Abstract
A capability that is key to increasing the performance of paper microfluidic devices is control of fluid transport in the devices. We present dissolvable bridges as a novel method of manipulating fluid volumes within paper-based devices. We demonstrate and characterize the operation of the bridges, including tunability of the volumes passed from 10 to 80 μL using parameters such as geometry and composition. We further demonstrate the utility of dissolvable bridges in the important context of automated delivery of different volumes of a fluid from a common source to multiple locations in a device for simple device loading and activation.
Introduction
There is a need for assays that are appropriate for use in resource-limited settings in multiple application areas, including human disease diagnosis, veterinary medicine, food safety, and environmental monitoring. These assays should have characteristics that make them usable by and useful for the end-users in those settings. In the context of diagnostics, this translates to assays that are ASSURED; affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free, and deliverable to the user1. Though lateral flow tests, the standard bioassy format in low-resource settings, fulfill many of the ASSURED criteria, they have been criticized for both their inability to multiplex (i.e., assay for multiple analytes from a single biosample) and their inability to perform multi-step sample processing with a resultant poor sensitivity for many analytes of clinical importance2.
In 2008, the Whitesides group introduced the use of microfluidic paper-based analytical devices (μPADS), two and three-dimensional paper-based structures that enable colorimetric assays (e.g. for detection of glucose and protein) with multiplexing capability3. Additional work in the area of paper-based assay development has included demonstrating alternate fabrication methods4, implementing multiplexed assays for the detection of additional biomarkers using one-step colorimetric reactions (e.g. nitrite, uric acid, and lactate)5, and performing the simultaneous analysis of multiple controls for on-device calibration6.
We have recently demonstrated two-dimensional paper networks (2DPNs) for autonomous multi-step sample processing. A key feature of the 2DPN assay is the configuration of the network, composed of multiple inlets per detection region, which functions as a program for the timed delivery of multiple fluid volumes within the network. 2DPNs that perform the processes of signal amplification7-9, sample dilution and mixing10, and small molecule extraction10, have been demonstrated. Critical to the operation of multi-step paper-based assays is a set of paper fluidic tools, i.e. analogs to the pump controls and valves of conventional microfluidics, to manipulate fluids within the network for precise timing of reagent delivery and metering of reagent volumes. We have previously demonstrated the utility of using geometry to control the flow rate of fluids in channels11,12, dissolvable barriers as programmable on-switches to flow11,13, and tools for metering fluid volumes within networks, e.g. based on pads of varying fluid capacity9. Additional useful tools for controlling flow include methods that rely on the modification of the wetting properties of the paper channel14 and simple user-activated mechanical on-switches15.
In the current report, we demonstrate novel dissolvable bridges as off-switches for the manipulation of independent fluid volumes in paper networks. We demonstrate their operation and capability to deliver a range of volumes, from 10 μL to 80 μL using several tuning parameters including geometry and composition of materials in the bridge circuit. We then demonstrate the utility of dissolvable bridges in the context of simple device loading and activation. Specifically, we demonstrate that the dissolvable bridges can be used to automatically meter different volumes of the same fluid from a common source for delivery to different pathways in an assay, e.g. user-added water for rehydration of multiple on-card dry reagents. Finally, we discuss the general utility of this valving method in paper-based devices for limited-resource settings.
