Abstract
Social status primarily determines male mammalian reproductive success, and hypotheses on the endocrinology of dominance have stimulated unprecedented investigation of its costs and benefits. Under the challenge hypothesis, male testosterone levels rise according to competitive need, while the social stress hypothesis predicts glucocorticoid (GC) rises in high ranking individuals during social unrest. Periods of social instability in group-living primates, primarily in baboons, provide evidence for both hypotheses, but data on social instability in seasonally-breeding species with marked social despotism but lower reproductive skew are lacking. We tested these hypotheses in seasonally-breeding rhesus macaques on Cayo Santiago, Puerto Rico. We documented male fecal GC and androgen levels over a 10 month period in relation to rank, age, natal status and group tenure length, including during a socially unstable period in which coalitions of lower-ranked males attacked higher-ranked males. Androgen but not GC levels rose during the mating season; older males had lower birth season levels but underwent a greater inter-season rise than younger males. Neither endocrine measure was related to rank except during social instability, when higher ranked individuals had higher and more variable levels of both. High ranking male targets had the highest GC levels of all males when targeted, and also had high and variable GC and androgen levels across the instability period. Our results provide evidence for both the challenge and social stress hypotheses.
Keywords: challenge hypothesis, social stress, male-male competition, social status, dominance
Introduction
Social status is the primary determinant of reproductive success among male mammals (Ellis 1995), and male-male competition for social status is an integral part of sexual selection (Darwin 1871). Our understanding of the dynamics of such competition has been greatly enhanced by investigations of its underlying hormonal mechanisms. For example, empirical tests of the challenge hypothesis, which proposes that male testosterone levels increase in response to competitive need (Wingfield et al. 1990), have elucidated both the proximate mechanisms and ultimate costs of male social competition in a number of species of mammals, as well as in birds and other vertebrates. Areas investigated within the challenge hypothesis framework include topics such as behavioral seasonality, territoriality, consortship formation, and aggression and dominance (for overviews, see Hirschenhauser et al. 2003 for birds and Hirschenhauser and Olivera 2006 for mammals).
In group-living sexually promiscuous vertebrates such as many primates, in which male-male competition manifests in the establishment of dominance hierarchies, we expect both within- and between-individual variation in circulating levels of androgen and glucocorticoid (GC) hormones in relation to seasonal changes in mating, and the social stress associated with the establishment and maintenance of social status (Sapolsky 2005). These changes may be functionally related to the key roles these hormones play in priming the body for competition, with GCs having an important role in the mobilization of energy (Sapolsky et al. 2000; Goymann and Wingfield 2004), while elevated androgens are associated with increased aggressive behavior (Sapolsky 1983; Wingfield et al. 1990; Muller and Wrangham 2004). Although the functional roles of GCs and androgens are phylogenetically conserved, there is likely to be inter-specific variation in patterns of male GC and androgen expression, depending on factors such as the degree of breeding seasonality and female estrus synchrony, and accompanying differences in male competitive regimes (Abbott et al. 2003; Sapolsky 2005).
Seasonal changes in male androgen and/or GC levels in relation to changes in male-male aggression and sexual activity occur widely among vertebrates (e.g. striped mice, Rhabdomys pumilio, Schradin 2008; nuthatches, Sitta europaea, Landys et al. 2010; alligators, Alligator mississippiensis, Hamlin et al. 2011), and such changes have been documented in detail in numerous seasonally breeding primate species (e.g. Verreaux´s sifakas, Propithecus verreauxii, Kraus et al. 1999; Fichtel et al. 2007; ring-tailed lemurs Lemur catta, Cavigelli and Pereira 2000; tufted capuchin monkeys, Cebus paella nigritus, Lynch et al. 2002; golden lion tamarins, Leontopithecus rosalia, Bales et al. 2006; Assamese macaques, Macaca assamensis, Ostner et al. 2008, 2011). However, data on the hormonal changes associated with male social instability in free-ranging primates are rarer. In yellow baboons (Papio cynocephalus) high-ranked males (those with most to lose from dominance changes) have been shown to experience lower GC levels than low-ranked males during social stability, but not during social instability (Sapolsky 1983), while high-ranked males have higher androgen levels than low-ranked males only during periods of social instability (Sapolsky 1983). GCs rise specifically in such individuals when instability threatens to decrease, rather than increase, their rank (Sapolsky 1992). The stressful effects of male rank instability may be experienced by both males and females (e.g. chacma baboons, Papio ursinus, males, Bergman et al. 2005; females, Beehner et al. 2005). In mandrills (Mandrillus sphinx), high- ranking males have higher GC levels than low-ranking males during social instability, whereas the reverse is true during social stability (Setchell et al. 2010).
The extent to which the effects of dominance stability on GC levels and other aspects of physiological condition can be generalized across primate species is unclear, and may be related to competitive regimes and modes of dominance acquisition (Abbott et al. 2003). For example, baboons are non-seasonal breeders, with female fertile periods distributed across the year. As a consequence, reproductive skew is typically very marked (e.g. chacma baboons, Cheney et al. 2004), as dominant males can monopolize these temporally dispersed fertile periods, and rank increases have a big reproductive pay-off for individuals. Similarly, reproductive skew is also extremely strong in mandrills (Charpentier et al. 2005; Setchell et al. 2005). How dominance stability relates to underlying GC and androgen levels in populations of seasonally breeding species where reproductive skew is less strong and losing rank position less reproductively costly (van Noordwijk and van Schaik 2004) is unknown. In part this is because rank instability is rare in such species, where males typically queue rather than fight for dominance (van Noordwijk and van Schaik 2004).
A good taxon for investigating such issues is the macaques (Macaca sp.), all species of which live in multi-male multi-female social groups and exhibit male-male competition for females, as demonstrated by marked sexual dimorphism (Plavcan 2001). Although macaque males of high social status generally have higher reproductive output (Rodriquez-Llanes et al. 2009), there is considerable variation in the percentage of sires obtained by alpha males between species. This ranges from relatively low levels in seasonal breeders such as Barbary macaques, Macaca sylvanus (6–25%: Paul et al. 1993; Kümmerli and Martin 2005) to high levels in non-strictly seasonal breeders such as long-tailed macaques, Macaca fascicularis (60–90%: De Ruiter et al. 1994; Engelhardt et al. 2006). As such, the relative reproductive pay-off for increases in male rank differs greatly between species, leading to big differences in male competitive regimes (van Noordwijk and van Schaik 2004).
Among macaques, species differences in male competitive regimes and reproductive skew appear to be associated with differences in dominance style, such that species with greater skew tend to be less despotic (i.e. they show more symmetrical dyadic agonistic interactions, greater social tolerance, and higher rates of post-conflict reconciliation; Thierry 2000), while species with low skew show the opposite pattern (Schülke and Ostner 2008, 2012). Whether or not the male competitive regime and degree of reproductive skew, or the social style of macaques, are associated with a particular pattern of rank-related male hormonal activity or differential hormonal responses to rank-related social instability and stress is unknown.
In this study, we present data from the free-ranging rhesus macaque (Macaca mulatta) population on Cayo Santiago, in which promiscuous mating occurs during a 6-month mating season and group males form linear dominance hierarchies. Groups in this population are very large (in some cases >200 individuals) and include numerous adult males (in some cases >30). Higher ranking males have greater reproductive success than lower-ranking males, but skew is low compared to many other macaque species or populations (20–30%: Berard et al. 1994; Widdig et al. 2004; Dubuc et al. 2011). Rhesus macaques have an extremely despotic social system, in which low-ranked individuals typically experience significant psychosocial stress resulting from the receipt of aggression and intimidation (Maestripieri 2007). Although rhesus males generally rise in rank gradually over time (Manson 1995), significant changes in dominance hierarchies initiated by lower-ranked males can sometimes occur during short periods of social instability (Higham and Maestripieri 2010).
