Abstract
Diabetic peripheral neuropathy (DPN) is the most common complication associated with diabetes. DPN can present as a loss of sensation, may lead to neuropathic ulcers, and is a leading cause of amputation. Reported estimates of the prevalence of DPN vary due to differences in study populations and diagnostic criteria. Furthermore, the epidemiology and clinical characteristics of DPN in Korean patients with type 2 diabetes mellitus (T2DM) are not as well understood as those of other complications of diabetes such as retinal and renal disease. Recently, the Diabetic Neuropathy Study Group of the Korean Diabetes Association (KDA) conducted a study investigating the impact of DPN on disease burden and quality of life in patients with T2DM and has published some data that are representative of the nation. This review investigated the prevalence and associated clinical implications of DPN in Korean patients with diabetes based on the KDA study.
Keywords: Diabetes, Peripheral nervous system diseases, Prevalence, Quality of life
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) is increasing in Korea [1] and has escalated from 1.5% to 9.9% over the last four decades. Major outcomes of the progression of T2DM include chronic complications that decrease quality of life (QoL), incur heavy burdens on the healthcare system, and increase diabetic mortality [2]. Therefore, it is clear that information regarding the prevalence of T2DM-related complications is important in the development of policies and practices for the management of T2DM patients so that they may receive the best care possible.
Diabetic peripheral neuropathy (DPN) is the most common complication associated with diabetes and it is estimated that 30% to 50% of diabetes patients are affected by this disorder [3-5]. Chronic sensorimotor distal symmetric polyneuropthy is the most common form of DPN [6] and can lead to substantial sensory loss, muscle weakness, and pain. The typical presentation of DPN is a gradual onset of sensory impairments that include burning and numbness in the feet. In fact, the onset is so gradual that the disease may go undiagnosed for years. Neuropathic pain may be severe when it is present but this type of pain is reported to occur in only 11% to 32% of individuals with DPN [7]. DPN leads to a number of impairments and functional limitations including foot ulceration and subsequent lower-extremity amputation [8]; in Korea between 2000 and 2002, 44.8% of foot amputee patients had diabetes [9]. In patients with diabetes, the presence of DPN is associated with a greater degree of health care use and an inability to work due to physical limitations [3]. Other potential complications of DPN, such as falls, are less clearly attributable to the disorder but also result in significant limitations of function.
Over the last five decades, several studies have reported the prevalence of DPN in Korea to be anywhere from 14.1% to 54.5%, depending on the study population and diagnostic criteria used [10]. It should be noted, however, that these studies were specifically designed to evaluate the prevalence of chronic complications associated with diabetes and that the diagnostic criteria for DPN were not clearly established during this time period. In 2009, the Diabetic Neuropathy Study Group of the Korean Diabetes Association (KDA) conducted a hospital-based nationwide survey to investigate the impact of DPN on disease burden and QoL in patients with T2DM. This cross-sectional study was carried out with T2DM patients from the diabetic clinics of 40 hospitals throughout Korea as has been described in previous reports, some of which have already been published [11-13]. In this review, we describe the prevalence and clinical implications of DPN in T2DM outpatients in Korea.
