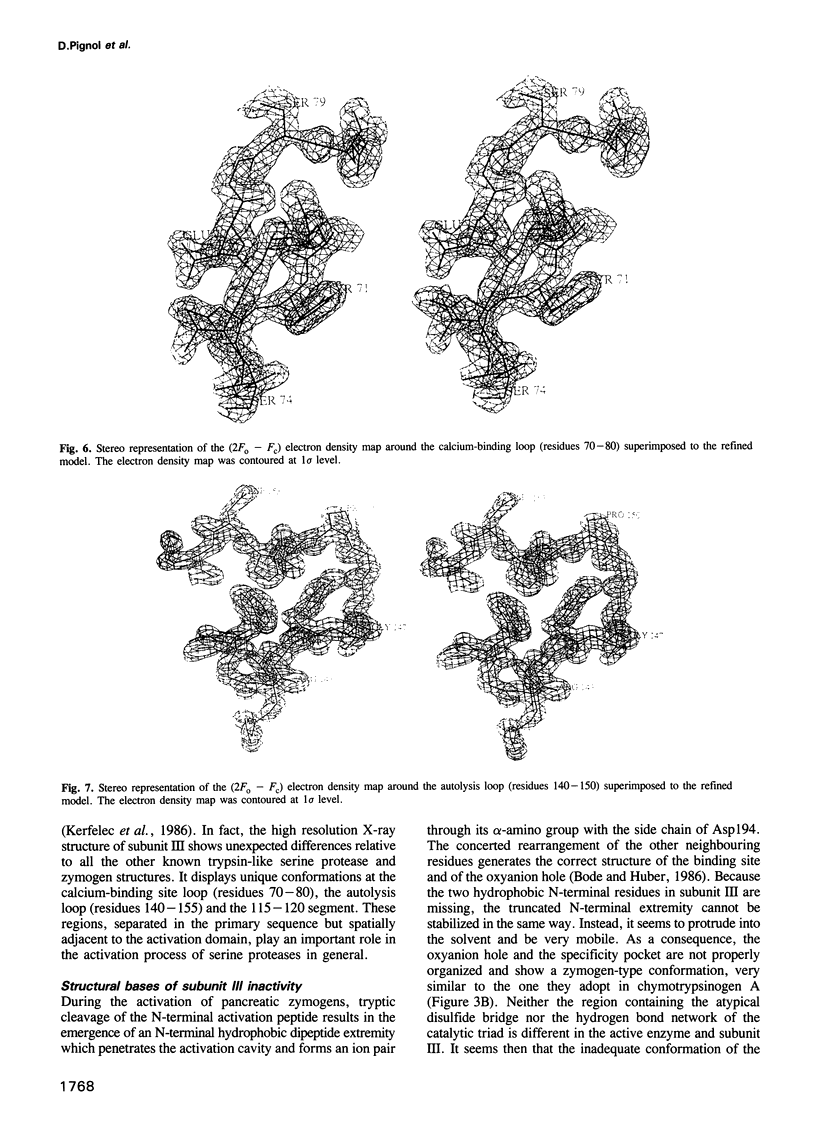
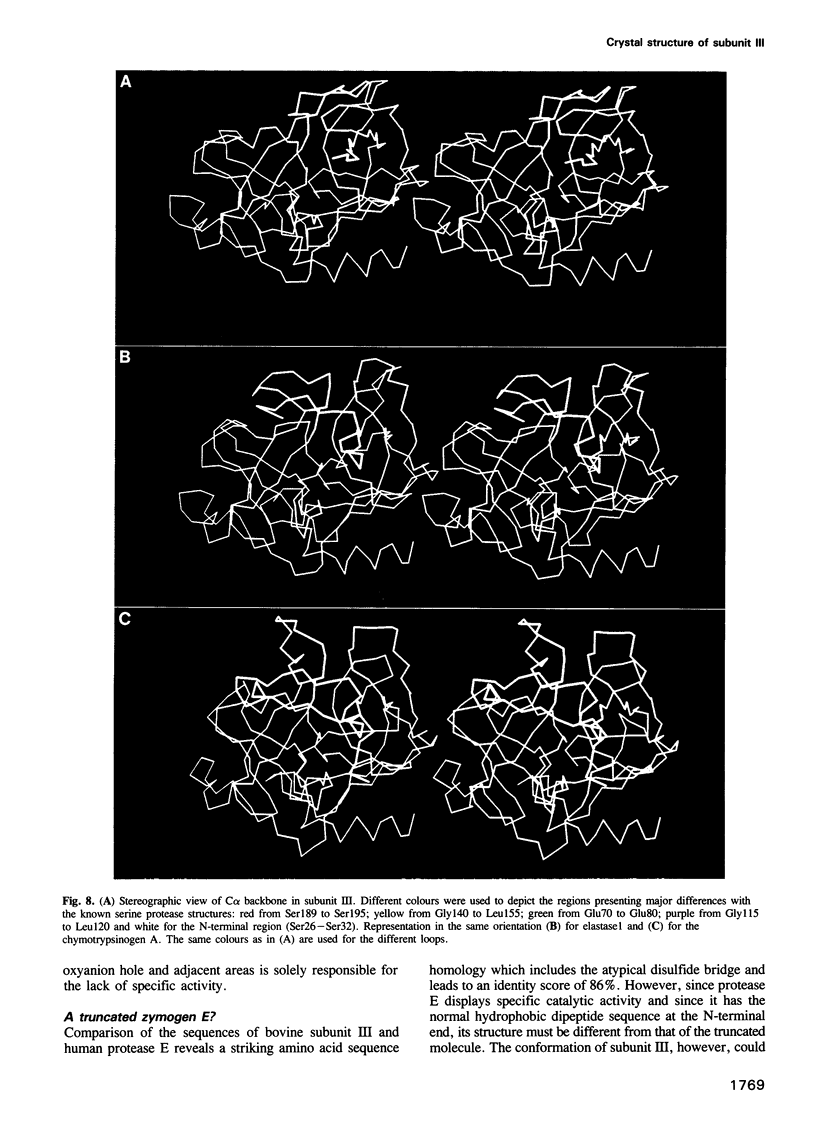
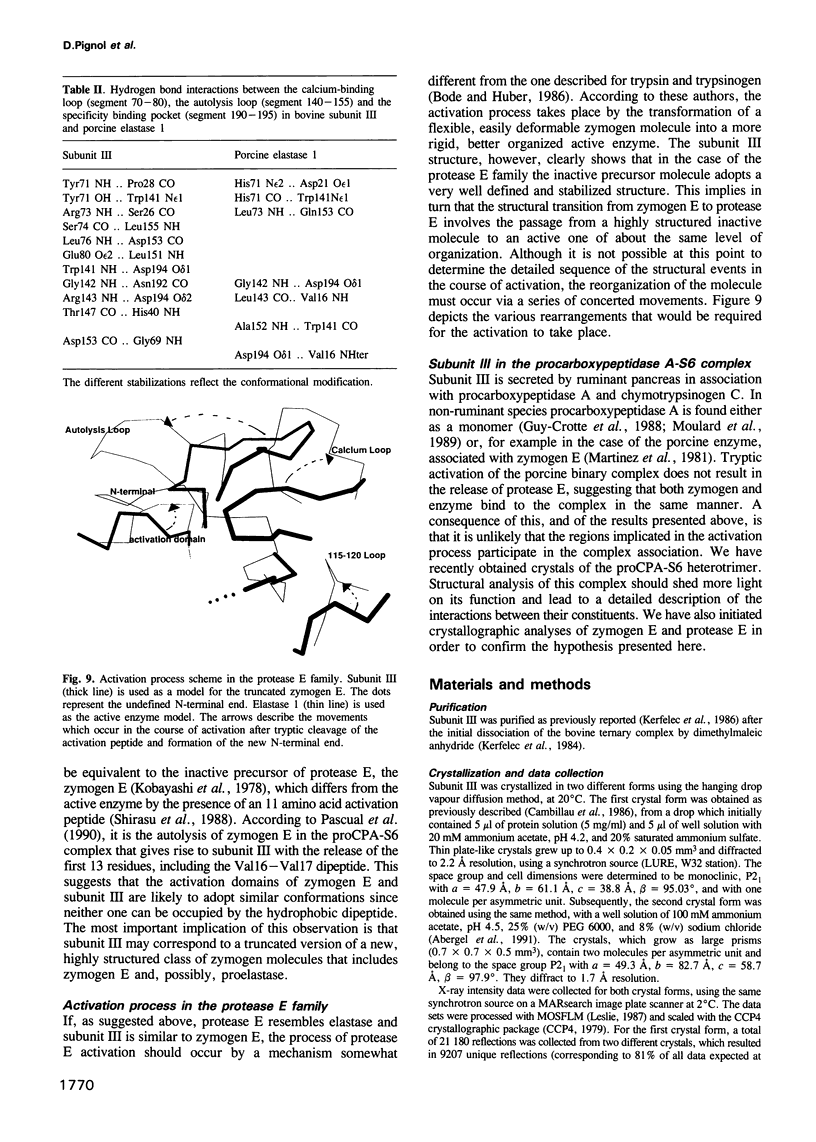
Abstract
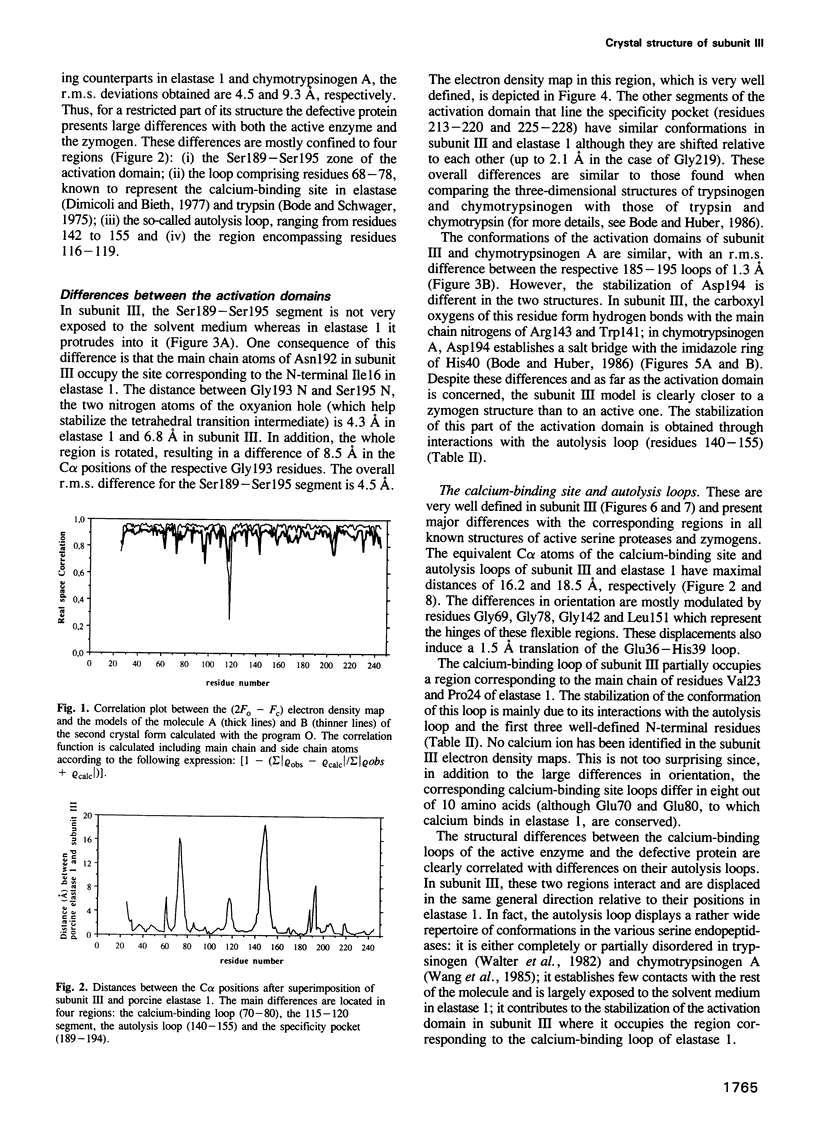
Subunit III, a defective serine endopeptidase lacking the typical N-terminal hydrophobic dipeptide is secreted by the pancreas of ruminant species as part of the bovine ternary complex procarboxypeptidase A-S6. Two monoclinic crystal forms were obtained and subsequently used to solve its X-ray structure. The highest resolution model of subunit III was refined at 1.7 A resolution to a crystallographic R-factor of 18.4%, with r.m.s. bond deviations from ideality of 0.012 A. About 80% of the model presents the characteristic architecture of trypsin-like proteases. The remaining zones, however, have well-defined, unique conformations. The regions from residues 70 to 80 and from 140 to 155 present maximum distances of 16 and 18 A relative to serine proteases and zymogens. Comparisons with the structures of porcine elastase 1 and chymotrypsinogen A indicate that the specific binding pocket of subunit III adopts a zymogen-like conformation and thus provide a basis for its inactivity. In general, the structural analysis of subunit III strongly suggests that it corresponds to a truncated version of a new class of highly structured elastase-like zymogen molecules. Based on the structures of subunit III and elastase 1, it is concluded that large concerted movements are necessary for the activation of zymogen E.