Previous studies of both captive and free-ranging rhesus macaques have shown that male testosterone levels are significantly higher during the mating than during the birth season (e.g. Plant et al. 1974; Robinson et al. 1975; Gordon et al. 1976, 1978; Herndon et al. 1996; Mehlman et al. 1997). Relationships between male hormone levels and dominance are less clear, with studies of captive rhesus macaques showing variable results. Some studies have shown no overall relationships between hormones and rank (Bernstein et al. 1991; subadult males, Bercovitch and Clarke 1995), while others have shown that higher ranked individuals exhibit higher androgen levels (Rose et al. 1971), or that lower-ranked individuals had the lowest (among juveniles, Mann et al. 1998). Despite this, a consistent finding even in studies finding otherwise no relationship between dominance and testosterone is that the highest ranked male had the highest androgen levels (Bernstein et al. 1991, Bercovitch and Clarke 1995), suggesting that high rank may to some extent reflected in higher levels of androgens. Studies of other free-ranging macaque populations have also produced mixed results, with no relationships between rank and androgen or GC levels in wild long-tailed macaques (Girard-Buttoz et al. 2009), no relationships between rank and androgen levels in Japanese macaques Macaca fuscata (Eaton and Resko 1974) and Assamese macaques (Ostner et al. 2011), but higher GC levels in high-ranked Japanese macaque males (Barrett et al. 2002), and low-ranked Assamese macaque males (Ostner et al. 2008).
We investigated the relationships between male androgen and glucocorticoid hormones, mating, and dominance. Using data collected over the course of a 10 month period, we assessed variation in male androgen and GC levels according to season, dominance rank, natal status, group residency length, and age. Furthermore, we took advantage of a rare period of male rank instability, during which males formed revolutionary coalitions and fought over dominance (Higham and Maestripieri 2010), to investigate the relationship between the two hormones and the social stress associated with dominance instability. We predicted that male androgen levels should be higher during the mating than the birth season, while seasonal differences in GC levels should either be absent or less marked. We also predicted that, when the dominance hierarchy is stable, rank should not be associated with variation in GC and androgen levels; during the period of rank instability, however, higher ranked males should have higher androgen levels consistent with the challenge hypothesis, and higher GC levels consistent with data from baboons on the social stress of instability. We predicted that, given that higher ranking males should be experiencing persistent authority challenges during these periods to which they may experience endocrine surges, they should also have more variable levels of both hormones. We predicted that older adult males would exhibit reduced androgen levels, consistent with data from humans (Ellison et al. 2002; Bribiescas 2006), chacma baboons and gelada (Beehner et al. 2009) and mouse lemurs (Aujard and Perret 1998).
Methods
Study site and population
The study took place on Cayo Santiago, a 15.2 ha island located 1 km off the East coast of Puerto Rico. A rhesus macaque colony was established on this island in 1938 from free-ranging individuals captured in India (Rawlins and Kessler 1986) and this is currently managed by the Caribbean Primate Research Center (CPRC) of the University of Puerto Rico. The animals are free-ranging and live in naturally-formed social groups, but are provisioned daily with commercial monkey chow. There is currently a 6-month mating season from March to August, followed by a 6-month birth season from September to February (Hoffman et al. 2008). Data presented in this study were collected from 20th October 2008 – 7th August 2009. During this time the population included approximately 1000 individuals living in 6 social groups. All data collection took place in Group R, which consisted of an average of 268 individuals (range = 243–307) during the study period (group size varied as a result of births, deaths, and male emigration/immigration). We collected dominance rank data (see below for rank calculations) on all adult males in Group R. Although the number of males in this group reached a maximum of 44 during the study period, only 35 of these males were permanent members of the group and were observed to interact with other males with sufficient regularity to be included in the group’s dominance hierarchy.
Focal behavioral data and fecal samples were collected on a subset of 15 males during the birth season (mean age 13.9 years, range 8.8–21.8), and 20 males during the mating season (mean age 12.9 years, range 5.5–22.4). Ranks of focal males ranged from 1–34 (birth season) and 1–35 (mating season), so representing the full range of ranks in the group. Of these males, 13 were the same in both seasons, and so sample sizes are slightly smaller for the one analysis in which we compare the same individual males between seasons. Males were chosen as focals on the basis of their central physical and apparent social positions in the group. Our mating season sample of 20 males included 11 of the 12 males involved as either targets or coalition members during periods of rank instability (Higham and Maestripieri 2010; see below). Data on male ages, group residency length (both measured in days on June 1st 2009) and natal status (i.e. born in group R or another group) were available for all males from long-term records.
During the 2009 mating season, a period of rank instability began at the start of June, which resulted in several high ranking males being attacked repeatedly over the course of the following weeks by coalitions of subordinates (Higham and Maestripieri 2010). Two males were targeted at the beginning of June, one male towards the end of June, and the final male in early-mid August. As a result of these coalitionary attacks, the aggressors were able to outrank their targets, some of which were permanently evicted from the group (Higham and Maestripieri 2010). In particular, the alpha male was attacked, badly beaten and permanently evicted from the group on August 10th, 3 days after the last fecal sample included in the present dataset was collected. Data in the present study were analyzed separately for three periods: 3 months of the birth season (October – December; no data were collected in January and February, when most animals were trapped by the colony management), the first 3 months of the mating season (March – May), before the onset of rank instability, and the 3 months of rank instability (June – August). These three periods are referred to as the BS, MS-stable, and MS-unstable period, respectively. The MS-stable period represents the peak of the mating season, when the majority of mating activity took place (Higham et al. 2011).
Fecal Sample Collection and Endocrine Analyses
Fecal samples were collected from males between 7:00 and 14:30, as previous studies have shown little (if any) diurnal variation in endocrine metabolite values measured from primate feces (e.g. glucocorticoids, Higham et al. 2009; androgens, Ostner et al. 2011; androgens and glucocorticoids, Girard-Buttoz et al. 2009). We collected 465 fecal samples in total, with a mean 8.7 ±0.6 (range 2–11) samples per male for the birth season, a mean 8.4 ±0.6 (range 3–14) samples per male for the MS-stable period, and a mean 7.6 ±0.7 (range 3–13) samples per male for the MS-unstable period. Samples for three of the target males were collected regularly throughout the MS-unstable period. Samples for one target male, who was permanently diarrheal during the MS-unstable period, were unavailable for this period only. Fecal samples (uncontaminated with urine) were collected only after defecation was observed directly. Samples were then homogenized, non-fecal debris was removed, and a small bolus of approximately 0.5–2 grams wet weight was placed in a 20 ml tube, which was in turn placed into a cooler containing ice packs (Hodges and Heistermann, 2003). Upon return to the mainland (at either 11:30 or 14:30), tubes were frozen at −20 °C until they were shipped on ice to the German Primate Center for hormone analysis. All fecal samples arrived in Germany still frozen.
Fecal samples were lyophilized and pulverized and an aliquot (50–70 mg) of the fecal powder was extracted with 3 ml 80% methanol by vortexing for 15 min (Heistermann et al. 1995). All fecal extracts were analyzed for cortisol (GC) and androgen metabolites using enzyme immunoassays for 5β-androstane-3a,11β-diol-17-one (3a,11β-dihydroxy-CM; Ganswindt et al. 2003) and epiandosterone (EA, Palme and Möstl 1994), respectively. The GC assay, carried out as described in detail in Heistermann et al. (2004), has been successfully applied to monitor glucocorticoid output in a variety of primate species (Heistermann et al. 2006; Fichtel et al. 2007; Weingrill et al. 2011), including baboons (Higham et al. 2009) and several species of macaques (Shutt et al. 2007; Ostner et al. 2008; Girard-Buttoz et al. 2009). It has previously also been validated for measuring adrenocortical activity in response to trapping stress in rhesus macaques (Hoffman et al. 2010). The measurement of EA, a major metabolite of testosterone in primate feces (Möhle et al. 2002; Girard-Buttoz et al. 2009), was carried out as described in detail elsewhere (Girard-Buttoz et al. 2009). The assay has previously been validated and successfully used to monitor androgen status in several species of primates including macaques (Girard-Buttoz et al. 2009; Ostner et al. 2011; Weingrill et al. 2011).
Inter-assay variation, determined by repeated measurement of high and low value quality controls in each assay, was 12.2% (High) and 12.5% (Low) (3a,11β-dihydroxy-CM), and 9.8% (High) and 16.6% (Low) (EA), while intra-assay variation was 6.2% (High) and 7.3% (Low) (3a,11β-dihydroxy-CM) and 7.1% (High) and 8.8% (Low) (EA).
