DOI] [PubMed] [Google Scholar]
- Lemansky P., Fatemi S. H., Gorican B., Meyale S., Rossero R., Tartakoff A. M. Dynamics and longevity of the glycolipid-anchored membrane protein, Thy-1. J Cell Biol. 1990 May;110(5):1525–1531. doi: 10.1083/jcb.110.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Huang K. S. Factors affecting the ability of glycosylphosphatidylinositol-specific phospholipase D to degrade the membrane anchors of cell surface proteins. Biochem J. 1991 Oct 15;279(Pt 2):483–493. doi: 10.1042/bj2790483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Prasad A. R. A phospholipase D specific for the phosphatidylinositol anchor of cell-surface proteins is abundant in plasma. Proc Natl Acad Sci U S A. 1988 Feb;85(4):980–984. doi: 10.1073/pnas.85.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. The glycosyl-phosphatidylinositol anchor of membrane proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):427–454. doi: 10.1016/0304-4157(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- Malik A. S., Low M. G. Conversion of human placental alkaline phosphatase from a high Mr form to a low Mr form during butanol extraction. An investigation of the role of endogenous phosphoinositide-specific phospholipases. Biochem J. 1986 Dec 1;240(2):519–527. doi: 10.1042/bj2400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson W. J., Raper J., Doering T. L., Hart G. W., Englund P. T. Fatty acid remodeling: a novel reaction sequence in the biosynthesis of trypanosome glycosyl phosphatidylinositol membrane anchors. Cell. 1990 Jul 13;62(1):73–80. doi: 10.1016/0092-8674(90)90241-6. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz C. N., Zhang Y. Y., Guo Y., Tsang T. C., Kochan J. P., Altszuler N., Davitz M. A. Production of the glycosylphosphatidylinositol-specific phospholipase D by the islets of Langerhans. J Biol Chem. 1991 Sep 25;266(27):17733–17736. [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Moran P., Beasley H., Gorrell A., Martin E., Gribling P., Fuchs H., Gillett N., Burton L. E., Caras I. W. Human recombinant soluble decay accelerating factor inhibits complement activation in vitro and in vivo. J Immunol. 1992 Sep 1;149(5):1736–1743. [PubMed] [Google Scholar]
- Moran P., Raab H., Kohr W. J., Caras I. W. Glycophospholipid membrane anchor attachment. Molecular analysis of the cleavage/attachment site. J Biol Chem. 1991 Jan 15;266(2):1250–1257. [PubMed] [Google Scholar]
- Pelham H. R. Multiple targets for brefeldin A. Cell. 1991 Nov 1;67(3):449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallon B. J., Fung W. J., Tsang T. C., Li S., Kado-Fong H., Huang K. S., Kochan J. P. Primary structure and functional activity of a phosphatidylinositol-glycan-specific phospholipase D. Science. 1991 Apr 19;252(5004):446–448. doi: 10.1126/science.2017684. [DOI] [PubMed] [Google Scholar]
- Shak S., Davitz M. A., Wolinsky M. L., Nussenzweig V., Turner M. J., Gurnett A. Partial characterization of the cross-reacting determinant, a carbohydrate epitope shared by decay accelerating factor and the variant surface glycoprotein of the African Trypanosoma brucei. J Immunol. 1988 Mar 15;140(6):2046–2050. [PubMed] [Google Scholar]
- Tausk F., Fey M., Gigli I. Endocytosis and shedding of the decay accelerating factor on human polymorphonuclear cells. J Immunol. 1989 Nov 15;143(10):3295–3302. [PubMed] [Google Scholar]
- Ting A. E., Pagano R. E. Detection of a phosphatidylinositol-specific phospholipase C at the surface of Swiss 3T3 cells and its potential role in the regulation of cell growth. J Biol Chem. 1990 Apr 5;265(10):5337–5340. [PubMed] [Google Scholar]
- Wileman T., Boshans R. L., Schlesinger P., Stahl P. Monensin inhibits recycling of macrophage mannose-glycoprotein receptors and ligand delivery to lysosomes. Biochem J. 1984 Jun 15;220(3):665–675. doi: 10.1042/bj2200665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. L., Rifkin D. B., Kelly F., Hannocks M. J., Gabrilove J. L. Basic fibroblast growth factor stimulates myelopoiesis in long-term human bone marrow cultures. Blood. 1991 Mar 1;77(5):954–960. [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Zhang F., Schmidt W. G., Hou Y., Williams A. F., Jacobson K. Spontaneous incorporation of the glycosyl-phosphatidylinositol-linked protein Thy-1 into cell membranes. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5231–5235. doi: 10.1073/pnas.89.12.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]