Abstract
Background
Previous studies have suggested an association between usual interstitial pneumonia (UIP) and lung cancer (Ca). However, clinical and histological information is not enough to determine such an association, due to the low incidence and short survival time of patients with both conditions.
Methods
We retrospectively reviewed the clinical and histological records of Ca patients with UIP between January 1999 and August 2013 at the Samsung Medical Center, Seoul, Korea. We found 43 patients who had Ca with UIP (UIP-Ca). Previously reported data of eighty-four patients with UIP-only were included as a comparison group.
Results
Smoking is related to poor prognosis in patients with UIP-Ca, and the number of patients with a high smoking index of more than 30 pack-years significantly increased in UIP-Ca patients compared with UIP-only patients. There is no significant prognostic differentiation between UIP-Ca patients and UIP-only patients. Microscopically, UIP-Ca patients showed characteristically heterogeneous histological patterns and degrees of differentiation. There were many foci of squamous metaplasia or dysplasia at the peripheral area of squamous cell carcinomas.
Conclusions
We report 43 cases of UIP-Ca. Our results suggest that smoking is related to cancer occurrence in UIP patients and poor prognosis in UIP-Ca patients.
Keywords: Idiopathic pulmonary fibrosis, Lung neoplasms, Metaplasia, Smoking, Etiology, Survival
Idiopathic interstitial pneumonia is classified into four categories: usual interstitial pneumonia (UIP), desquamative interstitial pneumonia, nonspecific interstitial pneumonia, and acute interstitial pneumonia.1,2 UIP, which is clinically known as idiopathic pulmonary fibrosis (IPF), is notorious for its poor prognosis, with a median survival of 2.8 to 5 years.3,4 Some studies have suggested an association between UIP and lung cancer,4-6 but reliable clinical and histological information regarding lung neoplasms in patients with UIP is scarce due to the rarity of the simultaneous occurrence of these conditions. Herein, we discuss the clinicopathological characteristics and analyze the survival probability of lung cancer patients with UIP.
MATERIALS AND METHODS
Case selection
We collected data from lung cancer patients with UIP between January 1999 and August 2013 from the Samsung Medical Center, Seoul, Korea. A total of 46 patients were detected. These patients showed clinically appropriate operability and resectability, and pneumonectomy, lobectomy, sleeve lobectomy or wedge resection was performed. Three patients with connective tissue disease, including one patient with systemic sclerosis, one patient with CREST syndrome, and one patient with Sjogren syndrome, which are known risk factors for interstitial lung disease, were excluded. The remaining 43 patients had no drug or occupational history. Preoperative computed tomographies were examined. Gross findings, hematoxylin and eosin and immunohistochemical slides of surgical specimens were reviewed by two pathologists. Simultaneous diagnosis of lung cancer and UIP was found in twenty of the 43 patients (47%) (Tables 1, 2). The remainder showed the diagnostic time of precedent UIP and subsequent lung cancer. The cancer-free period (CFP) is defined as the period of UIP before the detection of lung cancer (e.g., a CFP of zero means UIP and lung cancer were diagnosed at the same time).
Table 1.
Clinicohistological information of 43 usual interstitial pneumonia patients with lung cancer

LCNE, large cell neuroendocrine carcinoma.
Table 2.
Clinicohistological information of 43 usual interstitial pneumonia patients with lung cancer

SI, smoking index; py, packyears; PFT, pre-operative pulmonary function test; FEV1, forced expiratory volume at 1 second; FVC, forced vital capacity; CFP, cancer-free period; SP, survival period; M, male; SQ, squamous cell carcinoma; LLL, left lower lobe; S, current smoker; N/A, not available; P, pneumonectomy; E, ex-smoker; LCNE, large cell neuroendocrine carcinoma; RLL, right lower lobe; N, non-smoker; L, lobectomy; LUL, left upper lobe; Ad, adenocarcinoma; RUL, right upper lobe; LCC, large cell carcinoma; LCNE+SQ, combined large cell neuroendocrine carcinoma and squamous cell carcinoma; RML, right middle lobe; W, wedge resection; SCC, small cell carcinoma; NSCC, non-small cell carcinoma; ASQ, adenosquamous carcinoma; Pl, pleomorphic carcinoma; F, female.
aCensored.
Microscopically, all cases showed lung cancers at the background of UIP. The Institutional Review Board approved this study (SMC 2013-08-149-001).
