Abstract
Background
Human papillomavirus (HPV) is an oncogenic virus in cervical cancer and most invasive carcinomas (ICs) are caused by HPV16 and 18. However, the roles and contributions of other uncommon and rare genotypes remain uncertain.
Methods
HPV genotypes were retrospectively assessed using an HPV DNA chip that can specify up to 32 HPV genotypes. We arbitrarily regarded genotypes accounting for less than 6% of the total as uncommon and rare genotypes.
Results
A total of 3,164 HPV-positive cases were enrolled. In groups 2A, 2B, 3, and unclassified HPV genotypes, 2.4% of cases with uncommon HPV genotypes (68, 26, 34, 53, 66, 69, 70, 73, 40, 42, 43, 44, 54, 55, 61, 62, 6, and 11) showed high grade squamous intraepithelial lesions and ICs. There were no HPV32- and 57-infected cases.
Conclusions
We found that the uncommon and rare HPV genotypes may provide incremental etiologic contributions in cervical carcinogenesis, especially HPV68, 70, and 53. Further studies on these uncommon and rare HPV genotypes will be of importance in establishing the significance of genotypes in different regions, especially in planning a strategy for further vaccine development as well as follow-up on the effectiveness of the currently used vaccines.
Keywords: Human papillomavirus, Cervix uteri, Carcinogenesis, Genotype
Uterine cervical cancer is the second most common female malignancy worldwide1 and cervical cancer remains a major health problem in Korea, although the incidence has shown a decreasing trend due to the high quality of the national health project for cervical cancer screening and development of cervical vaccines.2 Regarding uterine cervical carcinogenesis, human papillomavirus (HPV) is one of the most important oncogenic causes in cervical carcinomas.3 HPV is a double-stranded DNA virus infecting basal epithelial cells of cutaneous or mucosal tissues.4 Epidemiologic investigations have clearly established a causal link between HPV infection and development of cervical cancer, and recent use of a sensitive molecular method for detection of HPV DNA revealed HPV infection in more than 80% of squamous intraepithelial lesions.3,5 More than 140 different HPV genotypes have been identified and sequenced, and approximately 40 HPV genotypes are known to act as oncogenic genotypes. HPV16 is by far the most carcinogenic type, followed by HPV18, 31, 33, and 45, which, together with HPV16, account for >90% of HPV-related cancers as a single genotype or one of multiple coinfected genotypes.6,7 However, little experimental work on the carcinogenicity of HPV types has been reported, except for HPV16 and HPV18, and currently, other rare and uncommon genotypes are poorly understood.8-11
In this study, we focused on the correlation of low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), or invasive carcinoma (IC) with rare and uncommon HPV genotypes in order to clarify the importance of these uncommon genotypes associated with uterine cervical dysplasia and carcinogenesis.
MATERIALS AND METHODS
Materials
Materials were collected from the pathology archives of Gachon University Gil Medical Center for the period between 2006 and 2012. During the six-year period, we collected 10,002 smears examined with HPV analysis as well as Papanicolaou staining from female patients in Incheon, Korea. We retrospectively selected HPV-positive patients based on the results of cytologic and histologic examination. A total of 3,164 cases were HPV-positive (31.6%). The authors arbitrarily regarded cases comprising less than 6.0% as uncommon and rare HPV genotypes. The total proportion of rare and uncommon HPV genotypes was 44.3% (1,666/3,758), however, we excluded the uncommon and rare group 1 HPV genotypes. A total of 404 cases were included in this study, comprised of groups 2A, 2B, 3, and unclassified HPV genotypes in cases excluding group 1-coinfection. After addition of each genotype of multiple infections, the total number of cases was 447.
