Abstract
Complementation group C of xeroderma pigmentosum (XP) represents one of the most common forms of this cancer-prone DNA repair syndrome. The primary defect is located in the subpathway of the nucleotide excision repair system, dealing with the removal of lesions from the non-transcribing sequences ('genome-overall' repair). Here we report the purification to homogeneity and subsequent cDNA cloning of a repair complex by in vitro complementation of the XP-C defect in a cell-free repair system containing UV-damaged SV40 minichromosomes. The complex has a high affinity for ssDNA and consists of two tightly associated proteins of 125 and 58 kDa. The 125 kDa subunit is an N-terminally extended version of previously reported XPCC gene product which is thought to represent the human homologue of the Saccharomyces cerevisiae repair gene RAD4. The 58 kDa species turned out to be a human homologue of yeast RAD23. Unexpectedly, a second human counterpart of RAD23 was identified. All RAD23 derivatives share a ubiquitin-like N-terminus. The nature of the XP-C defect implies that the complex exerts a unique function in the genome-overall repair pathway which is important for prevention of skin cancer.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Dubnick M., Kerlavage A. R., Moreno R., Kelley J. M., Utterback T. R., Nagle J. W., Fields C., Venter J. C. Sequence identification of 2,375 human brain genes. Nature. 1992 Feb 13;355(6361):632–634. doi: 10.1038/355632a0. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bailly V., Sommers C. H., Sung P., Prakash L., Prakash S. Specific complex formation between proteins encoded by the yeast DNA repair and recombination genes RAD1 and RAD10. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8273–8277. doi: 10.1073/pnas.89.17.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
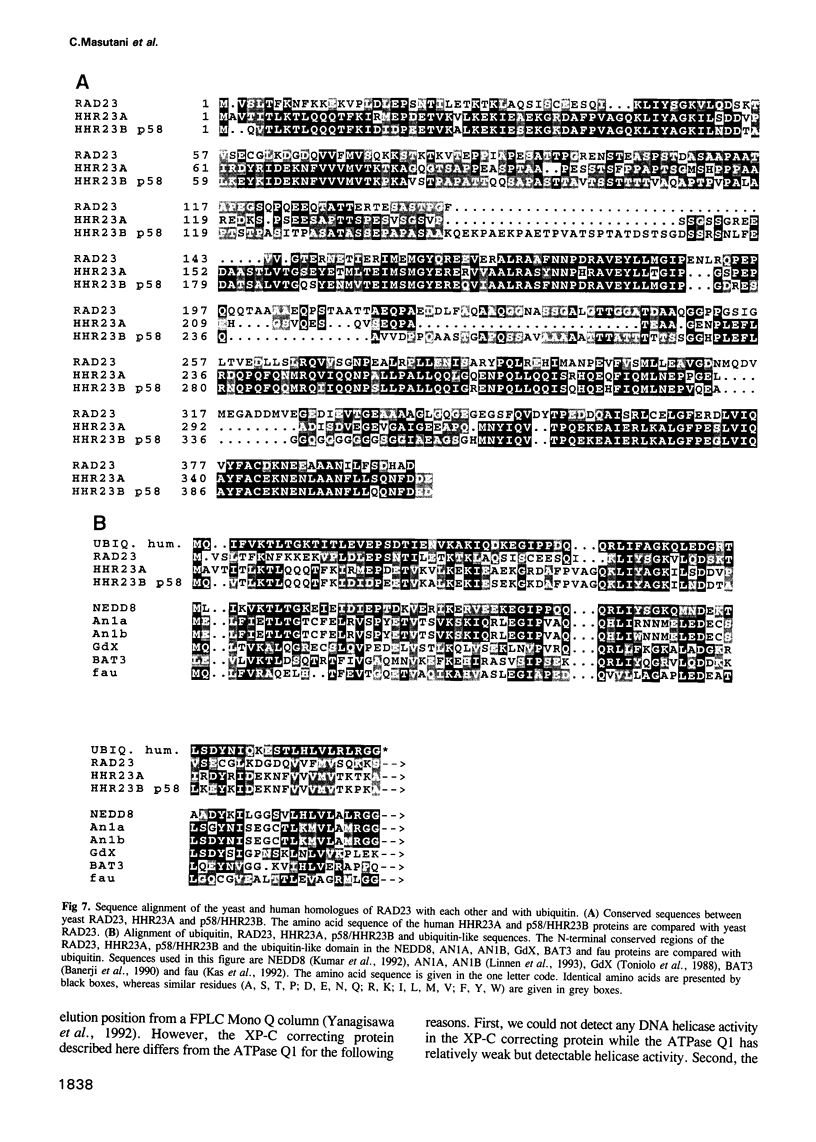
- Banerji J., Sands J., Strominger J. L., Spies T. A gene pair from the human major histocompatibility complex encodes large proline-rich proteins with multiple repeated motifs and a single ubiquitin-like domain. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2374–2378. doi: 10.1073/pnas.87.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell L., Cooper A. J., Friedberg E. C. Stable and specific association between the yeast recombination and DNA repair proteins RAD1 and RAD10 in vitro. Mol Cell Biol. 1992 Jul;12(7):3041–3049. doi: 10.1128/mcb.12.