Reference data as comparison group
Clinical data of eighty-four patients with UIP without lung cancer from a previous report was included for comparison (Table 3).7 These 84 patients were diagnosed at our hospital between July 1996 and June 2002. Fifty-seven of the 84 patients were histologically diagnosed by surgical lung biopsy and the remaining twenty-seven patients were identified by the nonhistological diagnostic criteria of the American Thoracic Society.8 Identical exclusion criteria were used for reference group selection.
Table 3.
Clinical information of 84 usual interstitial pneumonia patients as a reference group

N, non-smoker; E, ex-smoker; C, current smoker.
aThe dead:the censored=46:38.
Statistical analysis
The overall survival of patients with lung cancer and UIP and patients in the reference group was compared using the multivariate analysis of the Cox proportional hazard model. Correlation analysis was performed using chi-squared tests and independent t-tests. Values were considered statistically significant at a p-value less than .05. All statistical analyses were performed in SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Of the 43 patients, 41 (95%) were male and 34 (79%) were smokers. The smokers included 14 current and 20 ex-smokers. The mean age was 68 years. Thirty-seven (86%) patients received a lobectomy, and 33 (77%) instances of lung cancer were located in the lower lobe. The lung cancers consisted of 18 squamous cell carcinomas, 17 adenocarcinomas, 2 combined carcinomas, 2 large cell neuroendocrine carcinomas, and a number of other less common neoplasms. The combined carcinomas consisted of squamous cell carcinoma with a large cell neuroendocrine carcinoma, and small cell carcinoma with a non-small cell carcinoma.
Compared to the reference data, older age and male sex were significantly associated with lung cancer in patients with UIP (Table 4). In addition, positive smoking history and more than 30 pack-years of smoking (mean cigarette number per day multiplied by smoking years) were higher in the UIP with lung cancer group than in the UIP-only group (Table 4).
Table 4.
Comparison between the UIP with lung cancer group and the UIP-only group
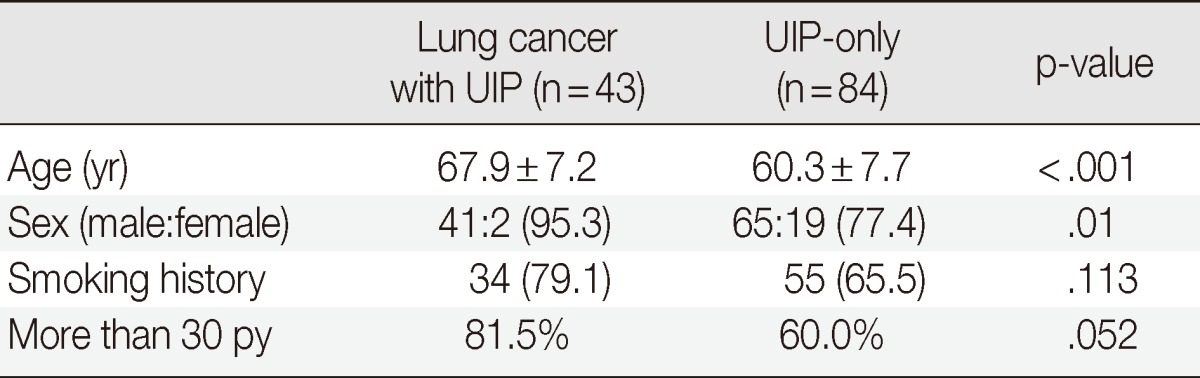
Values are presented as mean±standard deviation or number (%), unless indicated otherwise.
UIP, usual interstitial pneumonia; py, pack years.
Survival analysis results revealed a 1-year survival rate of 60% for patients with lung cancer and UIP. Multivariate analysis of the Cox proportional hazard model was performed with factors of age, stage, surgical procedure, smoking history, smoking index and pulmonary function test before surgery in the 43 UIP with lung cancer patients and in the total 127 patients, including the 84 UIP-only patients (Tables 5, 6). Except for smoking history, all factors showed no significant correlation with survival. In the 43 UIP with lung cancer patients, those with smoking history had an odds ratio of 12.610 (95% confidence interval [CI], 1.293 to 123.016; p=.029) relative to the nonsmoking patients. In the group of all 127 patients, the patients with smoking history had an odds ratio of 1.557 (95% CI, 0.799 to 3.033; p=.193) relative to the nonsmoking patients. In addition, an odds ratio of 0.461 was found between the UIP with cancer and the UIP-only patients.
Table 5.
Multivariate Cox proportional analysis of the 43 usual interstitial pneumonia with lung cancer patients

OR, odds ratio; CI, confidence interval; Smoking, ex-smoker and current smoker; py, pack years; FVC, forced vital capacity; W, wedge resection; L, lobectomy; P, pneumonectomy; CFP, cancer free period.
aMedian number.