Experimental
The “paper” (i.e. we use this term broadly to encompass all porous materials traditionally used in the lateral flow industry) circuits containing dissolvable bridges were composed of a glass fiber source pad (Ahlstrom, Helsinki, Finland), a glass fiber, polyester (Ahlstrom, Helsinki, Finland), or nitrocellulose (Millipore, Billerica, MA) piece as the “feeder” material, a sugar bridge (trehalose or mannose, Sigma-Aldrich, St. Louis, MO), a nitrocellulose piece for the delivery material, and Mylar™ (Fraylock, San Carlos, CA) as the housing. (See Table S1 in Supporting Information for more information on material properties including thickness, porosity and water capacity, provided by the manufacturers.) All components were cut to appropriate shapes on a CO2 laser system (Universal Laser Systems, Scottsdale, AZ). A web camera (Logitech, Fremont, CA) and a high-resolution scanner (Epson Perfection V700, Nagano, Japan) were used to acquire image data. A sugar bridge was fabricated by mixing 3 gm of sugar with 500 μL of water to create a granular paste. A four-sided mylar mold was secured over an adhesive section of the card and subsequently filled with the sugar-based paste. Excess paste was scraped off so that the top of the bridge was level with the top of the walls of the mold. The mold was removed, and the bridge was allowed to dry in a desiccator. For the experiments of Figures 3 and 5, the bridges were dried overnight, while for the experiment of Figure 4, the bridges were dried for 4 hours. The effect of bridge drying time on bridge function was investigated and the results are presented in Supporting Information. Briefly, the average volume of fluid delivered was smaller in the case of the one hour bridge drying time (28 μL) vs. the overnight bridge drying time (32 μL), and the reproducibility of the two cases are comparable. Either food coloring or fetal bovine serum, the latter with or without the recombinant malaria protein PfHRP2, was used as a sample fluid. Details of the reagent list for the malaria assay system have been published previously. Please see Supporting Information for details of the protocol used in this study9. All measurements were completed at a nominal laboratory operating temperature of 20 °C.
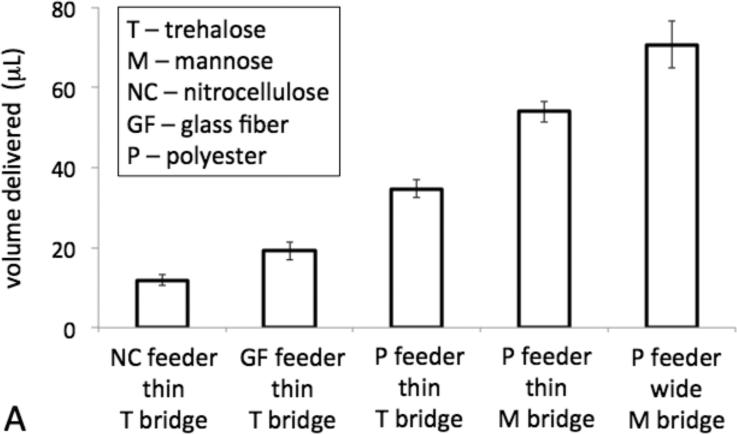
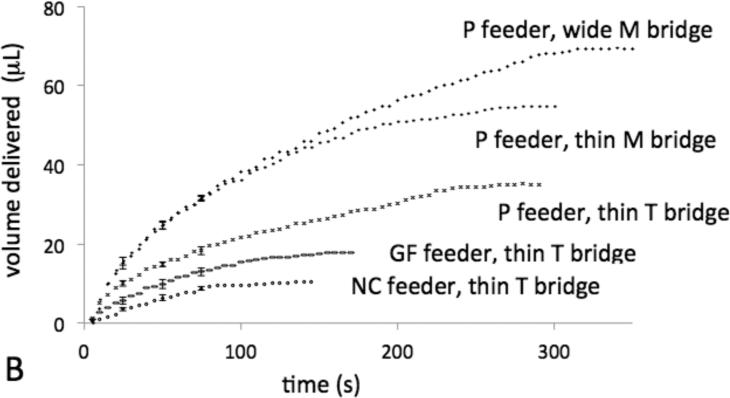
Figure 3.
Dissolvable bridges can be tuned to pass a well-defined volume of fluid. A) The bar chart displays the volume delivered for each type of dissolvable bridge circuit. The error bars represent the standard deviation of N=3 or 4 replicate measurements. “Wide” bridges are 6 mm × 7.5 mm × 0.6 mm and “thin” bridges are 3 mm × 7.5 mm × 0.6 mm. B) The plot displays the volume delivered vs. time for a representative of each type of circuit. Each error bar represents the standard deviation for N=4 replicate measurements at the given time point.