Calculation of dominance rank
As described elsewhere (Higham and Maestripieri 2010, Higham et al. 2011), we established two separate dominance hierarchies, one for the BS period and the other for the MS-stable period. Ranks were assigned on the basis of the following “winner-loser” agonistic interactions involving all adult (≥5.5 years) males: fear grins (winner is the individual grinned at); avoidance (winner is the individual avoided); displacements (winner is the displacer); and threats, chases and lunges (winner is the aggressor in all cases). We recorded 643 such interactions during the BS period and 812 interactions during the MS-stable period. We compiled interactions into winner-loser matrices and used Matman 1.1 (Noldus) to create the separate hierarchies. Following 10,000 iterations, significant linear hierarchies were produced (linearity test using Landau's linearity index corrected for unknown relationships; BS, p=0.011; MS-stable, p=0.003), which were highly directionally consistent (both seasons, Directional Consistency Index = 0.91). The ranks formed by this process were used in further analyses. During the MS-unstable period, ranks were by definition unstable. Given that the individuals being challenged during such a period, and those with most to lose, were those that were high ranking at the onset of the period, we analyzed male GC and androgen levels over the MS-unstable period against their ranks at the onset of this period (i.e. those from the MS-stable period). Analyses of dominance rank for the MS-unstable period are therefore discussed in terms of males who were formerly high or low ranking. One male not seen frequently interacting with others during the mating season was not ranked for this period.
Statistical Analyses
Males who had spent longer in the group had higher ranks, as indicated by a significant correlation between dominance rank and residency length (Spearman’s correlations, BS, r= −0.929, n=13, p<0.001; MS-stable, r= −0.921, n=19, p<0.001). These variables were not considered further separately, and analyses focused on rank only. As male age was independent of group tenure length (Pearson’s correlation, r=0.246, n=19, p=0.310), and dominance rank (Spearman’s correlation, r=0.075, n=19, p=0.759), both rank and age were considered in models. First, we used ANOVA to investigate variability in androgen and GC levels among all males regardless of season. Then, hormonal data were averaged for each of the 3 study periods and male mean values were compared across the 3 periods using Friedman tests, followed by Wilcoxon signed-rank tests to assess each period against the other separately (nonparametric tests were used due to small sample sizes). To assess whether male characteristics such as age (covariate), rank (covariate), or natal status (fixed effect) affected variation in androgen and GC levels, mean values for each study period were analyzed separately using General Linear Models (GLM), as this parametric method allowed us to evaluate all variables simultaneously and ensure that any significant results were independent of each other. However, given that sample sizes were small and that rank data are best analyzed with non-parametric statistics, we also used Spearman’s correlations (for age and rank effects on hormonal variables) and Mann Whitney U tests (for natal status). The results of these tests generally did not differ qualitatively in their significance or non-significance from the results of GLMs, and where this is the case only the GLM results are presented. In a few cases, results of non-parametric analyses differed slightly to those of GLMs; in these cases both sets of results are presented. Natal status was not included in analyses of the birth season, as our dataset only included one natal male during that period. During the MS-unstable period we also split males into two equal categories of high- and low-ranking and compared GC and androgen levels between the two categories for comparison with a previous study of baboons (Bergman et al. 2005). We undertook Mann Whitney U tests to compare whether, during the MS-unstable period, the androgen and GC levels were higher for: 1) male targets compared to other males; 2) males that launched coalitionary attacks compared to other males. Finally, we assessed whether the two male targets in the dataset for which we have data specifically for the periods in which they were primarily targeted showed specific effects at those times. To do this, we split the data during the MS-unstable period from June into two (1st–15th June, 16th–30th June) and averaged GC and androgen values for all males within these periods only. As attacks on male 54V began and were most intense in the first half of June, we assessed whether this male had higher GC and androgen levels than other males at this time using a one-sample Wilcoxon test. As attacks on male 83L began and were most intense in the second half of June, we did the same for this male for this period. Statistics were undertaken in SPSS 15.0, were two-tailed, and results were considered statistically significant when the p-value was <0.05.
Results
Consistency and seasonality in endocrine variables
Across the whole study period, there was significant inter-individual variation in both GC (ANOVA, F23,463 = 3.82, p<0.001) and androgen (F23,463 = 7.81, p<0.001) levels. Males’ mean GC levels ranged from 147.4 – 496.5 ng/g during the BS period, 131.7 – 775.2 ng/g during the MS-stable period, and 107.1 – 564.3 ng/g during the MS-unstable period. Males’ mean androgen levels ranged from 708.8 – 6654.6 ng/g during the BS period, 694.4 – 7609.7 ng/g during the MS-stable period, and 703.8 – 4846.4 ng/g during the MS-unstable period. Coefficients of variation across all samples for each male averaged 58.9 ±4.3% (range 34.7–120.8%) for GCs and 46.8 ±3.1% (range 27.0–91.9%) for androgens, suggesting that there was a relatively high degree of intra-individual variability.
Repeated measures comparisons of hormone levels across the three study periods revealed significant variation in androgens (Friedman test, χ2=14.00, df=2, p=0.001) but not in GCs (χ2=4.67, df=2, p=0.097). Post-hoc analyses indicated that males had significantly higher androgen levels during the MS-stable period (mean = 4464.5 ±437.8 ng/g) than during both the BS-period (mean = 3324.3 ±449.3 ng/g, Wilcoxon signed-ranks test, Z= −2.41, p=0.016) and the MS-unstable period (mean = 2946.3 ±261.4 ng/g, Z= −3.59, p<0.001). In contrast, there was no significant difference in male androgen levels between the BS and the MS-unstable periods (Z= −0.94, p=0.347).
Endocrinology of social status and rank-related social stress
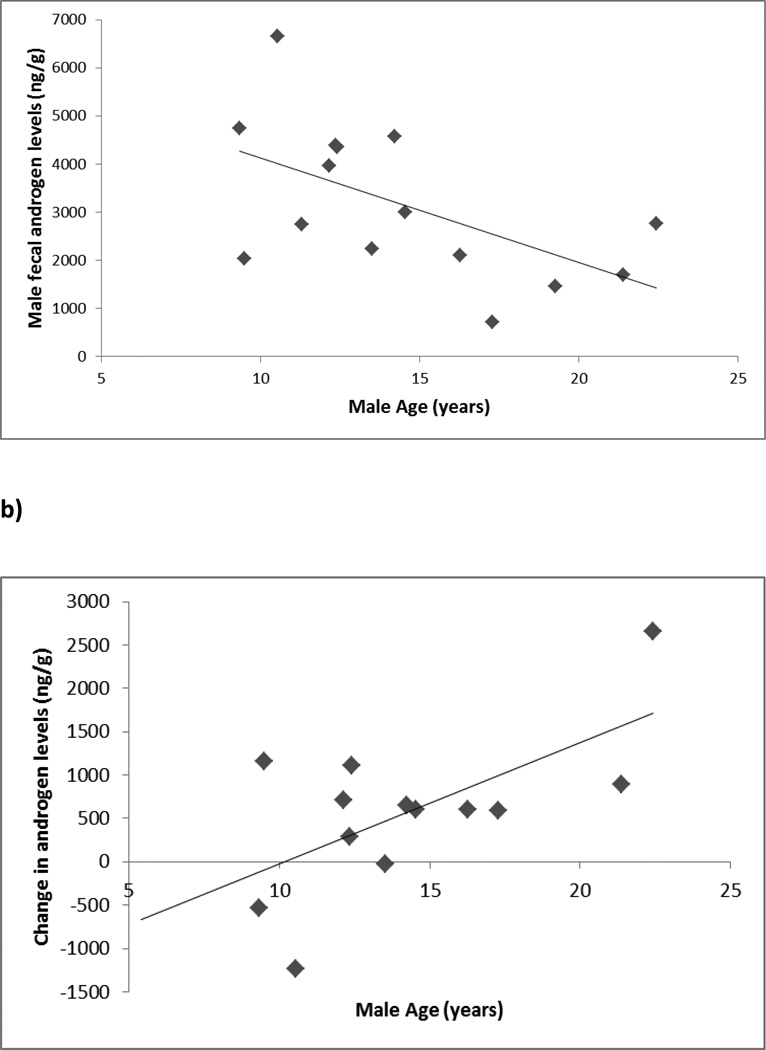
During the BS-period, GLMs showed no significant effects of rank (F1,12 = 0.30, p=0.597), or age (F1,12 = 1.21, p=0.292) on GC levels. Similarly, there were no effects of rank (F1,12 = 0.02, p=0.897) on androgen levels, but there was a significant effect of age, with older males exhibiting lower androgen levels (F1,12 = 5.56, p=0.036; Figure 1a). There were also significant effects of male age on the variance of both GC (F1,12 = 5.45, p=0.038) and androgen levels (F1,12 = 5.90, p=0.032), with older males exhibiting lower variability in both endocrine parameters. There were no significant effects of rank on variance of either GC (F1,12 = 0.05, p=0.516) or androgen levels (F1,12 = 1.04, p=0.329).