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggerstaff M., Szymkowski D. E., Wood R. D. Co-correction of the ERCC1, ERCC4 and xeroderma pigmentosum group F DNA repair defects in vitro. EMBO J. 1993 Sep;12(9):3685–3692. doi: 10.1002/j.1460-2075.1993.tb06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christiansen G., Landers T., Griffith J., Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977 Mar;21(3):1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Collins A. R. Mutant rodent cell lines sensitive to ultraviolet light, ionizing radiation and cross-linking agents: a comprehensive survey of genetic and biochemical characteristics. Mutat Res. 1993 Jan;293(2):99–118. doi: 10.1016/0921-8777(93)90062-l. [DOI] [PubMed] [Google Scholar]
- Finley D., Bartel B., Varshavsky A. The tails of ubiquitin precursors are ribosomal proteins whose fusion to ubiquitin facilitates ribosome biogenesis. Nature. 1989 Mar 30;338(6214):394–401. doi: 10.1038/338394a0. [DOI] [PubMed] [Google Scholar]
- Fleer R., Nicolet C. M., Pure G. A., Friedberg E. C. RAD4 gene of Saccharomyces cerevisiae: molecular cloning and partial characterization of a gene that is inactivated in Escherichia coli. Mol Cell Biol. 1987 Mar;7(3):1180–1192. doi: 10.1128/mcb.7.3.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flejter W. L., McDaniel L. D., Johns D., Friedberg E. C., Schultz R. A. Correction of xeroderma pigmentosum complementation group D mutant cell phenotypes by chromosome and gene transfer: involvement of the human ERCC2 DNA repair gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):261–265. doi: 10.1073/pnas.89.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Prakash S. Cloning and nucleotide sequence analysis of the Saccharomyces cerevisiae RAD4 gene required for excision repair of UV-damaged DNA. Gene. 1988 Dec 30;74(2):535–541. doi: 10.1016/0378-1119(88)90186-2. [DOI] [PubMed] [Google Scholar]
- Hanawalt P., Mellon I. Stranded in an active gene. Curr Biol. 1993 Jan;3(1):67–69. doi: 10.1016/0960-9822(93)90156-i. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Nucleotide excision repair I: from E. coli to yeast. Trends Genet. 1993 May;9(5):173–177. doi: 10.1016/0168-9525(93)90164-d. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Nucleotide excision repair. II: From yeast to mammals. Trends Genet. 1993 Jun;9(6):211–217. doi: 10.1016/0168-9525(93)90121-w. [DOI] [PubMed] [Google Scholar]
- Huang J. C., Svoboda D. L., Reardon J. T., Sancar A. Human nucleotide excision nuclease removes thymine dimers from DNA by incising the 22nd phosphodiester bond 5' and the 6th phosphodiester bond 3' to the photodimer. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3664–3668. doi: 10.1073/pnas.89.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch S. The ubiquitin-conjugation system. Annu Rev Genet. 1992;26:179–207. doi: 10.1146/annurev.ge.26.120192.001143. [DOI] [PubMed] [Google Scholar]