Full text
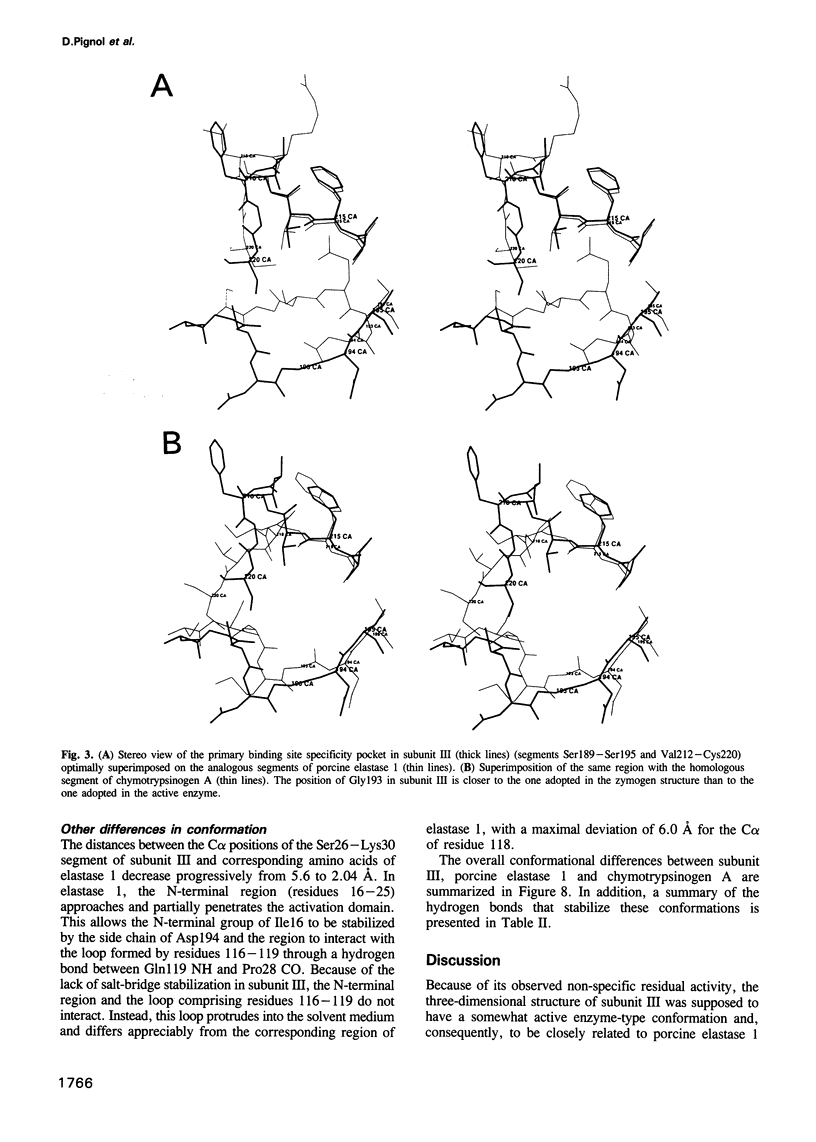
PDF

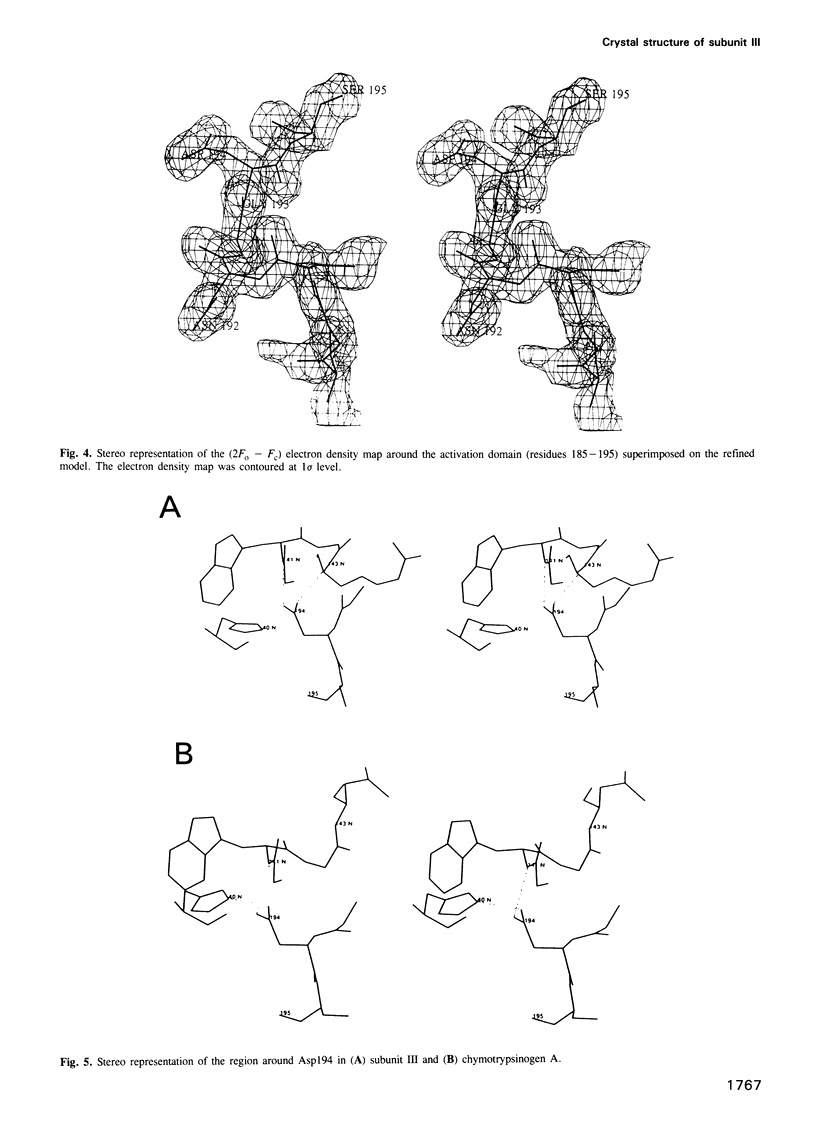

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abergel C., Moulard M., Moreau H., Loret E., Cambillau C., Fontecilla-Camps J. C. Systematic use of the incomplete factorial approach in the design of protein crystallization experiments. J Biol Chem. 1991 Oct 25;266(30):20131–20138. [PubMed] [Google Scholar]
- BROWN J. R., GREENSHIELDS R. N., YAMASAKI M., NEURATH H. THE SUBUNIT STRUCTURE OF BOVINE PROCARBOXYPEPTIDASE A-S6. CHEMICAL PROPERTIES AND ENZYMATIC ACTIVITIES OF THE PRODUCTS OF MOLECULAR DISAGGREGATION. Biochemistry. 1963 Jul-Aug;2:867–876. doi: 10.1021/bi00904a040. [DOI] [PubMed] [Google Scholar]
- Bode W., Schwager P., Huber R. The transition of bovine trypsinogen to a trypsin-like state upon strong ligand binding. The refined crystal structures of the bovine trypsinogen-pancreatic trypsin inhibitor complex and of its ternary complex with Ile-Val at 1.9 A resolution. J Mol Biol. 1978 Jan 5;118(1):99–112. doi: 10.1016/0022-2836(78)90246-2. [DOI] [PubMed] [Google Scholar]
- Bode W., Schwager P. The refined crystal structure of bovine beta-trypsin at 1.8 A resolution. II. Crystallographic refinement, calcium binding site, benzamidine binding site and active site at pH 7.0. J Mol Biol. 1975 Nov 15;98(4):693–717. doi: 10.1016/s0022-2836(75)80005-2. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Cambillau C., Kerfelec B., Foglizzo E., Chapus C. Crystallization and preliminary X-ray study of subunit III of the bovine pancreatic procarboxypeptidase A-S6 ternary complex. J Mol Biol. 1986 Jun 20;189(4):709–710. doi: 10.1016/0022-2836(86)90500-0. [DOI] [PubMed] [Google Scholar]
- Cambillau C., Kerfelec B., Sciaky M., Chapus C. Subunit III of ruminant procarboxypeptidase A-S6 complexes and pancreatic proteases E. A new family of pancreatic serine endopeptidases? FEBS Lett. 1988 May 9;232(1):91–95. doi: 10.1016/0014-5793(88)80392-2. [DOI] [PubMed] [Google Scholar]