Figure 1.
a) Mean androgen levels by male age during the birth season; b) Increase in androgen levels from the birth to the mating season by male age.
During the MS-stable period, there were no significant effects of any variable on either GC (rank, F1,14 = 3.35, p=0.089; natal status, F1,14 = 2.97, p=0.107; age, F1,14 = 1.37, p=0.261) or androgen levels (rank, F1,14 = 3.68, p=0.076; natal status, F1,14 = 2.21, p=0.159; age, F1,14 = 1.86, p=0.194). Older males exhibited a significantly greater increase in androgen levels from the BS to the MS-stable period compared to younger males (r=0.630, n=13, p=0.021; Figure 1b) and this may explain why the significant correlation between male age and androgens found in the BS period was not present in the MS-stable period. There was no effect of any variable on the variance in male GC levels (rank, F1,13 = 0.22, p=0.645; natal status, F1,13 = 1.02, p=0.330; age, F1,13 = 0.03, p=0.874), and no effect of age (F1,13 = 1.66, p=0.220) or natal status (F1,13 = 1.48, p=0.246) on variance in androgen levels. GLMs showed a significant effect of rank on androgen variance (F1,13 = 4.98, p=0.044), but such a relationship was not supported by the Spearman’s rank correlation (rs= − 0.360, n=17, p= 0.155).
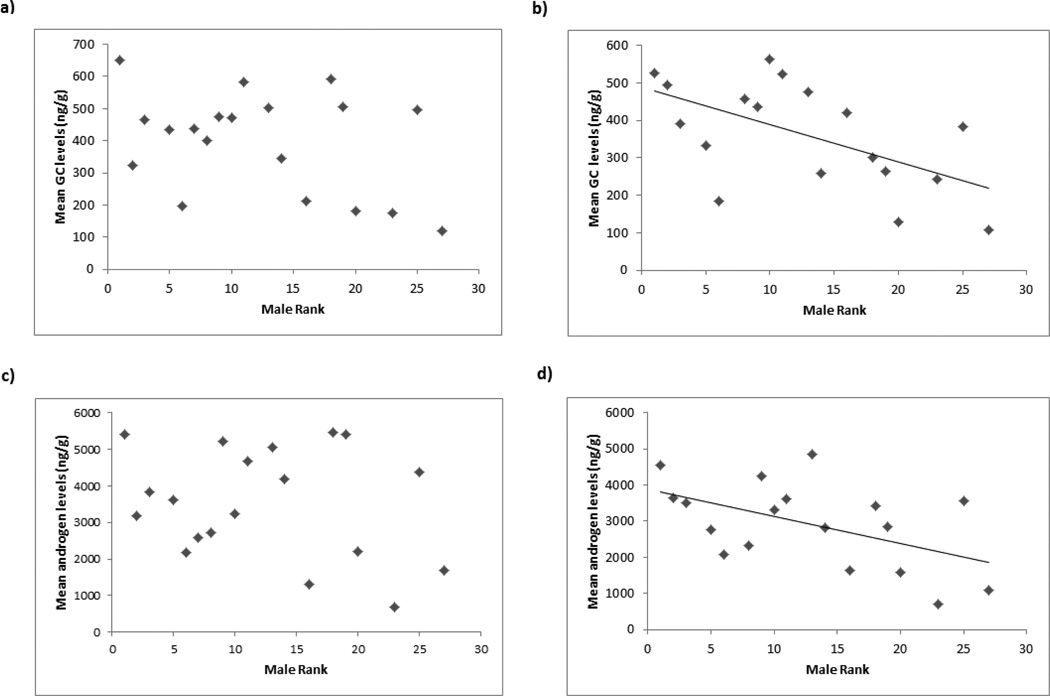
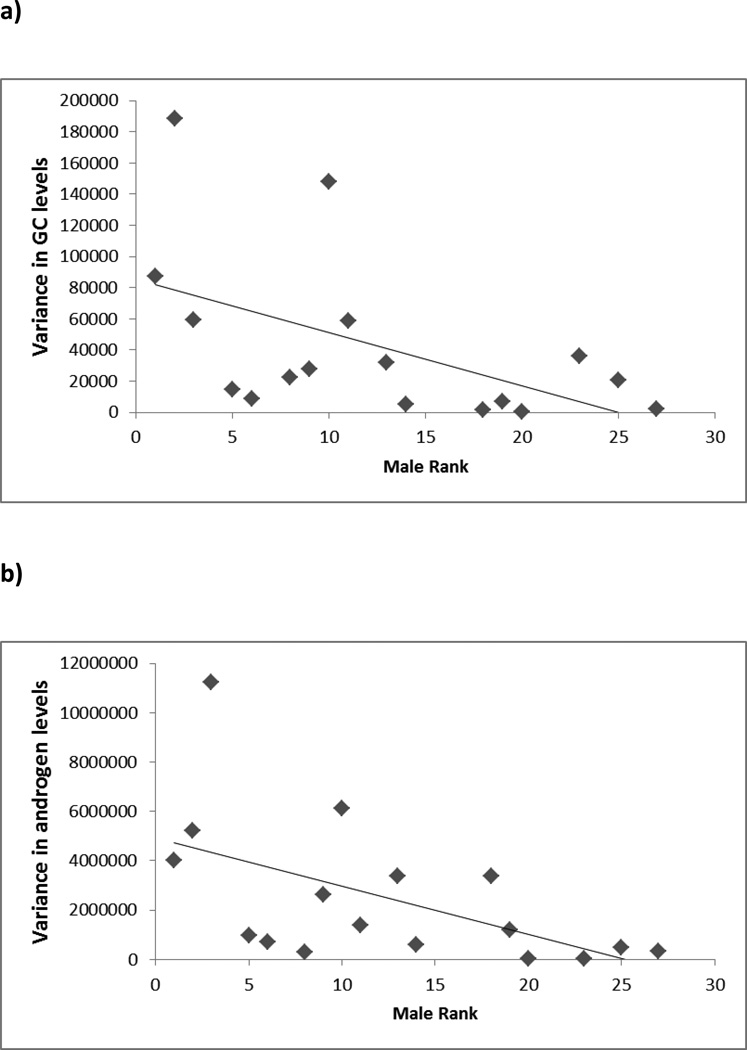
During the MS-unstable period, there were significant effects of male rank on GC (F1,14 = 8.07, p=0.013) and androgen levels (F1,14 = 7.53, p=0.016), with formerly higher-ranking males showing higher levels of both hormones than formerly lower-ranking males (Figure 2). Splitting the males into two equal-sized categories by rank (high, low) and averaging across males in these categories for comparison with previously published baboon data (see Discussion), shows that over this period the mean GC levels for formerly higher-ranking males were 434.7 ± 37.5 ng/g compared to 312.2 ± 45.0 ng/g for formerly lower-ranking males, and mean androgen levels for formerly higher-ranking males were 3338.0 ± 260.6 ng/g compared to 2593.7 ± 412.4 ng/g for formerly lower-ranking males. Unlike rank, age or natal status had no significant effects on hormonal variables during the MS-unstable period (age: GCs, F1,14 = 0.23, p=0.640; androgens, F1,14 = 3.51, p=0.082; natal status: GCs, F1,14 = 1.20, p=0.292; androgens, F1,14 = 3.70, p=0.075). There were no significant effects of any parameter on the variance of either GC (rank, F1,13 = 3.87, p=0.071; natal status, F1,13 = 2.70, p=0.124; age, F1,13 = 0.23, p=0.882) or androgen levels (rank, F1,13 = 4.52, p=0.053; natal status, F1,13 = 1.92, p=0.189; age, F1,13 = 1.17, p=0.299). However, the two close-to-significant rank relationships found in these GLMs of both endocrine parameters were significant in Spearman’s rank correlations, with higher-ranking males experiencing greater variability in both GC (rs= −0.596, n=17, p=0.012; Figure 3a) and androgen levels (rs= −0.620, n=17, p=0.008; Figure 3b). Further, the two males that were targets during the MS-unstable period were both high outliers for variability in GC levels, while the third highest variability belonged to the alpha male (Figure 3a), who was targeted within 3 days of cessation of fecal sample collection for the MS-unstable period. The GC levels of these 3 targets showed higher variance in levels than other males (Mann Whitney U test, z= −2.547, p=0.011). The highest variability in androgen levels belonged to the 3rd ranked male, who ultimately became the alpha male following the MS-unstable period by instigating an attack on the existing alpha. The 3 males (including the existing alpha) who were targets had the next 3 most variable androgen levels, and these 3 males showed higher variance in levels than other males (comparison includes the highly variable 3rd ranked male, Mann Whitney U test, z= −2.310, p=0.021).