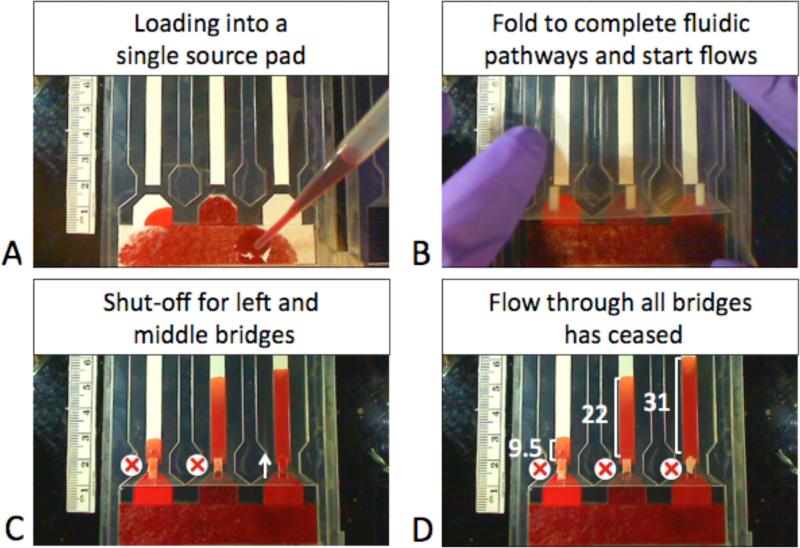
Figure 4.
Simple reagent loading enabled by dissolvable trehalose bridges. A) User adds excess colored fluid to the glass fiber source pad. The single source pad is connected to 3 different feeder materials – nitrocellulose (left), glass fiber (middle), and treated polyester (right). B) User then folds the device, simultaneously connecting each feeder type to its nitrocellulose outlet with sugar bridges. C) The sugar bridges attached to the nitrocellulose (left) and glass fiber (middle) feeder bridges have dissolved enough to halt flow, but the sugar bridge attached to the polyester feeder (right) still conducts fluid. D) Image showing final volumes transfered in μL.
Results and Discussion
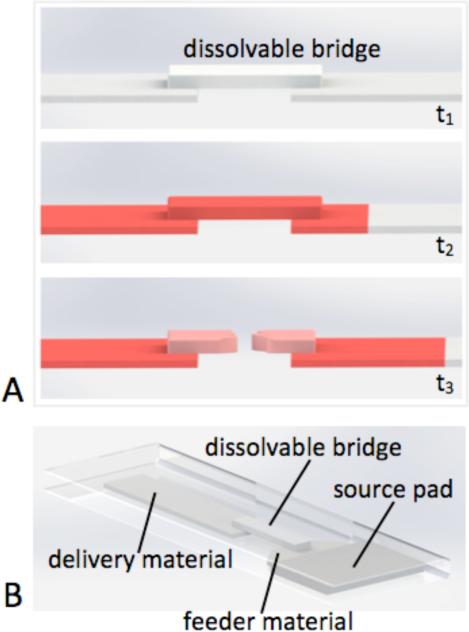
The operation of a dissolvable bridge is shown schematically in Figure 1.
Figure 1.
Operation of a dissolvable bridge. A) The bridge material dissolves to a permanent shut-off state after passing a well-defined volume of fluid from the feeder material to the delivery material. B) Folding card format used to implement a dissolvable bridge network.
The bridge was composed of a material that dissolved to a permanent shut-off state after passing a precise volume of fluid from the feeder material to the delivery material. The total volume of fluid passed by a given dissolvable bridge could be tuned using several experimental parameters, including cross-sectional area of the bridge, composition of the bridge, and the choice of the feeder material.