Table 6.
Multivariate Cox proportional analysis of the total 127 patients, including the 43 UIP with lung cancer and 84 UIP-only patients
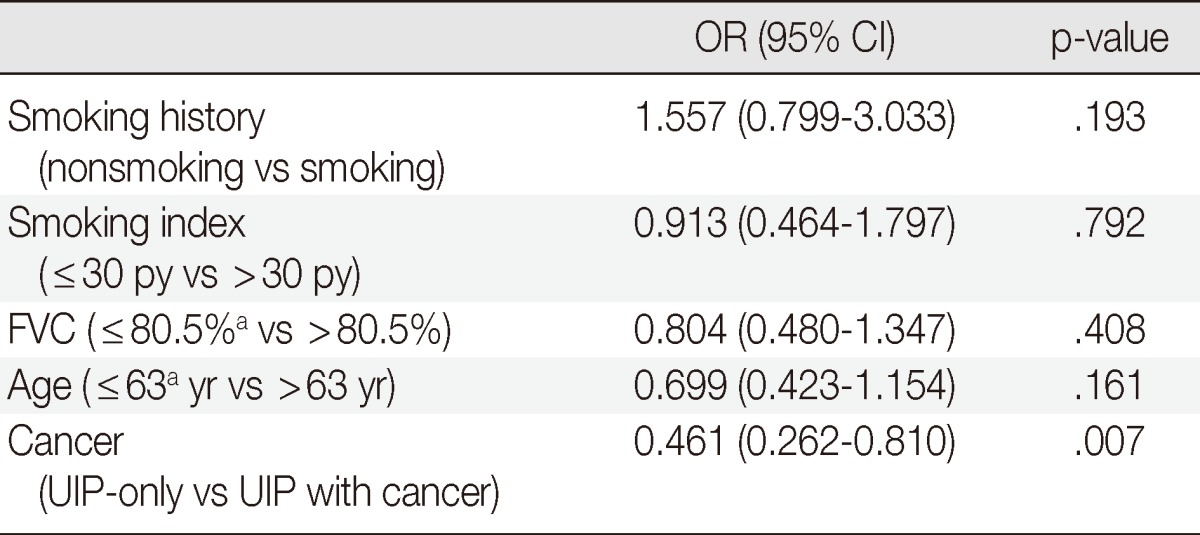
UIP, usual interstitial pneumonia; OR, odds ratio; CI, confidence interval; Smoking, ex-smoker and current smoker; py, pack years; FVC, forced vital capacity.
aMedian number.
The causes of death were examined. Metastasis of lung cancer occurred to the spine in one patient, to the pleura in one patient and to the lung in the other 5 patients. Thirteen patients died due to pulmonary failure with pneumonia. Acute respiratory distress syndrome and acute renal failure occurred in three patients with a survival period of less than 1 month. The remaining 23 patients were censored or had no clinical record.
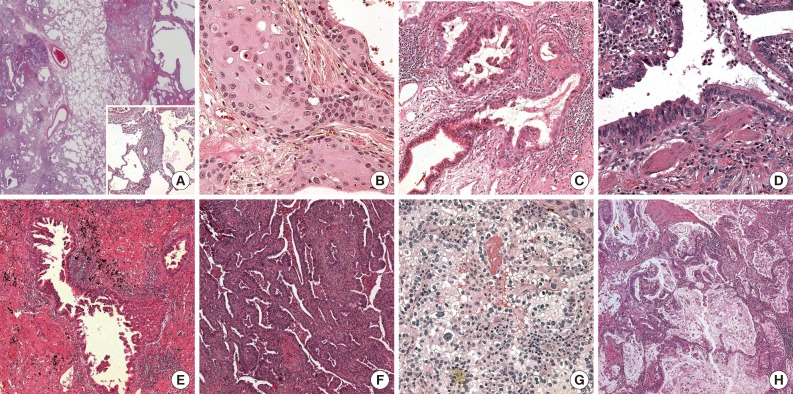
Histopathologically, on gross examination, all tumors were located at the UIP lesion. Almost all tumors were poorly circumscribed and were 0.4 to 7.5 cm in size. The UIP lesion was found to be firm and poorly circumscribed in appearance with a honeycombing pattern. Microscopically, UIP was detected in the peritumoral area in all cases. The UIP showed nonuniform interstitial fibrosis with a patchwork pattern, fibroblastic foci, mild inflammation and honeycomb changes (Figs. 1, 2A). Several areas with hyperplasia of bronchial smooth muscle were detected. There were frequent areas of brown pigmented macrophages in the air spaces and interstitia.
Fig. 1.