Figure 2.
Mean GC and androgen levels by male rank: a) GCs averaged across the whole year; b) GCs averaged across the MS-unstable period; c) androgens averaged across the whole year; d) androgens averaged across the MS-unstable period.
Figure 3.
Male variance in GC and androgen levels during the MS-unstable period showing significant correlations between rank and the variance of both variables: a) GC levels - note that the two high outliers are the two males targeted by revolutionary coalitions (Higham and Maestripeiri 2010) for which we have data during the MS-unstable period. The third highest variability belonged to the alpha male, who was targeted within 3 days of cessation of fecal sample collection for the MS-unstable period; b) androgen levels – note that the greatest variability belonged to the 3rd ranked male, who ultimately became the alpha male following the MS-unstable period by instigating an attack on the existing alpha male. The two males who were targets during the MS-unstable period, and the existing alpha male (targeted immediately after the end of this period), had the next 3 highest levels of variability.
Males involved in forming coalitions against higher-ranking males did not have higher androgen or GC levels than other males, either during the MS-stable (androgens, Mann Whitney U test, Z= −0.423, p=0.673; GCs, Z= −0.592, p=0.554) or during the MS-unstable period (androgens, Mann Whitney U test, Z= −0.676, p=0.499; GCs, Z= −0.592, p=0.554). Likewise, coalition males did not show higher variance in androgen or GC levels in either the MS-stable (androgens, Mann Whitney U test, Z= −1.132, p=0.258; GCs, Z= −0.498, p=0.618) or during the MS-unstable period (androgens, Mann Whitney U test, Z= −0.281, p=0.779; GCs, Z= −0.468, p=0.640). Of the two male targets for whom data are available for the specific fortnight period in which they were primarily targeted, both males exhibited the highest levels of GCs of any male at those times, with each male showing levels significantly higher than all other males during those two respective fortnights (54V, first half of June, one-sample Wilcoxon test, Z= −3.351, p=0.001; 83L, second half of June, Z= −3.059, p=0.002). Although neither male showed the highest androgen level in the group during their respective period of primary targeting, they nonetheless both showed levels that were significantly higher than those of other males overall (54V, first half of June, one-sample Wilcoxon test, Z= −2.379, p=0.017; 83L, second half of June, Z= −2.341, p=0.019).
Discussion
Our study provides new insights into the relationships between male-male competition for mating and dominance, the social stress associated with dominance stability, and GC and androgen levels in a socially living mammal, the rhesus macaque. Our results provide new evidence in support of the challenge hypothesis, indicating that male androgen levels rise during the mating season and in conjunction with challenges for social status, as well as evidence in support of the hypothesis that the social stress associated with struggles for dominance is accompanied by an elevation in GC hormones among high-ranking individuals in group-living male primates. These hormones play important roles in energy mobilization (GCs, Sapolsky et al. 2000; Goymann and Wingfield 2004), and in facilitating aggressive behavior (androgens, Wingfield et al. 1990), thus priming individuals for direct competition.
The seasonal patterns of androgen excretion documented in the present study are consistent with data from a wide range of vertebrates (Wingfield et al. 1990; Hirschenhauser et al. 2003; Hirschenhauser and Olivera 2006), including other seasonally breeding primates (Verreaux´s sifakas, Kraus et al. 1999; Fichtel et al. 2007; ring-tailed lemurs, Cavigelli and Pereira 2000; tufted capuchin monkeys, Lynch et al. 2002; golden lion tamarins, Bales et al. 2006; Assamese macaques, Ostner et al. 2008, 2011), as well as from captive (e.g. Plant et al. 1974; Robinson et al. 1975; Gordon et al. 1976, 1978; Herndon et al. 1996) and free-ranging (Mehlman et al. 1997) populations of rhesus macaques. Androgen levels were higher during the period of peak reproductive competition, consistent with the challenge hypothesis (Wingfield et al. 1990). In the present study, levels were highest during the peak mating MS-stable period (Higham et al. 2011), and these seasonal patterns may contribute to the high observed male mortality on Cayo Santiago at this time (Hoffman et al. 2008), given that increased testosterone has been linked to increased mortality in a number of studies of varied animal species (see Wingfield et al. 1990). The occurrence of peak reproductive competition in the MS-stable period may have led to relatively high GC levels at this time, and hence an absence of overall higher GC levels across all males during the MS-unstable period, in contrast to other studies, which have found raised levels across all males during unstable periods (e.g. mandrills, Setchell et al. 2010).
Our data also indicate an interaction between seasonality and age, with older males experiencing lower androgen levels than younger males during the birth season, which may be evidence of senescence. Studies of humans (e.g. Ellison et al. 2002, Bribiescas 2006) and chacma baboon and gelada males (Beehner et al. 2009) have shown significant declines in androgen levels as adult males age. Previous studies of captive rhesus macaques have found no differences in the testosterone levels of old and young males despite differences in mating rates between these males (Chambers et al. 1982). However, older males experience greater binding of testosterone to sex hormone-binding globulin than younger males (Chambers et al. 1981) and older males with higher binding levels have decreased mating rates. Our results add to a general picture of male senescence from a free-ranging seasonally-breeding primate population. They also highlight the importance of considering the annual cycle when looking for such effects among seasonal breeders – a focus solely on the mating season did not detect age-related differences in male androgen levels (Chambers et al. 1982) and such a focus would have failed to do so in the present study also. In addition to reduced androgen levels among older males, lower androgen levels are often documented among individuals likely to be fathers in species with paternal care (Wynne-Edwards 2001). As such, a further (non-mutually exclusive) potential explanation for lower androgen levels in older males during the birth season is that they have more offspring in the group and/or in the new birth cohort. However, it is not possible to assess this without paternity data. Older males underwent a significantly greater increase in androgen levels from the birth to the mating season than younger males did, such that the androgen levels of older and younger males in the mating season were comparable. This result provides further evidence for the challenge hypothesis (Wingfield et al. 1990), with the lower birth season androgen levels of older males rising to match those of younger males during the period of reproductive competition. These changes may be important in facilitating the necessary behavioral aggression in older males required to compete with younger individuals.
Consistent with much of the available data from macaques (Eaton and Resko 1974; Bernstein et al. 1991; Bercovitch and Clarke 1995; Girard-Buttoz et al. 2009; but see Rose et al. 1971; Barrett et al. 2002; Ostner et al. 2008), we found no significant relationships between dominance rank, androgen levels, or GC levels during periods when the dominance hierarchy was stable. Though our sample size is not large, significant relationships were nonetheless found during periods of rank instability, suggesting that small sample sizes are unlikely to be the explanation. Under circumstances of rank instability, higher-ranking males had significantly higher androgen levels than lower-ranking males. These results are consistent with data from baboons (Sapolsky 1983), and provide further support for the challenge hypothesis, with higher-ranked individuals showing increased androgen production that may facilitate aggressive behavior in response to competitive need (Wingfield et al. 1990).