- Kantor G. J., Barsalou L. S., Hanawalt P. C. Selective repair of specific chromatin domains in UV-irradiated cells from xeroderma pigmentosum complementation group C. Mutat Res. 1990 May;235(3):171–180. doi: 10.1016/0921-8777(90)90071-c. [DOI] [PubMed] [Google Scholar]
- Kas K., Michiels L., Merregaert J. Genomic structure and expression of the human fau gene: encoding the ribosomal protein S30 fused to a ubiquitin-like protein. Biochem Biophys Res Commun. 1992 Sep 16;187(2):927–933. doi: 10.1016/0006-291x(92)91286-y. [DOI] [PubMed] [Google Scholar]
- Koken M. H., Reynolds P., Jaspers-Dekker I., Prakash L., Prakash S., Bootsma D., Hoeijmakers J. H. Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8865–8869. doi: 10.1073/pnas.88.20.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Kraemer K. H., Lee M. M., Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987 Feb;123(2):241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Kumar S., Tomooka Y., Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992 Jun 30;185(3):1155–1161. doi: 10.1016/0006-291x(92)91747-e. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
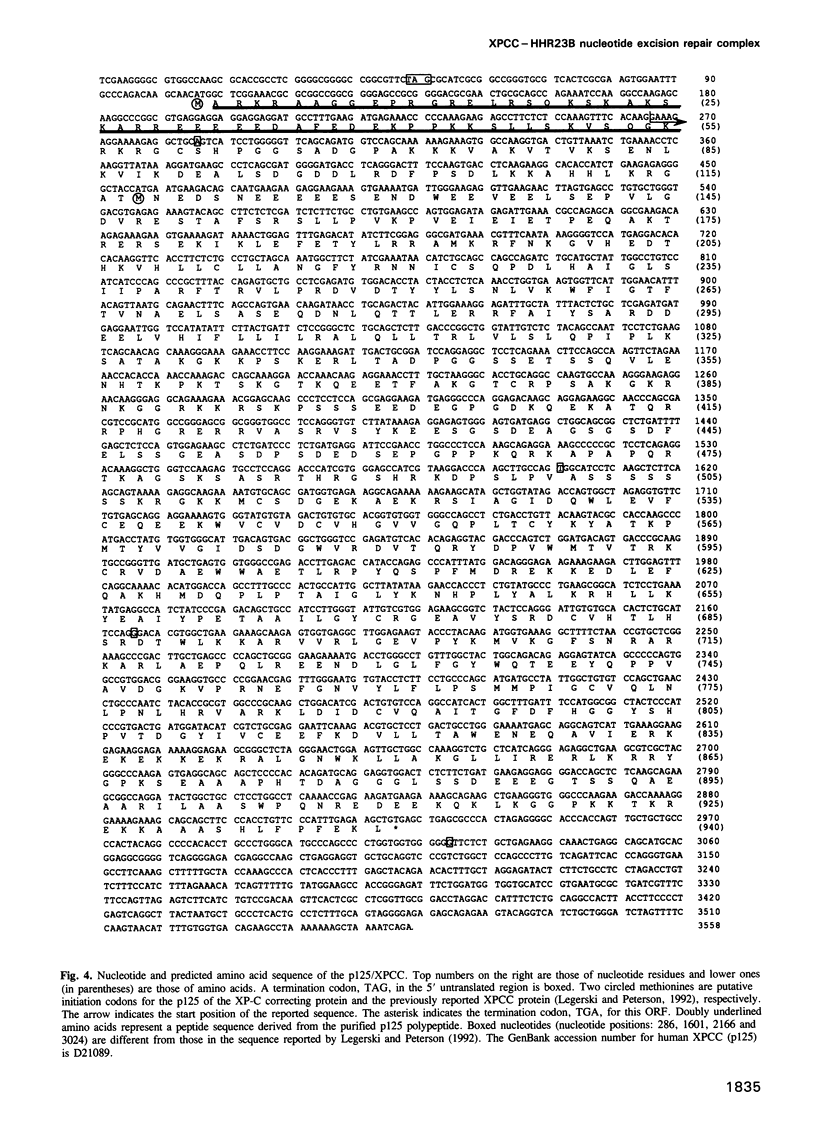
- Legerski R., Peterson C. Expression cloning of a human DNA repair gene involved in xeroderma pigmentosum group C. Nature. 1992 Sep 3;359(6390):70–73. doi: 10.1038/359070a0. [DOI] [PubMed] [Google Scholar]
- Li L., Bales E. S., Peterson C. A., Legerski R. J. Characterization of molecular defects in xeroderma pigmentosum group C. Nat Genet. 1993 Dec;5(4):413–417. doi: 10.1038/ng1293-413. [DOI] [PubMed] [Google Scholar]
- Linnen J. M., Bailey C. P., Weeks D. L. Two related localized mRNAs from Xenopus laevis encode ubiquitin-like fusion proteins. Gene. 1993 Jun 30;128(2):181–188. doi: 10.1016/0378-1119(93)90561-g. [DOI] [PubMed] [Google Scholar]
- Madura K., Prakash S. Transcript levels of the Saccharomyes cerevisiae DNA repair gene RAD23 increase in response to UV light and in meiosis but remain constant in the mitotic cell cycle. Nucleic Acids Res. 1990 Aug 25;18(16):4737–4742. doi: 10.1093/nar/18.16.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Samuels M., Sharp P. A. In vitro transcription: whole-cell extract. Methods Enzymol. 1983;101:568–582. doi: 10.1016/0076-6879(83)01038-1. [DOI] [PubMed] [Google Scholar]
- Masutani C., Sugasawa K., Asahina H., Tanaka K., Hanaoka F. Cell-free repair of UV-damaged simian virus 40 chromosomes in human cell extracts. II. Defective DNA repair synthesis by xeroderma pigmentosum cell extracts. J Biol Chem. 1993 Apr 25;268(12):9105–9109. [PubMed] [Google Scholar]
- Melnick L., Sherman F. The gene clusters ARC and COR on chromosomes 5 and 10, respectively, of Saccharomyces cerevisiae share a common ancestry. J Mol Biol. 1993 Oct 5;233(3):372–388. doi: 10.1006/jmbi.1993.1518. [DOI] [PubMed] [Google Scholar]