PREVALENCE OF DPN IN KOREA
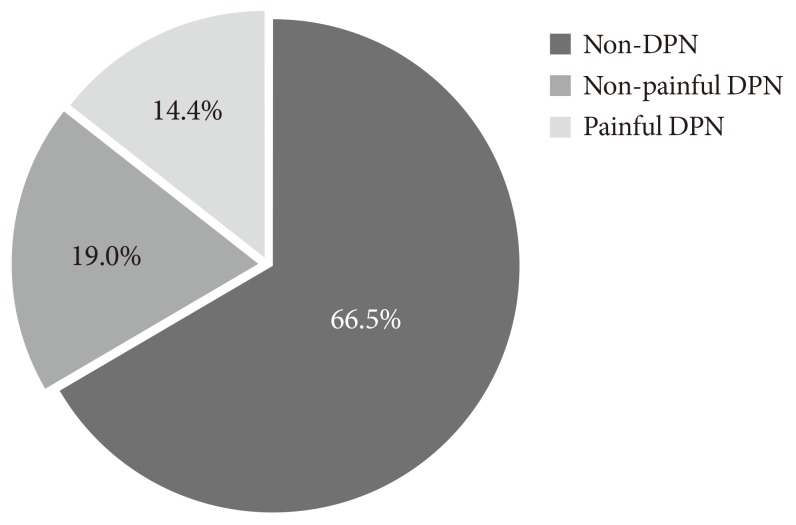
The prevalence of DPN in Korea varies from 13.1% to 61.8%, depending on the population being studied [4,14,15]. This inconsistency may be attributed to the various types of diabetes, differences in study design, sample selection, and diagnostic criteria, and variation in race, age, sex, and duration of diabetes in the populations studied. According to findings of the Diabetic Neuropathy Study Group of the KDA, the actual prevalence of DPN is 33.5% (n=1,338, study population=3,999) (Fig. 1) [11]. In the prior studies by that group, diagnostic criteria included the presence of documented DPN by quantitative sensory or nerve conduction studies, the presence of symptoms typically attributable to DPN after the exclusion of other causes of neuropathy, results from the Michigan Neuropathy Screening Instrument (MNSI, ≥3 score), and abnormal results on the 10 g monofilament test (2 out of 10). Thus, the high rate of DPN might partly be due to the inclusion of diverse diagnostic criteria [16-18]. In terms of a clinical diagnosis, it is generally agreed that DPN can be diagnosed by the presence of a combination of peripheral symptoms and neurological deficits [19]. Findings from the KDA study are compatible with the reported range in prevalence of a meta-analysis by the International Diabetes Federation, which estimated that the incidence of DPN in the West-Pacific Asia region varies from 9% to 45% [20]. Up to half of patients with DPN exhibit painful symptoms while the remainder may be asymptomatic but present with neurological deficits [16]. Among the patients with DPN in the KDA study, 43.1% (n=577) were found to have painful DPN based on the following criteria: the average daily pain intensity in the legs, feet, or hands lasted 48 hours; pain was rated ≥4 (moderate or strong pain) on the visual analog scale; patients were taking medication for their current pain [13]. Use of these criteria resulted in a prevalence of painful DPN of 14.4% for patients with T2DM. Some reports have suggested that the perception of pain may vary based on ethnic and cultural differences [21]. However, in this study, the prevalence of painful DPN was comparable to those of reports from Western countries [22].
Fig. 1.
The prevalence of diabetic peripheral neuropathy (DPN) (n=3,999).
CLINICAL CHARACTERISTICS OF PATIENTS WITH DPN
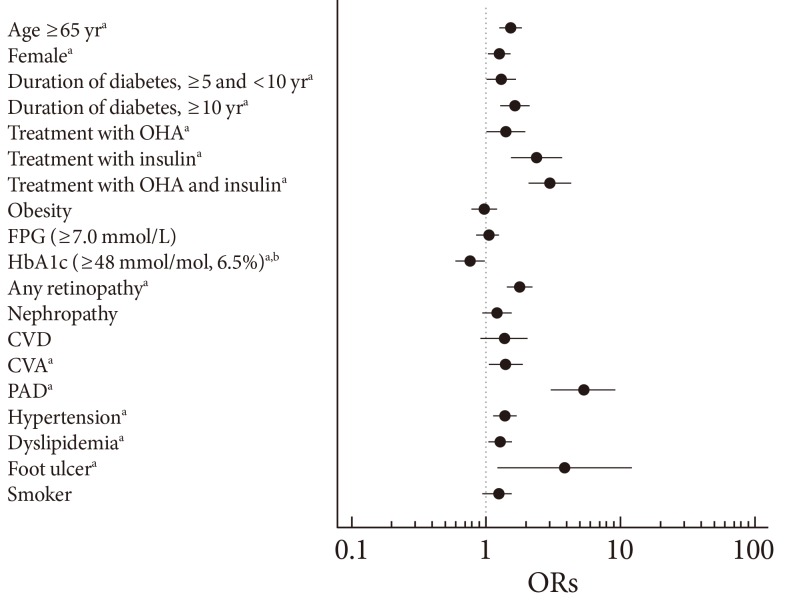
The primary risk factor for DPN is hyperglycemia and other independent risk factors include advanced age, longer duration of diabetes, cigarette smoking, hypertension, elevated triglyceride levels, a higher body mass index, alcohol consumption, and greater height [5,23]. To evaluate the clinical characteristics of patients with DPN, the results from the nationwide survey of the Diabetic Neuropathy Study Group of KDA were evaluated. A multivariate logistic regression analysis in patients with T2DM revealed that the clinical variables independently associated with DPN include older age, being female, a longer diabetes duration, lower hemoglobin A1c (HbA1c) level, the presence of retinopathy, a history of cerebrovascular accident, or peripheral arterial disease, the presence of hypertension or dyslipidemia, treatment with an oral hypoglycemic agent or insulin, and a history of foot ulcers (Fig. 2) [11]. As expected, patients identified in this study with painful DPN were older, typically female, had a longer duration of diabetes, showed more prevalent insulin use, and exhibited a greater incidence of other microvascular complications (retinopathy, nephropathy, or both) and hypertension compared to patients with nonpainful DPN [13].