Our study also found that under circumstances of rank instability, higher-ranking males had significantly higher GC levels than lower-ranking males. Studies of other vertebrates, including hyenas (van Meter et al. 2009) and birds (Japanese quail, Guibert et al. 2010) have shown raised GC levels in response to social instability. However, to our knowledge, directly comparable results showing a GC effect on instability specific to male rank have only been found in primates. Moreover, such effects have been shown previously only in species exhibiting strong reproductive skew by rank (baboons, Sapolsky 1983, 1992; Bergman et al. 2005; Gesquiere et al. 2011; mandrills, Setchell et al. 2010), in which reproductive gains and losses associated with increases and decreases in rank relatively high. In the most directly comparable data, where individuals were split into two equal sized categories of dominants/subordinates (as in the present study), dominants experienced GC levels around 122% of subordinates (Bergman et al. 2005), compared to 139% in the present study. This comparison is too small and idiosyncratic to draw conclusions from, and more studies are needed that test endocrine responses to rank instability in different species with diverse male-male competitive regimes (i.e. differences in the degree of direct contest competition over dominance). Our data suggest that the stress of rank instability for high-ranking males may be at least as strong in rhesus macaques as in baboons. This is despite the moderate levels of skew found in this rhesus macaque population (Berard et al. 1994; Widdig et al. 2004; Dubuc et al. 2011), which indicates that the reproductive consequence of dropping rank is reduced. In part, this could be because dominance instability is much rarer in large groups of seasonally breeding species such as rhesus macaques, in which males undergo less direct male-male contest competition (as indicated by relatively low sexual dimorphism in body size and weaponry such as canines, Plavcan 2001), and in which males typically queue for rank (Manson 1995; van Noordwijk and van Schaik 2004). This may mean that physiological and behavioral mechanisms for coping with such instability may be less established (Abbott et al. 2003). It may also reflect the high psychosocial stress and aggression that low-ranking individuals are typically thought to suffer in such a socially despotic species (Maestripieri 2007). Relatedly, high social despotism may make dominance perturbations especially brutal, as individuals may be particularly aggressive during challenges for status (Higham and Maestripieri 2010). The instability in the present study was certainly dramatic; several individuals were chased into the sea and seriously injured, and the alpha male was expelled from the group completely (Higham and Maestripieri 2010).
In addition to the general effects of instability on male endocrine levels related to rank, there were also specific effects on the individual targets, with the three targets for which endocrine data were available experiencing higher levels of both GCs and androgens than other males. These males were subject to a number of highly aggressive group attacks, in which they were badly beaten (Higham and Maestripieri 2010). What is particularly striking is that the two males for whom endocrine data were available for the specific two-week period in which they were initially targeted, and targeted most intensely, exhibited the highest levels of GCs of any male at those times. This probably reflects the high levels of stress these males were under at these times, with GC increases reflecting increased energy mobilization and facilitating increased responsiveness to threats. These individuals also had overall significantly higher androgen levels than other males at these times (though not the absolutely highest levels). They also experienced far greater variation in their levels of GCs than any other male, as well as high variation in their androgen levels. High levels of variability could be indicative of specific variation in dominance status and challenges from day-to-day during this period, or may indicate high levels of aggression, energy mobilization, or stress more generally. Collectively these results indicate very specific costs to the targets of such attacks. Such individual-specific effects of instability were also noted in baboons (Sapolsky 1992). Increased variability in endocrine levels was also generally experienced by higher-ranking individuals during the MS-unstable period which may again indicate that effects of increased social fluctuation are being reflected by increased fluctuation in underlying endocrinology. Unlike these extensive effects observed in male targets, we found no evidence that coalition members, many of whom increased their ranks due to these attacks on higher-ranked individuals (Higham and Maestripieri 2010), had higher androgen levels than other individuals. This is in contrast to results from baboons, which suggested that individuals on trajectories of increasing rank through contest competition had higher testosterone levels than other males (Beehner et al. 2006).
In conclusion, our data provide new evidence for the stressful effects of dominance instability for seasonally-breeding male primates living in multi-male multi-female groups with relatively low reproductive skew. In response to instability, GC levels were raised in higher-ranking individuals, as well as specifically in the individuals being targeted. These males also experienced increased variability in their GC and androgen levels. Our data also provide several separate pieces of evidence in support of the challenge hypothesis (Wingfield et al. 1990): androgen levels are higher during the period of peak reproductive competition; higher-ranking males exhibited higher androgen levels during periods of rank instability; and despite having lower androgen levels in the birth season, old adult males experienced greater increases in levels in the transition from the birth to the competitive mating season. These endocrine changes are likely to have played important roles in the mobilization of energy (Sapolsky et al. 2000; Goymann and Wingfield 2004), and in the facilitation of aggressive behavior (Wingfield et al. 1990), related to male-male competition. Given the high mortality levels of Cayo Santiago males in the mating season (Hoffman et al. 2008), further studies should attempt to combine investigation of the costly seasonal physiological effects experienced by males of different ages and ranks. Studies of mating season increases in androgen levels (this study) and decreases in energetic status and physical condition (Higham et al. 2011), could be added to new studies of immune function and disease to determine whether variation in male reproductive and social strategies leads directly to changes in physiological condition and health, which in turn drive patterns of seasonal mortality.
Table 1.
Summary of effects of seasonality, rank and age on male androgen and GC levels (p<0.05 in bold).
| Androgens (ng/g) | Glucocorticoids (GCs) (ng/g) | |
|---|---|---|
| Male means |
Birth: 708.8 – 6654.6 MS-stable: 694.4 – 7609.7 MS-unstable: 703.8 – 4846.4 |
Birth: 147.4 – 496.5 MS-stable: 131.7 – 775.2 MS-unstable: 107.1 – 564.3 |
| Seasonality means |
Birth: 3324.3 MS-stable: 4464.5 MS-unstable: 2946.3 χ2=14.00, df=2, p=0.001 |
Birth: 322.8 MS-stable: 468.0 MS-unstable: 370.2 χ2=4.67, df=2, p=0.097 |
| Age relationship |
Birth: F1,12 = 5.56, p=0.036 MS-stable: F1,14 = 1.86, p=0.194 MS-unstable: F1,14 = 3.51, p=0.082 |
Birth: F1,12 = 1.21, p=0.292 MS-stable: F1,14 = 1.37, p=0.261 MS-unstable: F1,14 = 0.23, p=0.640 |
| Rank relationship |
Birth: F1,12 = 0.02, p=0.897 MS-stable: F1,14 = 3.68, p=0.076 MS-unstable: F1,14 = 7.53, p=0.016 |
Birth: F1,12 = 0.30, p=0.597 MS-stable: F1,14 = 3.35, p=0.089 MS-unstable: F1,14 = 8.07, p=0.013 |
Acknowledgements
We thank Doreen Hess, Jenna Goldfein, Maria Rakhovskaya and the staff of the Caribbean Primate Research Center for logistical support in the field and assistance with animal capturing and handling. We are extremely grateful to Andrea Heistermann and Petra Kiesel for analyzing the fecal samples, and to Tara Mandalaywala and the Caribbean Primate Research Center for assisting with their transportation to Germany. We are deeply indebted to John Addicott for helping us to set up our Access database. JH also thanks Antje Engelhardt and Constance Dubuc for general discussion on macaque social styles and competitive regimes. We would also like to thank Thore Bergman for providing raw data from his 2005 Animal Behaviour study. Finally, the MS was improved by comments from Joan Silk and two anonymous reviewers. This research was supported by NIH grant R21-AG029862 to D.M. This publication was made possible by grant number CM-5-P40RR003640 from the NIH National Center for Research Resources (NCRR) to the Caribbean Primate Research Center of the University of Puerto Rico. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Ethical Standards
This study was conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and conformed to all laws of Puerto Rico, the United States, and Germany. The protocol for this study was approved by the Institutional Animal Care and Use Committee, Medical Sciences Department, University of Puerto Rico.