(A) Computed tomography horizontal view reveals a pulmonary mass with usual interstitial pneumonia (UIP). (B) The surgical specimen shows a poorly-circumscribed gray and white soft mass with UIP.
Fig. 2.
(A) On the low-power field, usual interstitial pneumonia (UIP) shows non-uniform interstitial fibrosis with a patchwork pattern, honeycomb changes, fibroblastic foci (inset) and mild inflammation (inset). (B) There are many dysplastic foci of the epithelium in the peripheral portion of this squamous cell carcinoma with UIP. (C) Adenocarcinomas also show many foci of transformation from normal bronchiolar epithelium to dysplastic cells at the periphery of the tumor. (D) Dysplastic cells show more hyperchromatic and larger nuclei than normal reactive bronchiolar epithelial cells. Adenocarcinomas have variable patterns described as micropapillary (E) and cleft-like acinar (F). (G) Most adenocarcinomas contain variable areas of clear cell change. (H) Several tumors show variable area of mucin production.
Squamous cell carcinoma with UIP
Eighteen of the 43 lung cancers were squamous cell carcinomas. Twelve cases were moderately differentiated, 5 cases were poorly differentiated and 1 case was well differentiated. The patient with a well-differentiated squamous cell carcinoma (case no. 43) had a comparatively longer survival period. Microscopically, the structure of UIP was maintained in the tumoral areas, and cancer cell proliferation was observed along the cavernous hall of the honeycombing. The tumors showed variable degrees of differentiation. Wide dense fibrotic stroma was detected between the clusters of the squamous cell carcinomas. Peritumoral UIP lesions occasionally showed squamous metaplasia or dysplastic epithelial cells (Fig. 2). The foci of squamous metaplasia were haphazardly distributed along the cavernous hall of honeycombing.
Adenocarcinoma with UIP
Seventeen of the 43 lung cancers were adenocarcinomas. Microscopically, dense fibrotic stroma and large cavities of honeycombing were discovered in the tumoral areas. The adenocarcinomas also grew along the cavernous halls of honeycombing rather than invading the fibrotic stroma. The growth patterns varied at tumors and were described as acinar, papillary, micropapillary or solid with cavitation.9,10 The acinar pattern with branching was the most frequently observed (Fig. 2F), followed by papillary and micropapillary patterns (Fig. 2E). One case showed a mainly solid pattern. Most of the adenocarcinomas accompanied clear cell changes (Fig. 2G). Clear cells had distinct cell borders that had plump clear to pinkish cytoplasm and round nuclei with smooth and slightly thickened membranes. Several areas of intracellular or extracellular mucin production (Fig. 2H) were detected. In addition, there were many foci of transformation from normal bronchiolar epithelium to adenocarcinomas in the peripheral tumor areas (Fig. 2C, D).
DISCUSSION
UIP is a fatal interstitial fibrosing disease of the lung. In recent studies, a similarity between UIP and lung cancer has been suggested based on epigenetic and genetic abnormalities, pathogenetic sequence, a shared feature of uncontrolled proliferation and abnormalities of specific signaling pathways in UIP.11,12 UIP is also known to increase the risk of lung cancer.6 Although there are only a few reports regarding lung cancer in patients with UIP, they commonly describe a predominance of squamous cell carcinoma, male sex and a positive history of smoking.5 Our study also showed a significantly higher incidence in older or male patients compared to a reference group. UIP patients with lung cancer showed higher rates of smoking history (p=.113) and more than 30 pack-years of smoking (p=.052). According to several previous reports,5,6,13 smoking ought to be related to UIP with lung cancer. Our study also shows such a relationship. We also found many foci of squamous metaplasia at the periphery of squamous cell carcinomas. Considering the well-known relationship between smoking and squamous metaplasia, we suggest smoking as a pathogen of lung cancer in UIP patients. Calabrese et al.14 described the overexpression of squamous cell carcinoma antigens in IPF. They demonstrated that metaplastic epithelial cells play an important role in the tumorigenesis of UIP. There were many foci of squamous metaplasia in honeycombing epithelium in the present study. According to a report of Hironaka and Fukuyama,15 IPF patients with lung cancer showed more frequent foci of squamous metaplasia than IPF patients without lung cancer (p=.002). They suggested that squamous metaplasia may not be a precursor of lung cancer itself, but could cause a susceptibility to developing lung carcinoma on the basis of Ki67 and p53 indexes. As a result, smoking may promote tumorigenesis, and metaplastic and dysplastic foci of the UIP lesion suggest progression to lung cancer. We recommend that the amount of metaplastic or dysplastic foci be mentioned in the pathologic report of lung biopsies, and that UIP patients who have frequently metaplastic foci and more than 30 pack-years of smoking receive close follow-up.