- Chapus C., Kerfelec B., Dimicoli J. L. Binding of terbium and of an elastase inhibitor to bovine pancreatic subunit III, an inactive protease E. J Biol Chem. 1990 Mar 5;265(7):3726–3730. [PubMed] [Google Scholar]
- Chapus C., Puigserver A., Kerfélec B. The bovine pro-carboxypeptidase A-S6 ternary complex: a rare case of a secreted protein complex. Biochimie. 1988 Sep;70(9):1143–1151. doi: 10.1016/0300-9084(88)90179-4. [DOI] [PubMed] [Google Scholar]
- Dimicoli J. L., Bieth J. Location of the calcium ion binding site in porcine pancreatic elastase using a lanthanide ion probe. Biochemistry. 1977 Dec 13;16(25):5532–5537. doi: 10.1021/bi00644a022. [DOI] [PubMed] [Google Scholar]
- Granon S., Kerfelec B., Chapus C. Spectrofluorimetric investigation of the interactions between the subunits of bovine pancreatic procarboxypeptidase A-S6. J Biol Chem. 1990 Jun 25;265(18):10383–10388. [PubMed] [Google Scholar]
- Guy-Crotte O., Barthe C., Basso D., Fournet B., Figarella C. Characterization of two glycoproteins of human pancreatic juice: P35, a truncated protease E and P19, precursor of protein X. Biochem Biophys Res Commun. 1988 Oct 14;156(1):318–322. doi: 10.1016/s0006-291x(88)80842-8. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Keil-Dlouha V., Puigserver A., Marie A., Keil B. On subunit II of bovine procarboxypeptidase A. Enzymatic specificity after tryptic activation. Biochim Biophys Acta. 1972 Aug 28;276(2):531–535. doi: 10.1016/0005-2744(72)91013-3. [DOI] [PubMed] [Google Scholar]
- Kerfelec B., Cambillau C., Puigserver A., Chapus C. The inactive subunit of ruminant procarboxypeptidase A-S6 complexes. Structural basis of inactivity and physiological role. Eur J Biochem. 1986 Jun 16;157(3):531–538. doi: 10.1111/j.1432-1033.1986.tb09699.x. [DOI] [PubMed] [Google Scholar]
- Kerfelec B., Chapus C., Puigserver A. Existence of ternary complexes of procarboxypeptidase A in the pancreas of some ruminant species. Eur J Biochem. 1985 Sep 16;151(3):515–519. doi: 10.1111/j.1432-1033.1985.tb09132.x. [DOI] [PubMed] [Google Scholar]