Fig. 2.
Odds ratios (ORs) of factors associated with the presence of diabetic peripheral neuropathy according to a logistic-regression analysis. Standardized ORs (95% confidence intervals) are expressed per standard deviation increase for each continuous variable. ORs for categorical variables have a reference group without the respective factor. OHA, oral hypoglycemic agents; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; CVD, cardiovascular disease; CVA, cerebrovascular accident; PAD, peripheral arterial disease. aP<0.05 vs. control, bHbA1c measurements in International Federation of Clinical Chemistry (mmol/mol) and Diabetes Control and Complications Trial (%) units. Adapted from Won JC, et al. Diabet Med 2012;29:e290-6, with permission from John Wiley and Sons [11].
These findings suggest that much more attention should be paid to high-risk diabetic patients to exert better control over DPN, especially in those who are elderly and/or have a long history of diabetes. Moreover, patients with DPN exhibit a higher prevalence of other diabetes-related complications or comorbidities. Although higher HbA1c levels did not show a significant relationship with DPN, the proportion of DPN patients significantly increased in conjunction with higher HbA1c levels according to an analysis of variance. In addition, diabetes treatment (i.e., the proportion of patients being treated with insulin) could be seen as a surrogate for disease severity [24,25]. Therefore, these data suggest that factors other than hyperglycemia, such as the duration of diabetes, are involved in the development of DPN and that these factors may obscure any associations between the prevalence of DPN and glycemic status. As a result, the pathogenesis of diabetic complications is composed not only of hyperglycemic status but also of other factors associated with metabolic control [26]. According to cohort studies, each 1% reduction in HbA1c levels is associated with a 37% decrease in the risk for microvascular complications [27]. Although it may not be concluded from the KDA study that poor glycemic control results in chronic complications, including DPN, these findings serve as a warning to healthcare professionals that there is an urgent need for better glycemic control to prevent the development of chronic complications due to T2DM. Furthermore, the management of DPN and other diabetes-related complications or co-morbidities should be an important and integrated component of diabetes disease management.
TREATMENT
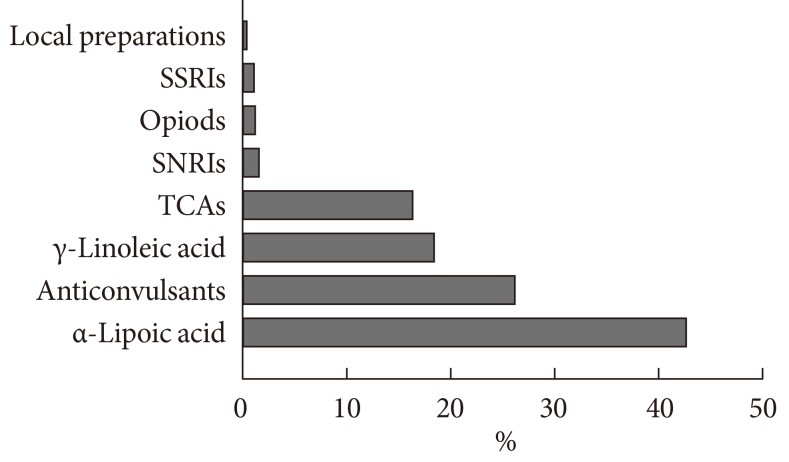
Treatment strategies for DPN are intended to improve the symptoms of patients, to modify the pathophysiology of DPN, including metabolic control, or both. Unfortunately, these approaches do not appear to be sufficient because the underlying mechanisms of DPN and measures for treatment efficacy have yet to be clearly elucidated. The KDA study assessed the medications prescribed to patients by their physicians for the management of DPN. Of the 1,338 patients diagnosed with DPN, 69.8% (n=934) were currently prescribed medications for DPN including α-lipoic acid (40.2% of prescriptions, n=396), anticonvulsant drugs (24.6% of prescriptions, n=242), γ-linoleic acid (17.2% of prescriptions, n=169), tricyclic antidepressants (15.2% of prescriptions, n=150), serotonin-norepinephrine reuptake inhibitors (1.2% of prescriptions, n=12), opioids (0.8% of prescriptions, n=8), selective serotonin reuptake inhibitors (0.7% of prescriptions, n=7), and local preparations (0.1% of prescriptions, n=1). Approximately 20.1% (n=187) of patients received more than one medication (Fig. 3) [11].
