Abstract
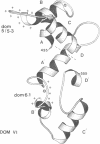
In order to identify a binding site for ligand intercellular adhesion molecule-1 (ICAM-1) on the beta 2 integrin lymphocyte function-associated antigen-1 (LFA-1), protein fragments of LFA-1 were made by in vitro translation of a series of constructs which featured domain-sized deletions starting from the N-terminus of the alpha subunit of LFA-1. Monoclonal antibodies and ICAM-1 were tested for their ability to bind to these protein fragments. Results show that the putative divalent cation binding domains V and VI contain an ICAM-1 binding site. A series of consecutive peptides covering these domains indicated two discontinuous areas as specific contact sites: residues 458-467 in domain V and residues 497-516 in domain VI. A three-dimensional model of these domains of LFA-1 was constructed based on the sequence similarity to known EF hands. The two regions critical for the interaction of LFA-1 with ICAM-1 lie adjacent to each other, the first next to the non-functional EF hand in domain V and the second coinciding with the potential divalent cation binding loop in domain VI. The binding of ICAM-1 with the domain V and VI region in solution was not sensitive to divalent cation chelation. In short, a critical motif for ICAM-1 binding to the alpha subunit of LFA-1 is shared between two regions of domains V and VI.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu Y. S., Bugg C. E., Cook W. J. Structure of calmodulin refined at 2.2 A resolution. J Mol Biol. 1988 Nov 5;204(1):191–204. doi: 10.1016/0022-2836(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Berendt A. R., McDowall A., Craig A. G., Bates P. A., Sternberg M. J., Marsh K., Newbold C. I., Hogg N. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1-binding site. Cell. 1992 Jan 10;68(1):71–81. doi: 10.1016/0092-8674(92)90207-s. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Cabañas C., Hogg N. Lymphocyte-fibroblast adhesion. A useful model for analysis of the interaction of the leucocyte integrin LFA-1 with ICAM-1. FEBS Lett. 1991 Nov 4;292(1-2):284–288. doi: 10.1016/0014-5793(91)80885-7. [DOI] [PubMed] [Google Scholar]
- Cook W. J., Ealick S. E., Babu Y. S., Cox J. A., Vijay-Kumar S. Three-dimensional structure of a sarcoplasmic calcium-binding protein from Nereis diversicolor. J Biol Chem. 1991 Jan 5;266(1):652–656. [PubMed] [Google Scholar]
- Corbi A. L., Miller L. J., O'Connor K., Larson R. S., Springer T. A. cDNA cloning and complete primary structure of the alpha subunit of a leukocyte adhesion glycoprotein, p150,95. EMBO J. 1987 Dec 20;6(13):4023–4028. doi: 10.1002/j.1460-2075.1987.tb02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Burke T. A., Plow E. F. The ligand binding site of the platelet integrin receptor GPIIb-IIIa is proximal to the second calcium binding domain of its alpha subunit. J Biol Chem. 1990 Feb 25;265(6):3440–3446. [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Matsueda G. R., Plow E. F. A discrete sequence in a platelet integrin is involved in ligand recognition. Nature. 1991 Mar 7;350(6313):66–68. doi: 10.1038/350066a0. [DOI] [PubMed] [Google Scholar]
- Dekker N., Cox M., Boelens R., Verrijzer C. P., van der Vliet P. C., Kaptein R. Solution structure of the POU-specific DNA-binding domain of Oct-1. Nature. 1993 Apr 29;362(6423):852–855. doi: 10.1038/362852a0. [DOI] [PubMed] [Google Scholar]
- Dempsey C. E., Bazzo R., Harvey T. S., Syperek I., Boheim G., Campbell I. D. Contribution of proline-14 to the structure and actions of melittin. FEBS Lett. 1991 Apr 9;281(1-2):240–244. doi: 10.1016/0014-5793(91)80402-o. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993 Jan;120(2):545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I., Buckle A. M., Hogg N. Early events of the immune response mediated by leukocyte integrins. Immunol Rev. 1990 Apr;114:29–44. doi: 10.1111/j.1600-065x.1990.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Dransfield I., Cabañas C., Craig A., Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992 Jan;116(1):219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I., Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin alpha subunits. EMBO J. 1989 Dec 1;8(12):3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Gariépy J., Hodges R. S. Primary sequence analysis and folding behavior of EF hands in relation to the mechanism of action of troponin C and calmodulin. FEBS Lett. 1983 Aug 22;160(1-2):1–6. doi: 10.1016/0014-5793(83)80924-7. [DOI] [PubMed] [Google Scholar]
- Gulino D., Boudignon C., Zhang L. Y., Concord E., Rabiet M. J., Marguerie G. Ca(2+)-binding properties of the platelet glycoprotein IIb ligand-interacting domain. J Biol Chem. 1992 Jan 15;267(2):1001–1007. [PubMed] [Google Scholar]