- Kerfelec B., Chapus C., Puigserver A. Two-step dissociation of bovine 6S procarboxypeptidase A by dimethylmaleylation. Biochem Biophys Res Commun. 1984 May 31;121(1):162–167. doi: 10.1016/0006-291x(84)90701-0. [DOI] [PubMed] [Google Scholar]
- Lamzin V. S., Wilson K. S. Automated refinement of protein models. Acta Crystallogr D Biol Crystallogr. 1993 Jan 1;49(Pt 1):129–147. doi: 10.1107/S0907444992008886. [DOI] [PubMed] [Google Scholar]
- Martínez M. C., Avilés F. X., Sansegundo B., Cuchillo C. M. Comparison between the monomeric and binary-complex forms of procarboxypeptidase A from whole pig pancreas. Biochem J. 1981 Jul 1;197(1):141–147. doi: 10.1042/bj1970141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E., Cole G., Radhakrishnan R., Epp O. Structure of native porcine pancreatic elastase at 1.65 A resolutions. Acta Crystallogr B. 1988 Feb 1;44(Pt 1):26–38. doi: 10.1107/s0108768187007559. [DOI] [PubMed] [Google Scholar]
- Michon T., Granon S., Sauve P., Chapus C. The activation peptide of pancreatic procarboxypeptidase A is the keystone of the bovine procarboxypeptidase A-S6 ternary complex. Biochem Biophys Res Commun. 1991 Nov 27;181(1):449–455. doi: 10.1016/s0006-291x(05)81440-8. [DOI] [PubMed] [Google Scholar]
- Michon T., Sari J. C., Granon S., Kerfelec B., Chapus C. Microcalorimetric investigation of the interactions between the subunits of the bovine pancreatic procarboxypeptidase A-S6 complex. Eur J Biochem. 1991 Oct 1;201(1):217–222. doi: 10.1111/j.1432-1033.1991.tb16277.x. [DOI] [PubMed] [Google Scholar]
- Moulard M., Kerfelec B., Mallet B., Chapus C. Identification of a procarboxypeptidase A-truncated protease E binary complex in human pancreatic juice. FEBS Lett. 1989 Jul 3;250(2):166–170. doi: 10.1016/0014-5793(89)80712-4. [DOI] [PubMed] [Google Scholar]
- Pascual R., Vendrell J., Avilés F. X., Bonicel J., Wicker C., Puigserver A. Autolysis of proproteinase E in bovine procarboxypeptidase A ternary complex gives rise to subunit III. FEBS Lett. 1990 Dec 17;277(1-2):37–41. doi: 10.1016/0014-5793(90)80804-r. [DOI] [PubMed] [Google Scholar]