For the demonstrations here, we used sugars as the dissolvable bridge material. Sugars have been routinely used for the preservation of the dried gold label conjugated to antibody in conventional lateral flow assays. The compatibility of higher concentrations of trehalose solutions with the generation of a downstream gold particle signal was investigated using a lateral flow assay for the malaria protein PfHRP2. Trehalose solutions were used as the diluent for recombinant protein to create mock samples of 20 ng/mL PfHRP2 and up to 30% by weight trehalose (N=4 for each trehalose concentration). The signal intensity in the detection region was lower for higher trehalose concentrations at 25 minutes, e.g. the signal from 20 ng/mL of protein in a 30% trehalose solution was ~60% of the signal from the control of 20 ng/mL of protein in a 0% trehalose solution. However, by 45 minutes, the signal intensity was comparable for all trehalose concentrations. (Please see Supporting Information.) These preliminary results indicate that the main effect of the trehalose is to slow down the timescale of signal generation at higher trehalose concentrations, and that a comparable signal to the control with 0% trehalose can be achieved by increasing the time to result.
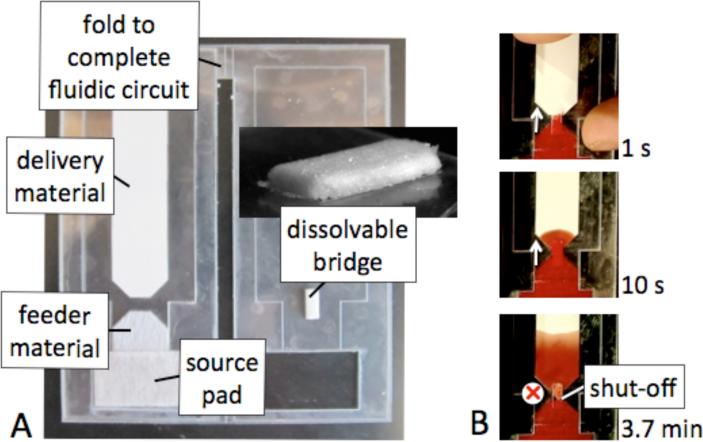
Images of a dissolvable bridge (before use) in a simple fluidic circuit used to characterize volume metering are shown in Figure 2A. Fluid is loaded into the source pad and the card is closed bringing the dissolvable bridge into contact with the upstream feeder material and the downstream delivery material such that the fluidic circuit is complete. Within the circuit, fluid flows by capillarity from the source pad (glass fiber) to the feeder material (polyester, glass fiber, or nitrocellulose), to the dissolvable bridge (trehalose or mannose), and finally to the delivery material (nitrocellulose). The image series in Figure 2B shows the operation of the dissolvable bridge as it first passes fluid until the bridge has dissolved to a shut-off state.
Figure 2.
A fluidic circuit containing a dissolvable bridge. A) Image showing the top view of a fluidic circuit with a dissolvable bridge. The circuit was completed by folding the card closed. B) Sequence of images of the circuit while fluid was being passed by the dissolvable bridge (1s and 10 s) and after the bridge had shut-off (3.7 min).
The approximate volume of fluid passed by the bridge was estimated using the location of the fluid front within the nitrocellulose at the time of bridge shut-off. The area occupied by the fluid parallel to flow, A, is approximately related to the volume delivered, V, at each time point, t, by the relation V (t) ~ PhA(t), where P is the porosity (0.8, specified by the manufacturer for the nitrocellulose used) and h is the height (135 μm). ImageJ (Research Services Branch, NIH) was used to estimate A as a function of time. Note that since the saturation of fluid within the porous matrix is known to be less than 100% and decreases with increasing distance from the source16, the formula overestimates the volume delivered at later times.