Fig. 3.
Pattern of medication for neuropathy in patients with diabetic peripheral neuropathy (n=934). SSRIs, selective serotonin reuptake inhibitors; SNRIs, serotonin-norepinephrine reuptake inhibitors; TCAs, tricyclic antidepressants.
More than half of patients with DPN were being treated with pathophysiology-based medication rather than symptomatic treatments, and in line with international and Korean guidelines, anticonvulsant, and tricyclic antidepressant drugs were commonly prescribed for symptomatic management [28,29]. On average, patients receiving treatment for DPN had a longer duration of diabetes and greater incidences of retinopathy, nephropathy, and hypertension. Moreover, the proportion of patients using insulin was greater in DPN patients on medication compared to those without treatment for DPN (43.5% vs. 36.4%, respectively; P=0.0157). Interestingly, the MNSI score was slightly higher in patients receiving treatment than in those not receiving treatment, but the mean number of patients unable to feel the 10 g monofilament was higher in those without treatment compared to those with treatment [11]. These findings suggest that a substantial number of patients with a higher risk for foot disease were not being properly managed by their physicians and that quantitative sensory nerve tests should be a routine inclusion in clinical practice to identify high-risk patients.
EFFECTS OF PAIN ON QOL IN PATIENTS WITH DPN
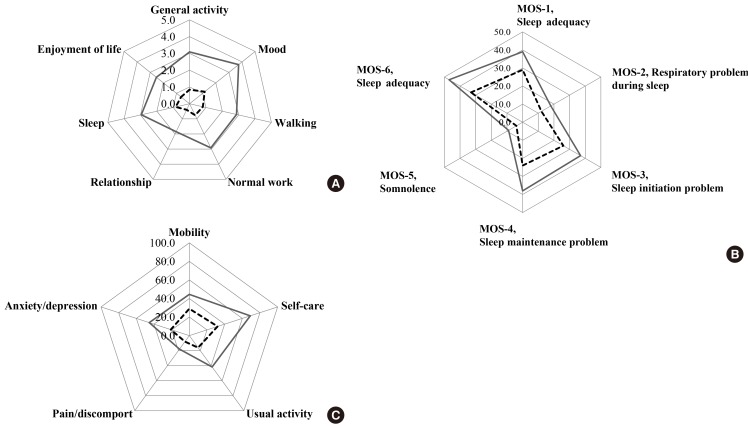
DPN has serious detrimental effects on the physical, emotional, and social functioning of patients [30]. Many DPN patients experience pain or discomfort, anxiety, depression, and limitations in activity, and may lose workdays or show decreased productivity as a result [31]. Although there is a growing awareness of the influence of DPN on QoL and the diseased status of patients with diabetes, neither the exact magnitude of this contribution nor the characteristics of patients most likely to be affected have been determined. In this study, all items assessing pain interference (Fig. 4A) and results from the Medical Outcomes Study Sleep Scale (Fig. 4B) revealed that patients with painful DPN suffer from pain during daily activities and sleep, relative to patients with nonpainful DPN [12,13]. In addition, the percentage of respondents reporting more than some problem for each dimension on the EQ-5D was significantly higher in patients with painful DPN compared to those with nonpainful DPN (Fig. 4C). Therefore, patients with painful DPN have greater discomfort during daily activities and sleep, and reduced QoL compared to patients with nonpainful DPN. These results provide some data on the nature and extent of the impact of pain on QoL in patients with painful DPN.
Fig. 4.