Histologically, the major lung neoplasms identified in patients with UIP were squamous cell carcinoma and adenocarcinoma. These lesions showed a characteristically variable degree of differentiation and histological pattern. These disparate findings may be explained by two broad hypotheses regarding pathogenesis. 1) UIP background: UIP has heterogeneous microscopic features that lead to heterogeneous structural patterns and a variable degree of differentiation. 2) Synchronous dysplastic foci: many metaplastic and dysplastic foci were found in peritumoral UIP lesions. A neoplastic mass may be composed of several synchronous tumors originating from a single UIP lesion. This hypothesis of conjoined synchronous tumors can explain the variable degree of differentiation and structural patterns, as well as the incidence of combined tumors and tumors that are found soon after or at the same time patients are diagnosed with UIP.
In the survival analysis, the odds ratio between the cancer group and the no cancer group was 0.461. This indicates that there is no significant prognostic differentiation between the UIP with lung cancer group and the UIP-only group. Even though this study has a limitation of the previously reported comparison group, such a low value of the odds ratio has implications for clinical prognostic guidelines and Korean data.
In conclusion, we report a rare 43 cases of UIP with lung cancer. Smoking is related to poor prognosis in UIP patients with lung cancer, and the number of patients with high a smoking index of more than 30 pack-years significantly increased in UIP with lung cancer patients compared with UIP-only patients. There is no significant prognostic differentiation between UIP with lung cancer patients and UIP-only patients in multivariate analysis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Katzenstein AL, Myers JL. Nonspecific interstitial pneumonia and the other idiopathic interstitial pneumonias: classification and diagnostic criteria. Am J Surg Pathol. 2000;24:1–3. doi: 10.1097/00000478-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 3.Plones T, Osei-Agyemang T, Elze M, et al. Morbidity and mortality in patients with usual interstitial pneumonia (UIP) pattern undergoing surgery for lung biopsy. Respir Med. 2013;107:629–632. doi: 10.1016/j.rmed.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Poletti V, Ravaglia C, Buccioli M, et al. Idiopathic pulmonary fibrosis: diagnosis and prognostic evaluation. Respiration. 2013;86:5–12. doi: 10.1159/000353580. [DOI] [PubMed] [Google Scholar]
- 5.Archontogeorgis K, Steiropoulos P, Tzouvelekis A, Nena E, Bouros D. Lung cancer and interstitial lung diseases: a systematic review. Pulm Med. 2012;2012:315918. doi: 10.1155/2012/315918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J, Kim DS, Shim TS, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2001;17:1216–1219. doi: 10.1183/09031936.01.99055301. [DOI] [PubMed] [Google Scholar]
- 7.Jeon K, Chung MP, Lee KS, et al. Prognostic factors and causes of death in Korean patients with idiopathic pulmonary fibrosis. Respir Med. 2006;100:451–457. doi: 10.1016/j.rmed.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 9.Ha SY, Roh MS. The new 2011 international Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in resected specimens: clinicopathologic relevance and emerging issues. Korean J Pathol. 2013;47:316–325. doi: 10.4132/KoreanJPathol.2013.47.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. 2011;8:381–385. doi: 10.1513/pats.201107-042ST. [DOI] [PubMed] [Google Scholar]
- 11.Vancheri C, Failla M, Crimi N, Raghu G. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J. 2010;35:496–504. doi: 10.1183/09031936.00077309. [DOI] [PubMed] [Google Scholar]
- 12.Chilosi M, Poletti V, Rossi A. The pathogenesis of COPD and IPF: distinct horns of the same devil? Respir Res. 2012;13:3. doi: 10.1186/1465-9921-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Seneviratne CK, Koss M. Idiopathic pulmonary fibrosis and malignancy. Curr Opin Pulm Med. 2001;7:278–282. doi: 10.1097/00063198-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese F, Lunardi F, Giacometti C, et al. Overexpression of squamous cell carcinoma antigen in idiopathic pulmonary fibrosis: clinicopathological correlations. Thorax. 2008;63:795–802. doi: 10.1136/thx.2007.088583. [DOI] [PubMed] [Google Scholar]
- 15.Hironaka M, Fukayama M. Pulmonary fibrosis and lung carcinoma: a comparative study of metaplastic epithelia in honeycombed areas of usual interstitial pneumonia with or without lung carcinoma. Pathol Int. 1999;49:1060–1066. doi: 10.1046/j.1440-1827.1999.00989.x. [DOI] [PubMed] [Google Scholar]