The ability to tune the volume of fluid delivered by the bridge before shut-off is shown in Figure 3A. The dissolvable bridges can be used to meter a range of volumes, between 10 to 80 μL, with between 5 and 12% coefficient of variation using the various experimental parameters investigated here. The plot of Figure 3B shows volume delivered vs. time for representative bridges. The flow profile of fluid exiting the bridge is affected by the flow-rate of fluid into the bridge from the feeder material, i.e. the choice of the feeder material, and the composition of the bridge, while changing the cross-sectional area of the bridge affects the time of flow along the same flow profile, and thus total volume delivered.
The uility of the dissolvable bridge was demonstrated in the context of a method of user loading fluid for multiple reagents in a single step. Specifically, dissolvable bridges were used to automatically dispense different fluid volumes to multiple pathways for downstream processing in a network from a single user-filled source pad. Previous work demonstrating paper network assays required multiple fluid loading steps8,9. In contrast, two significant advantages of this method are that it requires only a single user loading step and the exact volume of fluid added by the user to the source pad, as long as it is above a minimum, is not critical. Figure 4 shows a series of images demonstrating the application. In this example, the three fluid volumes delivered were 9.5 μL, 22 μL, and 31μL for the three dissolvable bridge circuits. Note that all of these fall within the ranges established for a particular type of bridge circuit (Figure 3).
As proof-of-concept that the dissolvable bridges are compatible with assay measurements, the reproducbility of the volumes delivered by the bridges when using a more demanding sample than colored fluids was investigated. Specifically, the reproducibility of the bridges (feeder material of treated polyester was evaluated using a complex sample matrix of fetal bovine serum. The average volume delivered was 32±2.1 μL (N=3). The average volume of fluid delivered and the coefficient of variation of 7% are both comparable to the colored fluid case for the same type of bridge circuit (see the data of Fig. 3). As further proof-of-concept that the dissolvable bridges are compatible with assay measurements, the bridges were implemented upstream of a sandwich format malaria assay and the reproducibility of a positive signal evaluated. Measurement of the positive signal from 80 ng/mL of recombinant malaria protein spiked into fetal bovine serum produced a background subtracted signal of 7500±720 (N=4). This corresponds to a reasonable coefficient of variation of 10%.
The dissolvable bridges valving method has general utility for the automated metering of reagents within any assay that makes use of porous materials. In addition to the demonstrated application of simple reagent loading, the dissolvable bridges can be used in alternate contexts for controlling fluid volumes in paper networks. Specifically, these turn-off valves can be used further downstream in the paper network to meter volumes of reagents for interactions such as chemical dilution or reaction. Compared to our previous methods of volume metering demonstrated, the current method does not require that the user position the device in a specific orientation for operation17, or that the user perform multiple addition steps7. Though we demonstrated a range of volumes metered from 10 to 80 μL (as a range that is useful for application to our current assays), it would be straightforward to extend this range by implementation of the dissolvable bridge circuit in alternate materials. Future work will include further characterization of the method, e.g. investigation of the effect of operating temperature on bridge function, and incorporation of this valving method into paper network assays as is useful.
Conclusion
Dissolvable bridges can serve as shut-off valves with a tunable range of total fluid volumes passed and flow profiles. The incorporation of these valves in paper-based devices can enable the automated controlled manipulation of multiple fluid volumes for simple user loading with the potential for the creation of higher performance assays suitable for use in limited-resource settings.
Supplementary Material
Acknowledgements
We acknowledge funding support from the Department of Bioengineering at the University of Washington and NIH Grant R01AI096184. In addition, J.H. and T.L. acknowledge funding support through Mary Gates Research Scholarships and T.L. acknowledges support through a Levinson Emerging Scholar Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supporting Information
Three pieces of supporting information are available. First, data on the effect of high concentrations of trehalose (up to 30% by weight) on an assay signal generated from gold particle labels is provided. Second, data on the effect of bridge drying time on the reproducibility of the volume delivered by the bridge is provided. And third, a table of material properties, including thickness, porosity, and water absorption capacity, specified by the manufacturers of the materials is provided. This supporting material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kettler H, White K, Hawkes S. World Health Organization. 2004.