Conflict of Interest
The authors declare no conflict of interest.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Medoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Aujard F, Perret M. Age-related effects on reproductive function and sexual competition in the male prosimian primate, Microcebus Murinus . Physiol Behav. 1998;64:513–519. doi: 10.1016/s0031-9384(98)00087-0. [DOI] [PubMed] [Google Scholar]
- Bales KL, French JA, McWilliams J, Lake RA, Dietz JM. Effects of social status, age, and season on androgen and cortisol levels in wild male golden lion tamarins (Leontopithecus rosalia) Horm Behav. 2006;49:88–95. doi: 10.1016/j.yhbeh.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Barrett GM, Shimizu K, Bardi M, Asaba S, Mori A. Endocrine correlates of rank, reproduction, and female-directed aggression in male Japanese macaques (Macaca fuscata) Horm Behav. 2002;42:85–96. doi: 10.1006/hbeh.2002.1804. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL. Effects of male social instability of female glucocorticoid levels in chacma baboons. Anim Behav. 2005;69:1211–1221. [Google Scholar]
- Beehner JC, Bergman TJ, Cheney DL, Seyfarth RM, Whitten PL. Testosterone predicts future dominance rank and mating activity among male chacma baboons. Behav Ecol Sociobiol. 2006;59:469–479. [Google Scholar]
- Beehner JC, Gesquiere L, Cheney DL, Seyfarth RM, Altmann J. Fecal testosterone related to age and life-history stages in male baboons and geladas. Horm Behav. 2009;56:472–480. doi: 10.1016/j.yhbeh.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard JD, Nürnberg P, Epplen JT, Schmidtke J. Alternative reproductive tactics and reproductive success in male rhesus macaques. Behaviour. 1994;129:177–201. [Google Scholar]
- Bercovitch FB, Clarke AS. Dominance rank, cortisol concentrations, and reproductive maturation in male rhesus macaques. Physiol Behav. 1995;58:215–221. doi: 10.1016/0031-9384(95)00055-n. [DOI] [PubMed] [Google Scholar]
- Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM, Whitten PL. Correlates of stress in free-ranging male chacma baboons. Anim Behav. 2005;70:703–713. [Google Scholar]
- Bernstein IS, Ruehlmann TE, Judge PG, Lindquist T, Weed JL. Testosterone changes during the period of adolescence in male rhesus monkeys (Macaca mulatta) Am J Primatol. 1991;24:29–38. doi: 10.1002/ajp.1350240104. [DOI] [PubMed] [Google Scholar]
- Bribiescas RG. On the evolution, life history, and proximate mechanisms of human male reproductive senescence. Evol Anthropol. 2006;15:132–141. [Google Scholar]
- Cavigelli S, Pereira ME. Mating season aggression and fecal testosterone levels in male ring-tailed lemurs (Lemur catta) Horm Behav. 2000;37:246–255. doi: 10.1006/hbeh.2000.1585. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Hess DL, Phoenix CH. Relationship of free and bound testosterone to sexual behavior in old rhesus males. Physiol Behav. 1981;27:615–620. doi: 10.1016/0031-9384(81)90231-6. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Resko JA, Phoenix CH. Correlation of diurnal changes in hormones with sexual behavior and age in male rhesus macaques. Neurobiol Aging. 1982;3:37–42. doi: 10.1016/0197-4580(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Charpentier M, Peignot P, Hossaert-McKey M, Gimenez O, Setchell JM, Wickings EJ. Constraints on control: factors influencing reproductive success in male mandrills. Behav Ecol. 2005;16:614–623. [Google Scholar]
- Cheney DL, Seyfarth RM, Fischer J, Beehner JC, Bergman TJ, Johnson SE, Kitchen DM, Palombit RA, Rendall D, Silk JB. Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. Int J Primatol. 2004;25:401–428. [Google Scholar]
- Darwin C. The descent of man and selection in relation to sex. London: J Murray; 1871. [Google Scholar]
- de Ruiter JR, Van Hooff JARAM, Scheffrahn W. Social and genetic aspects of paternity in wild long-tailed macaques (Macaca fascicularis) Behaviour. 1994;129:203–224. [Google Scholar]
- Dubuc C, Muniz L, Heistermann M, Engelhardt A, Widdig A. Testing the Priority-of Access model in a seasonally breeding primate species (Macaca mulatta) Behav Ecol Sociobiol. 2011;65:1615–1627. doi: 10.1007/s00265-011-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton GG, Resko JA. Plasma testosterone and male dominance in a Japanese macaque (Macaca fuscata) troop compared with repeated measures of testosterone in laboratory males. Horm Behav. 1974;5:251–259. doi: 10.1016/0018-506x(74)90033-6. [DOI] [PubMed] [Google Scholar]
- Ellis L. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol Sociobiol. 1995;16:257–333. [Google Scholar]
- Ellison PT, Bribiescas RG, Bentley GR, Campbell BC, Lipson SF, Panter-Brick C, Hill K. Population variation in age-related decline in male salivary testosterone. Hum Reprod. 2002;17:3251–3253. doi: 10.1093/humrep/17.12.3251. [DOI] [PubMed] [Google Scholar]
- Engelhardt A, Heistermann M, Hodges JK, Nürnberg P, Niemitz C. Determinants of male reproductive success in wild long-tailed macaques (Macaca fascicularis) – male monopolisation, female mate choice or post-copulatory mechanisms? Behav Ecol Sociobiol. 2006;59:740–52. [Google Scholar]
- Fichtel C, Kraus C, Ganswindt A, Heistermann M. Influence of reproductive season and rank on fecal glucocorticoid levels in free-ranging male Verreaux's sifakas (Propithecus verreauxi) Horm Behav. 2007;51:640–648. doi: 10.1016/j.yhbeh.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Ganswindt A, Palme R, Heistermann M, Borragan S, Hodges JK. Non-invasive assessment of adrenocortical function in the male African elephant (Loxodonta africana) and its relation to musth. Gen Comp Endocrinol. 2003;134:156–166. doi: 10.1016/s0016-6480(03)00251-x. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm Behav. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Girard-Buttoz C, Heistermann M, Krummel S, Engelhardt A. Seasonal and social influences on fecal androgen and glucocorticoid excretion in wild male long-tailed macaques (Macaca fascicularis) Physiol Behav. 2009;98:168–175. doi: 10.1016/j.physbeh.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Gordon TP, Rose RM, Bernstein IS. Seasonal rhythm in plasma testosterone levels in the rhesus monkey (Macaca mulatta): A three-year study. Horm Behav. 1976;7:229–243. doi: 10.1016/0018-506x(76)90050-7. [DOI] [PubMed] [Google Scholar]
- Gordon TP, Bernstein IS, Rose RM. Social and seasonal influences on testosterone secretion in the male rhesus monkey. Physiol Behav. 1978;21:623–627. doi: 10.1016/0031-9384(78)90140-3. [DOI] [PubMed] [Google Scholar]
- Goymann W, Wingfield JC. Allostatic load, social status and stress hormones: the costs of social status matter. Anim Behav. 2004;67:591–602. [Google Scholar]
- Guibert F, Richard-Yris M-A, Lumineau S, Kotrschal K, Guémené D, Bertin A, Möstl E, Houdelier C. Social instability in laying quail: consequences on yolk steroids and offspring's phenotype. PLoS ONE. 2010;5:e14069. doi: 10.1371/journal.pone.0014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin HJ, Lowers RH, Guillette LJ. Seasonal androgen cycles in adult male American alligators (Alligator mississippiensis) from a barrier island population. Biol Reprod. 2011;85:1108–1113. doi: 10.1095/biolreprod.111.092692. [DOI] [PubMed] [Google Scholar]
- Heistermann M, Ademmer C, Kaumanns W. Ovarian cycle and effect of social changes on adrenal and ovarian function in Pygathrix nemaeus . Int J Primatol. 2004;25:689–708. [Google Scholar]
- Heistermann M, Finke M, Hodges JK. Assessment of female reproductive status in captive-housed Hanuman langurs (Presbytis entellus) by measurement of urinary and fecal steroid excretion patterns. Am J Primatol. 1995;37:275–284. doi: 10.1002/ajp.1350370402. [DOI] [PubMed] [Google Scholar]
- Heistermann M, Palme R, Ganswindt A. Comparison of different enzymeimmunoassays for assessment of adrenocortical activity in primates based on fecal analysis. Am J Primatol. 2006;68:257–273. doi: 10.1002/ajp.20222. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Bein ML, Nordmeyer DL, Turner JJ. Seasonal testicular function in male rhesus monkeys. Horm Behav. 1996;30:266–271. doi: 10.1006/hbeh.1996.0032. [DOI] [PubMed] [Google Scholar]
- Higham JP, Hughes KD, Brent LJN, Dubuc C, Engelhardt A, Heistermann M, Maestripieri D, Santos LR, Stevens M. Familiarity affects assessment of facial signals of female fertility by free-ranging male rhesus macaques. Proc R Soc Lond B. 2011;278:3452–3458. doi: 10.1098/rspb.2011.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, MacLarnon AM, Ross C, Heistermann M, Semple S. Baboon sexual swellings: Information content of size and color. Horm Behav. 2008;53:452–462. doi: 10.1016/j.yhbeh.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Higham JP, Maestripieri D. Revolutionary coalitions in male rhesus macaques. Behaviour. 2010;147:1889–1908. [Google Scholar]