Mean value of measures related to the clinical impact of pain on (A) pain interference items using the Brief Pain Inventory-Short Form, (B) the Medical Outcomes Study Sleep Scale, and (C) the quality of life (QoL) in patients with painful diabetic peripheral neuropathy (DPN) (linear line) and nonpainful DPN (dotted line). (A) A 0 to 10 numeric rating scale was used for each item and was anchored at 0 (does not interfere) and 10 (completely interferes). (B) Item responses were assigned scores using conventional rules where higher scores indicate a greater severity of sleep disturbance (range, 0 to 100). (C) Data are expressed as percentages of respondents reporting more than some problems or greater (score of 2 or 3). Each question had three levels: no problem, score of 1; some problems, score of 2; and severe problems, score of 3. (A-C) P<0.01 for all items between painful DPN and nonpainful DPN. Adapted from Kim SS, et al. Diabetes Res Clin Pract. Forthcoming 2014, with permission from Elsevier [13].
AWARENESS AND EXPERIENCE OF PATIENTS REGARDING EDUCATION FOR THE MANAGEMENT OF FOOT SYMPTOMS
It is possible to prevent the amputation of limbs through regular screening and treatment. The complications of diabetes incur high costs on the healthcare system and foot care screening programs have been shown to not only be cost-effective but also to impact QoL in patients with T2DM [32]. The KDA recommends that patients with T2DM undergo an annual foot examination to proactively assess sensation in the feet, foot structure, biomechanics, circulation, and skin integrity [28]. In the KDA study, 72.8% of patients with DPN had been educated about DPN and 72.2% had heard about foot management, but the awareness of patients with DPN regarding their condition was only 12.6% (n=169). In other words, only one-eighth of patients with DPN reported having had DPN [11]; this is a concern. A lack of awareness regarding DPN and the neglectful care of its symptoms could lead to a high degree of unnecessary morbidity, substantial rates of hospitalization, and increased healthcare costs. Although more than two-thirds of patients with DPN recalled having foot problems and having received education about foot management over the past few years, the experience of diabetes education did not equate to their having knowledge of the disease. This disconnect likely occurs because many patients with DPN remained unaware that this complication is often asymptomatic. Therefore, a comprehensive program designed to increase awareness of DPN should be implemented and the role of primary care providers in screening for DPN needs to be developed.
CONCLUSIONS
The present review provides detailed estimates of the prevalence of DPN in patients with T2DM in Korea as well as the clinical characteristics of this disease. It is worth noting that a high proportion of T2DM patients suffer from DPN and the painful symptoms they experience are associated with a decreased QoL. The high prevalence of DPN and other long-term complications probably represent the current reality of diabetes care in a hospital setting since many patients with these complications tend to be treated at the hospital. The presence of DPN may indicate that patients have an increased severity of diabetes, which is also supported by the increased prevalence of other diabetes-related complications and comorbidities. All of these medical issues lead to increased resource utilization. It is believed that the findings of the current study will be used as a basis for future epidemiological studies, randomized controlled trials, and additional well-designed studies that will investigate methods with which to ameliorate the prevalence of diabetic complications in Korea. In addition, given the disease burden associated with DPN, future research may benefit from a focus on efficacy to differentiate treatment strategies. These results suggest that strategies aimed at the prevention and management of DPN should be put to the forefront of research and that the management of DPN must not be neglected.