- O'Donovan A., Wood R. D. Identical defects in DNA repair in xeroderma pigmentosum group G and rodent ERCC group 5. Nature. 1993 May 13;363(6425):185–188. doi: 10.1038/363185a0. [DOI] [PubMed] [Google Scholar]
- Okubo K., Hori N., Matoba R., Niiyama T., Fukushima A., Kojima Y., Matsubara K. Large scale cDNA sequencing for analysis of quantitative and qualitative aspects of gene expression. Nat Genet. 1992 Nov;2(3):173–179. doi: 10.1038/ng1192-173. [DOI] [PubMed] [Google Scholar]
- Perozzi G., Prakash S. RAD7 gene of Saccharomyces cerevisiae: transcripts, nucleotide sequence analysis, and functional relationship between the RAD7 and RAD23 gene products. Mol Cell Biol. 1986 May;6(5):1497–1507. doi: 10.1128/mcb.6.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboni R., Botta E., Stefanini M., Numata M., Yasui A. Identification of the eleventh complementation group of UV-sensitive excision repair-defective rodent mutants. Cancer Res. 1992 Dec 1;52(23):6690–6691. [PubMed] [Google Scholar]
- Sancar A., Hearst J. E. Molecular matchmakers. Science. 1993 Mar 5;259(5100):1415–1420. doi: 10.1126/science.8451638. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Prakash S. RAD10, an excision repair gene of Saccharomyces cerevisiae, is involved in the RAD1 pathway of mitotic recombination. Mol Cell Biol. 1990 Jun;10(6):2485–2491. doi: 10.1128/mcb.10.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibghatullah, Husain I., Carlton W., Sancar A. Human nucleotide excision repair in vitro: repair of pyrimidine dimers, psoralen and cisplatin adducts by HeLa cell-free extract. Nucleic Acids Res. 1989 Jun 26;17(12):4471–4484. doi: 10.1093/nar/17.12.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa K., Masutani C., Hanaoka F. Cell-free repair of UV-damaged simian virus 40 chromosomes in human cell extracts. I. Development of a cell-free system detecting excision repair of UV-irradiated SV40 chromosomes. J Biol Chem. 1993 Apr 25;268(12):9098–9104. [PubMed] [Google Scholar]
- Suzuki M., Enomoto T., Masutani C., Hanaoka F., Yamada M., Ui M. DNA primase-DNA polymerase alpha assembly from mouse FM3A cells. Purification of constituting enzymes, reconstitution, and analysis of RNA priming as coupled to DNA synthesis. J Biol Chem. 1989 Jun 15;264(17):10065–10071. [PubMed] [Google Scholar]