- 2.Posthuma-Trumpie G, Korf J, van Amerongen A. Anal Bioanal Chem. 2009;393:569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- 3.Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Angew Chem Int Edit. 2007;46:1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]; Martinez AW, Phillips ST, Whitesides GM. P Natl A Sci USA. 2008;105:19606–19611. doi: 10.1073/pnas.0810903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Y, Shi WW, Jiang L, Qin JH, Lin BC. Electrophoresis. 2009;30:1497–1500. doi: 10.1002/elps.200800563. [DOI] [PubMed] [Google Scholar]; Fenton EM, Mascarenas MR, Lopez GP, Sibbett SS. ACS App Mat Interf. 2009;1:124–129. doi: 10.1021/am800043z. [DOI] [PubMed] [Google Scholar]; Abe K, Suzuki K, Citterio D. Anal Chem. 2008;80:6928–6934. doi: 10.1021/ac800604v. [DOI] [PubMed] [Google Scholar]; Spicar-Mihalic P, Toley B, Houghtaling J, Liang T, Yager P, Fu E. J Micromech Microeng. 2013;23:067003. (6pp) [Google Scholar]
- 5.Li X, Tian JF, Shen W. Anal Bioanal Chem. 2010;396:495–501. doi: 10.1007/s00216-009-3195-9. [DOI] [PubMed] [Google Scholar]; Dungchai W, Chailapakul O, Henry CS. Anal Chim Acta. 2010;674:227–233. doi: 10.1016/j.aca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Wu WY, Wang W, Zhu JJ. J Chromatogr A. 2010;1217:3896–3899. doi: 10.1016/j.chroma.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Fu E, Kauffman P, Lutz B, Yager P. Sensor Actuat B-Chem. 2010;149:325–328. doi: 10.1016/j.snb.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu E, Liang T, Houghtaling J, Ramachandran S, Ramsey SA, Lutz B, Yager P. Anal Chem. 2011;83:7941–7946. doi: 10.1021/ac201950g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu E, Liang T, Spicar-Mihalic P, Houghtaling J, Ramachandran S, Yager P. Anal Chem. 2012;84:4574–4579. doi: 10.1021/ac300689s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborn JL, Lutz B, Fu E, Kauffman P, Stevens DY, Yager P. Lab Chip. 2010;10:2659–2665. doi: 10.1039/c004821f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu E, Lutz B, Kauffman P, Yager P. Lab Chip. 2010;10:918–920. doi: 10.1039/b919614e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu E, Ramsey SA, Kauffman P, Lutz B, Yager P. Microfluid Nanofluid. 2011;10:29–35. doi: 10.1007/s10404-010-0643-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kauffman P, Fu E, Lutz B, Yager P. Lab Chip. 2010;10:2614–2617. doi: 10.1039/c004766j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz B, Liang T, Fu E, Ramachandran S, Kauffman P, Yager P. Lab Chip. 2013;13:2840–2847. doi: 10.1039/c3lc50178g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noh N, Phillips ST. Anal Chem. 2010;82:4181–4187. doi: 10.1021/ac100431y. [DOI] [PubMed] [Google Scholar]; Chen H, Cogswell J, Anagnostopoulos C, Faghri M. Lab Chip. 2012;12:2909–2913. doi: 10.1039/c2lc20970e. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Tian JF, Shen W. Cellulose. 2010;17:649–659. [Google Scholar]; Martinez AW, Phillips ST, Nie Z, Cheng C-M, Carrilho E, Wiley BJ, Whitesides GM. Lab Chip. 2010;10:2499–2504. doi: 10.1039/c0lc00021c. [DOI] [PubMed] [Google Scholar]
- 16.Williams R. J Colloid Interf Sci. 1981;79:287–288. [Google Scholar]
- 17.Lutz BR, Trinh P, Ball C, Fu E, Yager P. Lab Chip. 2011;11:4274–4278. doi: 10.1039/c1lc20758j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.