- Puigserver A., Desnuelle P. Dissociation of bovine 6S procarboxypeptidase A by reversible condensation with 2,3-dimethyl maleic anhydride: application to the partial characterization of subunit III. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2442–2445. doi: 10.1073/pnas.72.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver A., Desnuelle P. Reconstitution of bovine procarboxypeptidase A-S6 from the free subunits. Biochemistry. 1977 May 31;16(11):2497–2501. doi: 10.1021/bi00630a028. [DOI] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Shen W. F., Fletcher T. S., Largman C. Primary structure of human pancreatic protease E determined by sequence analysis of the cloned mRNA. Biochemistry. 1987 Jun 16;26(12):3447–3452. doi: 10.1021/bi00386a030. [DOI] [PubMed] [Google Scholar]
- Shirasu Y., Takemura K., Yoshida H., Sato Y., Iijima H., Shimada Y., Mikayama T., Ozawa T., Ikeda N., Ishida A. Molecular cloning of complementary DNA encoding one of the human pancreatic protease E isozymes. J Biochem. 1988 Aug;104(2):259–264. doi: 10.1093/oxfordjournals.jbchem.a122454. [DOI] [PubMed] [Google Scholar]
- Venot N., Sciaky M., Puigserver A., Desnuelle P., Laurent G. Amino acid sequence and disulfide bridges of subunit III, a defective endopeptidase present in the bovine pancreatic 6 S procarboxypeptidase A complex. Eur J Biochem. 1986 May 15;157(1):91–99. doi: 10.1111/j.1432-1033.1986.tb09642.x. [DOI] [PubMed] [Google Scholar]
- Wang D., Bode W., Huber R. Bovine chymotrypsinogen A X-ray crystal structure analysis and refinement of a new crystal form at 1.8 A resolution. J Mol Biol. 1985 Oct 5;185(3):595–624. doi: 10.1016/0022-2836(85)90074-9. [DOI] [PubMed] [Google Scholar]
- Wicker C., Puigserver A. Further studies on subunit III of bovine procarboxypeptidase A. Structure and reactivity of the weakly functional active site. FEBS Lett. 1981 Jun 1;128(1):13–16. doi: 10.1016/0014-5793(81)81067-8. [DOI] [PubMed] [Google Scholar]