- Herzberg O., James M. N. Refined crystal structure of troponin C from turkey skeletal muscle at 2.0 A resolution. J Mol Biol. 1988 Oct 5;203(3):761–779. doi: 10.1016/0022-2836(88)90208-2. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E., Gotch F. M., Hildreth P. D., McMichael A. J. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983 Mar;13(3):202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- Humphries M. J. The molecular basis and specificity of integrin-ligand interactions. J Cell Sci. 1990 Dec;97(Pt 4):585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Large T. H., Houde M., Tawil J. W., Barton A., Esch F., Carbonetto S., Reichardt L. F. Molecular cloning of the rat integrin alpha 1-subunit: a receptor for laminin and collagen. J Cell Biol. 1990 Aug;111(2):709–720. doi: 10.1083/jcb.111.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S. C., Dustin M. L., Hibbs M. L., Springer T. A. On the species specificity of the interaction of LFA-1 with intercellular adhesion molecules. J Immunol. 1990 Aug 15;145(4):1181–1187. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. D., Lee L., Edwards B. F. Refined crystal structure of calcium-liganded carp parvalbumin 4.25 at 1.5-A resolution. Biochemistry. 1990 Feb 13;29(6):1404–1412. doi: 10.1021/bi00458a010. [DOI] [PubMed] [Google Scholar]
- Landis R. C., Bennett R. I., Hogg N. A novel LFA-1 activation epitope maps to the I domain. J Cell Biol. 1993 Mar;120(6):1519–1527. doi: 10.1083/jcb.120.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson R. S., Corbi A. L., Berman L., Springer T. Primary structure of the leukocyte function-associated molecule-1 alpha subunit: an integrin with an embedded domain defining a protein superfamily. J Cell Biol. 1989 Feb;108(2):703–712. doi: 10.1083/jcb.108.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewit-Bentley A., Morera S., Huber R., Bodo G. The effect of metal binding on the structure of annexin V and implications for membrane binding. Eur J Biochem. 1992 Nov 15;210(1):73–77. doi: 10.1111/j.1432-1033.1992.tb17392.x. [DOI] [PubMed] [Google Scholar]
- MacArthur M. W., Thornton J. M. Influence of proline residues on protein conformation. J Mol Biol. 1991 Mar 20;218(2):397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Michishita M., Videm V., Arnaout M. A. A novel divalent cation-binding site in the A domain of the beta 2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell. 1993 Mar 26;72(6):857–867. doi: 10.1016/0092-8674(93)90575-b. [DOI] [PubMed] [Google Scholar]
- Plumb J. A., Milroy R., Kaye S. B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989 Aug 15;49(16):4435–4440. [PubMed] [Google Scholar]
- Ross L., Hassman F., Molony L. Inhibition of Molt-4-endothelial adherence by synthetic peptides from the sequence of ICAM-1. J Biol Chem. 1992 Apr 25;267(12):8537–8543. [PubMed] [Google Scholar]
- Sekharudu Y. C., Sundaralingam M. A structure-function relationship for the calcium affinities of regulatory proteins containing 'EF-hand' pairs. Protein Eng. 1988 Jul;2(2):139–146. doi: 10.1093/protein/2.2.139. [DOI] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Cheresh D. A. Integrin (alpha v beta 3)-ligand interaction. Identification of a heterodimeric RGD binding site on the vitronectin receptor. J Biol Chem. 1990 Feb 5;265(4):2168–2172. [PubMed] [Google Scholar]
- Smith J. W., Cheresh D. A. Labeling of integrin alpha v beta 3 with 58Co(III). Evidence of metal ion coordination sphere involvement in ligand binding. J Biol Chem. 1991 Jun 25;266(18):11429–11432. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Erickson H. P., Springer T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990 Apr 20;61(2):243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Takada Y., Hemler M. E. The primary structure of the VLA-2/collagen receptor alpha 2 subunit (platelet GPIa): homology to other integrins and the presence of a possible collagen-binding domain. J Cell Biol. 1989 Jul;109(1):397–407. doi: 10.1083/jcb.109.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckwell D. S., Brass A., Humphries M. J. Homology modelling of integrin EF-hands. Evidence for widespread use of a conserved cation-binding site. Biochem J. 1992 Jul 1;285(Pt 1):325–331. doi: 10.1042/bj2850325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufty R. M., Kretsinger R. H. Troponin and parvalbumin calcium binding regions predicted in myosin light chain and T4 lysozyme. Science. 1975 Jan 17;187(4172):167–169. doi: 10.1126/science.1111094. [DOI] [PubMed] [Google Scholar]
- Vyas N. K., Vyas M. N., Quiocho F. A. A novel calcium binding site in the galactose-binding protein of bacterial transport and chemotaxis. Nature. 1987 Jun 18;327(6123):635–638. doi: 10.1038/327635a0. [DOI] [PubMed] [Google Scholar]
- van Eerd J. P., Takahshi K. Determination of the complete amino acid sequence of bovine cardiac troponin C. Biochemistry. 1976 Mar 9;15(5):1171–1180. doi: 10.1021/bi00650a033. [DOI] [PubMed] [Google Scholar]
- van der Merwe P. A., Brown M. H., Davis S. J., Barclay A. N. Affinity and kinetic analysis of the interaction of the cell adhesion molecules rat CD2 and CD48. EMBO J. 1993 Dec 15;12(13):4945–4954. doi: 10.1002/j.1460-2075.1993.tb06188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]