- Higham JP, Semple S, MacLarnon A, Heistermann M, Ross C. Female reproductive signaling, and male mating behavior, in the olive baboon. Horm Behav. 2009;55:60–67. doi: 10.1016/j.yhbeh.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Oliveira RF. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim Behav. 2006;71:265–277. [Google Scholar]
- Hirschenhauser K, Winkler H, Oliveira RF. Comparative analyses of male androgen responsiveness to social environment in birds: the effects of mating system and paternal incubation. Horm Behav. 2003;43:508–519. doi: 10.1016/s0018-506x(03)00027-8. [DOI] [PubMed] [Google Scholar]
- Hodges JK, Heistermann M. Field Endocrinology: monitoring hormonal changes in free-ranging primates. In: Setchell J, Curtis D, editors. Field and Laboratory methods in primatology. 2nd edn. Cambridge: Cambridge University Press; 2011. pp. 282–294. [Google Scholar]
- Hoffman CL, Higham JP, Heistermann M, Prendergast B, Coe C, Maestripieri D. Immune function and HPA axis activity in free-ranging rhesus macaques. Physiol Behav. 2011;104:507–514. doi: 10.1016/j.physbeh.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CL, Ruiz-Lambides AV, Davila E, Maldonado E, Gerald MS, Maestripieri D. Sex differences in survival costs of reproduction in a promiscuous primate. Behav Ecol Sociobiol. 2008;62:1711–1718. doi: 10.1007/s00265-008-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus C, Heistermann M, Kappeler PM. Physiological suppression of sexual function of subordinate males: a subtle form of intrasexual competition among male sifakas (Propithecus verreauxii)? Physiol Behav. 1999;66:855–861. doi: 10.1016/s0031-9384(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Kuemmerli R, Martin RD. Male and female reproductive success in Barbary macaques (Macaca sylvanus) in Gibraltar: No evidence for rank dependence. Int J Primatol. 2005;26:1229–1249. [Google Scholar]
- Landys MM, Goymann W, Scwabl I, Trapschuh M, Slagsvold T. Impact of seasonal and social challenge on testosterone and corticosterone levels in a year-round territorial bird. Horm Behav. 2010;58:317–325. doi: 10.1016/j.yhbeh.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Ziegler TE, Strier KB. Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus apella nigritus . Horm Behav. 2002;41:275–287. doi: 10.1006/hbeh.2002.1772. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Macachiavellian intelligence: how rhesus macaques and humans have conquered the world. Chicago: The University of Chicago Press; 2007. [Google Scholar]
- Mann DR, Akinbami MA, Gould KG, Wallen K. Sexual maturation in male rhesus monkeys: importance of neonatal testosterone exposure and social rank. J Endocrinol. 1998;156:493–501. doi: 10.1677/joe.0.1560493. [DOI] [PubMed] [Google Scholar]
- Manson JH. Do Female Rhesus Macaques Choose Novel Males? Am J Primatol. 1995;37:285–296. doi: 10.1002/ajp.1350370403. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Fernald BJ, Sallee FR, Suomi SJ, Linnoila M. CSF 5-HIAA, testosterone, and sociosexual behaviors in free-ranging male rhesus macaques in the mating season. Psychiat Res. 1997;72:89–102. doi: 10.1016/s0165-1781(97)00084-x. [DOI] [PubMed] [Google Scholar]
- Muller MN, Wrangham RW. Dominance, aggression and testosterone in wild chimpanzees: a test of the ‘challenge hypothesis’. Anim Behav. 2004;67:113–123. [Google Scholar]
- Ostner J, Heistermann M, Schülke O. Male competition and its hormonal correlates in wild Assamese macaques (Macaca assamensis) Horm Behav. 2011;59:105–113. doi: 10.1016/j.yhbeh.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Ostner J, Nunn CL, Schülke O. Female reproductive synchrony predicts skewed paternity across primates. Behav Ecol. 2008;19:1150–1158. doi: 10.1093/beheco/arn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme R, Möstl E. Biotin-straptavidin enzyme immunoassay for the determination of oestrogens and androgens in boar faeces. In: Görög S, editor. Advances in Steroid Analysis. Budapest: Akadémiai Kiadó; 1994. pp. 11–117. [Google Scholar]
- Paul A, Kuester J, Timme A, Arnemann J. The association between rank, mating effort, and reproductive success in male Barbary macaques (Macaca sylvanus) Primates. 1993;34:491–502. [Google Scholar]
- Plant TM, Zumpe D, Sauls M, Michael RP. An annual rhythm in the plasma testosterone of adult male rhesus monkeys maintained in the laboratory. J Endocrinol. 1974;62:403–404. doi: 10.1677/joe.0.0620403. [DOI] [PubMed] [Google Scholar]
- Plavcan JM. Sexual dimorphism in primate evolution. Am J Phys Anthropol. 2001;116:25–53. doi: 10.1002/ajpa.10011.abs. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Scheffler G, Eisele SG, Goy RW. Effects of age and season on sexual behavior and plasma testosterone and dihdrotestosterone concentrations of laboratory-housed male rhesus monkeys (Macaca mulatta) Biol Reprod. 1975;13:203–210. doi: 10.1095/biolreprod13.2.203. [DOI] [PubMed] [Google Scholar]
- Rodriquez-Llanes JM, Verbeke G, Finlayson C. Reproductive benefits of high social status in male macaques (Macaca) Anim Behav. 2009;78:643–649. [Google Scholar]
- Rose RM, Holaday JW, Bernstein IS. Plasma testosterone, dominance rank and aggressive behaviour in male rhesus monkeys. Nature. 1971;231:366–368. doi: 10.1038/231366a0. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Endocrine aspects of social instability in the olive baboon (Papio anubis) Am J Primatol. 1983;5:365–379. doi: 10.1002/ajp.1350050406. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992;17:701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticosteroids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schülke O, Ostner J. Male reproductive skew, paternal relatedness and female social relationships. Am J Primatol. 2008;70:1–4. doi: 10.1002/ajp.20546. [DOI] [PubMed] [Google Scholar]
- Schülke O, Ostner J. Mitani J, Call J, Kappeler P, Palombit R, Silk J, editors. Ecological and social influences on sociality. The evolution of primate societies. 2012 In press. [Google Scholar]
- Schradin C. Seasonal changes in testosterone and corticosterone levels in four social categories of a desert dwelling sociable rodent. Horm Behav. 2008;53:573–579. doi: 10.1016/j.yhbeh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Charpentier M, Wickings EJ. Mate-guarding and paternity in mandrills (Mandrillus sphinx): factors influencing monopolisation of females by the alpha male. Anim Behav. 2005;70:1105–1120. [Google Scholar]
- Setchell JM, Smith T, Wickings EJ, Knapp LA. Stress, social behaviour and secondary sexual traits in a male primate. Horm Behav. 2010;58:720–728. doi: 10.1016/j.yhbeh.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Shutt K, MacLarnon A, Heistermann M, Semple S. Grooming in Barbary macaques: better to give than receive? Biol Lett. 2007;3:231–233. doi: 10.1098/rsbl.2007.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry B, Iwaniuk AN, Pellis SM. The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithecidae, genus Macaca) Ethology. 2000;106:713–728. [Google Scholar]
- van Noordwijk MA, van Schaik CP. Sexual selection and the careers of primate males: paternity concentration, dominance-acquisition tactics and transfer decisions. In: Kappeler P, van Schaik CP, editors. Sexual selection in primates. Cambridge: Cambridge University Press; 2004. pp. 208–229. [Google Scholar]
- van Meter PE, French JA, Dloniak SM, Watts HE, Kolowski JM, Holekamp KE. Fecal glucocorticoids reflect socio-ecological and anthropogenic stressors in the lives of wild spotted hyenas. Horm Behav. 2009;55:329–337. doi: 10.1016/j.yhbeh.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingrill T, Willems EP, Zimmermann N, Steinmetz H, Heistermann M. Species-specific patterns in fecal glucocorticoid and androgen levels in zoo-living orangutans (Pongo spp.) Gen Comp Endocrinol. 2011;172:446–457. doi: 10.1016/j.ygcen.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Widdig A, Bercovitch FB, Streich WJ, Nürnberg P, Krawczak M. A longitudinal analysis of reproductive skew in male rhesus macaques. Proc R Soc Lond B. 2004;271:819–826. doi: 10.1098/rspb.2003.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The “Challenge Hypothesis”: theoretical implications for patterns of testosterone excretion, mating systems and breeding strategies. Am Nat. 1990;136:829–846. [Google Scholar]
- Wynne-Edwards KE. Hormonal changes in mammalian fathers. Horm Behav. 2001;40:139–145. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]