- Tanaka K., Miura N., Satokata I., Miyamoto I., Yoshida M. C., Satoh Y., Kondo S., Yasui A., Okayama H., Okada Y. Analysis of a human DNA excision repair gene involved in group A xeroderma pigmentosum and containing a zinc-finger domain. Nature. 1990 Nov 1;348(6296):73–76. doi: 10.1038/348073a0. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Persico M., Alcalay M. A "housekeeping" gene on the X chromosome encodes a protein similar to ubiquitin. Proc Natl Acad Sci U S A. 1988 Feb;85(3):851–855. doi: 10.1073/pnas.85.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troelstra C., van Gool A., de Wit J., Vermeulen W., Bootsma D., Hoeijmakers J. H. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell. 1992 Dec 11;71(6):939–953. doi: 10.1016/0092-8674(92)90390-x. [DOI] [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J., van Hoffen A., Karcagi V., Natarajan A. T., van Zeeland A. A., Mullenders L. H. Xeroderma pigmentosum complementation group C cells remove pyrimidine dimers selectively from the transcribed strand of active genes. Mol Cell Biol. 1991 Aug;11(8):4128–4134. doi: 10.1128/mcb.11.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J., van Hoffen A., Natarajan A. T., van Zeeland A. A., Mullenders L. H. The residual repair capacity of xeroderma pigmentosum complementation group C fibroblasts is highly specific for transcriptionally active DNA. Nucleic Acids Res. 1990 Feb 11;18(3):443–448. doi: 10.1093/nar/18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar S., Bugg C. E., Wilkinson K. D., Vierstra R. D., Hatfield P. M., Cook W. J. Comparison of the three-dimensional structures of human, yeast, and oat ubiquitin. J Biol Chem. 1987 May 5;262(13):6396–6399. [PubMed] [Google Scholar]
- Watkins J. F., Sung P., Prakash L., Prakash S. The Saccharomyces cerevisiae DNA repair gene RAD23 encodes a nuclear protein containing a ubiquitin-like domain required for biological function. Mol Cell Biol. 1993 Dec;13(12):7757–7765. doi: 10.1128/mcb.13.12.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988 Apr 8;53(1):97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- Yanagisawa J., Seki M., Ui M., Enomoto T. Alteration of a DNA-dependent ATPase activity in xeroderma pigmentosum complementation group C cells. J Biol Chem. 1992 Feb 25;267(6):3585–3588. [PubMed] [Google Scholar]
- van Duin M., Vredeveldt G., Mayne L. V., Odijk H., Vermeulen W., Klein B., Weeda G., Hoeijmakers J. H., Bootsma D., Westerveld A. The cloned human DNA excision repair gene ERCC-1 fails to correct xeroderma pigmentosum complementation groups A through I. Mutat Res. 1989 Mar;217(2):83–92. doi: 10.1016/0921-8777(89)90059-1. [DOI] [PubMed] [Google Scholar]
- van Vuuren A. J., Appeldoorn E., Odijk H., Yasui A., Jaspers N. G., Bootsma D., Hoeijmakers J. H. Evidence for a repair enzyme complex involving ERCC1 and complementing activities of ERCC4, ERCC11 and xeroderma pigmentosum group F. EMBO J. 1993 Sep;12(9):3693–3701. doi: 10.1002/j.1460-2075.1993.tb06044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]