ACKNOWLEDGMENTS
This study was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2010-0020224), and by the Inje Research and Scholarship Foundation in 2012 (J.C.W.).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kim DJ. The epidemiology of diabetes in Korea. Diabetes Metab J. 2011;35:303–308. doi: 10.4093/dmj.2011.35.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.K. Prospective Diabetes Study Group. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37) Diabetes Care. 1999;22:1125–1136. doi: 10.2337/diacare.22.7.1125. [DOI] [PubMed] [Google Scholar]
- 3.Candrilli SD, Davis KL, Kan HJ, Lucero MA, Rousculp MD. Prevalence and the associated burden of illness of symptoms of diabetic peripheral neuropathy and diabetic retinopathy. J Diabetes Complications. 2007;21:306–314. doi: 10.1016/j.jdiacomp.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L 1999-2000 national health and nutrition examination survey. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 5.Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20:1162–1167. doi: 10.2337/diacare.20.7.1162. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 7.Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43:957–973. doi: 10.1007/s001250051477. [DOI] [PubMed] [Google Scholar]
- 8.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513–521. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 9.Task Force Team for Basic Statistical Study of Korean Diabetes Mellitus of Korean Diabetes Association. Park IeB, Kim J, Kim DJ, Chung CH, Oh JY, Park SW, Lee J, Choi KM, Min KW, Park JH, Son HS, Ahn CW, Kim H, Lee S, Lee IB, Choi I, Baik SH. Diabetes epidemics in Korea: reappraise nationwide survey of diabetes "diabetes in Korea 2007". Diabetes Metab J. 2013;37:233–239. doi: 10.4093/dmj.2013.37.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Won JC, Ko KS. The epidemiology of diabetic neuropathy in Korea. Korean Clin Diabetes. 2010;11:177–183. [Google Scholar]
- 11.Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, Cha BY. Prevalence and clinical characteristics of diabetic peripheral neuropathy in hospital patients with type 2 diabetes in Korea. Diabet Med. 2012;29:e290–e296. doi: 10.1111/j.1464-5491.2012.03697.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, Cha BY. Validity of the medical outcomes study sleep scale in patients with painful diabetic peripheral neuropathy in Korea. J Diabetes Invest. 2013;4:405–409. doi: 10.1111/jdi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, Cha BY. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes Res Clin Pract. 2013 Dec 25; doi: 10.1016/j.diabres.2013.12.003. Epub http://dx.doi.org/10.1016/j.diabres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Tapp RJ, Shaw JE, de Courten MP, Dunstan DW, Welborn TA, Zimmet PZ AusDiab Study Group. Foot complications in Type 2 diabetes: an Australian population-based study. Diabet Med. 2003;20:105–113. doi: 10.1046/j.1464-5491.2003.00881.x. [DOI] [PubMed] [Google Scholar]
- 15.Lu B, Yang Z, Wang M, Yang Z, Gong W, Yang Y, Wen J, Zhang Z, Zhao N, Zhu X, Hu R. High prevalence of diabetic neuropathy in population-based patients diagnosed with type 2 diabetes in the Shanghai downtown. Diabetes Res Clin Pract. 2010;88:289–294. doi: 10.1016/j.diabres.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng WY, Jiang YD, Chuang LM, Huang CN, Heng LT, Wu HP, Tai TY, Lin BJ. Quantitative sensory testing and risk factors of diabetic sensory neuropathy. J Neurol. 1999;246:394–398. doi: 10.1007/s004150050370. [DOI] [PubMed] [Google Scholar]
- 18.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 19.Boulton AJ, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med. 1998;15:508–514. doi: 10.1002/(SICI)1096-9136(199806)15:6<508::AID-DIA613>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34:2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004;21:976–982. doi: 10.1111/j.1464-5491.2004.01271.x. [DOI] [PubMed] [Google Scholar]
- 23.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 24.el-Shazly M, Abdel-Fattah M, Scorpiglione N, Benedetti MM, Capani F, Carinci F, Carta Q, Cavaliere D, De Feo EM, Taboga C, Tognoni G, Nicolucci A The Italian Study Group for the Implementation of the St. Vincent Declaration. Risk factors for lower limb complications in diabetic patients. J Diabetes Complications. 1998;12:10–17. doi: 10.1016/s1056-8727(97)00001-9. [DOI] [PubMed] [Google Scholar]
- 25.Franklin GM, Shetterly SM, Cohen JA, Baxter J, Hamman RF. Risk factors for distal symmetric neuropathy in NIDDM. The San Luis Valley Diabetes Study. Diabetes Care. 1994;17:1172–1177. doi: 10.2337/diacare.17.10.1172. [DOI] [PubMed] [Google Scholar]
- 26.Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 27.Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, Matthews DR, Stratton IM, Holman RR UK Prospective Diabetes Study (UKDPS) Group. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68) Diabetologia. 2004;47:1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 28.Korean Diabetes Association. 2011 Treatment guideline for type 2 diabetes. 4th ed. Seoul: Korean Diabetes Association; 2011. [Google Scholar]
- 29.Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68:1178–1182. doi: 10.1212/01.wnl.0000259085.61898.9e. [DOI] [PubMed] [Google Scholar]
- 31.Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients' perspectives. J Pain. 2006;7:892–900. doi: 10.1016/j.jpain.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]