HPV DNA detection
According to the carcinogenic potential, HPV types were classified as high risk, low risk, and probably high risk. Among the HPV genotypes, only 12 are categorized as carcinogens of the uterine cervix by the Working Group of the World Health Organization (WHO) International Agency for Research on Cancer (IARC).12 HPV genotypes are classified as carcinogens (group 1; HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59), probable carcinogens (group 2A; HPV68), possible carcinogens (group 2B, HPV26, 30, 34, 53, 66, 67, 69, 70, 73, 82, 85, and 97), and not classifiable as to its carcinogenicity to humans (group 3; HPV6 and 11).
We used the MyHPV chip kit (MyGene Co., Seoul, Korea) for detection of HPV. The HPV test was performed according to the instructions of the manufacturers. The chip kit was originally able to detect 19 HPV types, and 13 types (HPV26, 32, 53, 55, 57, 59, 61, 62, 66, 68, 69, 70, and 73) were additionally included in May 2010 so the total 32 HPV types in the MyGene Chip kit included group 1 (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59), group 2A (HPV 68), group 2B (HPV26, 34, 53, 66, 69, 70, and 73), and group 3 (HPV6 and 11). Unclassified genotypes (HPV32, 40, 42, 43, 44, 54, 55, 57, 61, and 62) were also detectable. Thirty-two oligonucleotide probes (5' amine, 30-mer sized) specific for each of the 32 HPV subtypes were fixated. After obtaining fixated DNA specimens using a DNA isolation kit (MyGene), the L1 region, which is the target of HPV DNA, was amplified and dyed with indocarbocyanine-dUTP using GP5+/GP6+primer. The control group was β-globin amplified using polymearse chain reaction (PCR). DNA amplified by PCR was detected by electrophoresis using a 2% agarose gel. After amplification of HPV DNA, 10 mL of the product was denatured for 5 minutes at 95℃, and was placed inside the chamber with hybridization solution. The hybridization was performed for 90 minutes at 43℃ and then washed twice using 2× saline-sodium citrate (SSC) with 0.1% sodium dodecyl sulfate for 5 minutes, twice using 0.2× SSC for 5 minutes, and once using 1× SSC for 5 minutes. Finally, the product was dried at normal room temperature. Hybridated HPV DNA was detected using a Chip scanner (GenePix 4100A, Axon Instruments, Sunnyvale, CA, USA). Two spots indicate positivity. In the current study, cases showing HPV-negativity by chip scanner but positivity under electrophoresis, i.e., not specified, were categorized as 'other' after one repeated examination.
Cytologic and histologic results
We included all specimens on which both Papanicolaou cervicovaginal smears and HPV genotyping were performed. The pathologic results of smears, punch biopsy, or conization at the time of HPV genotyping were reviewed. The highest grade of diagnosed pathology was attached to the associated HPV type because of predictable variable errors, including histologic and/or cytologic under- or over-diagnoses. In this study, we analyzed cases showing reactive or inflammatory pathology as within normal limits (WNL).
Statistical analysis
Data analysis was performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). For estimation of the HPV genotype distribution, multiple HPV infections were considered separately. Here, we evaluated genotype-specific HPV prevalence, however, HPV-positive cases with multiple co-infections by group 1 were not counted. A stratified analysis of the relative overall HPV and genotype-specific contribution according to patient age at diagnosis and histological characteristics was performed using Pearson chi-square test and Fisher's exact test. To eliminate the bias of the small sample size, we set the number of each group to over 20 for statistical analysis. Differences were considered significant when the p-value was less than 0.05.
RESULTS
Overall prevalence of rare and uncommon HPV types relating to the cytologic or histologic diagnosis
A total of 447 cases comprised groups 2A, 2B, 3, and unclassified HPV genotypes in cases excluding group 1-coinfection (Table 1). One hundred one cases were LSILs (25.0%), 33 cases were HSILs (8.2%), and 11 cases were ICs (2.7%). Among them, 64.1% showed normal cytologic or histologic diagnosis (259/447).
Table 1.
The pathologic distribution of rare and uncommon HPV types, excluding group 1-coinfection
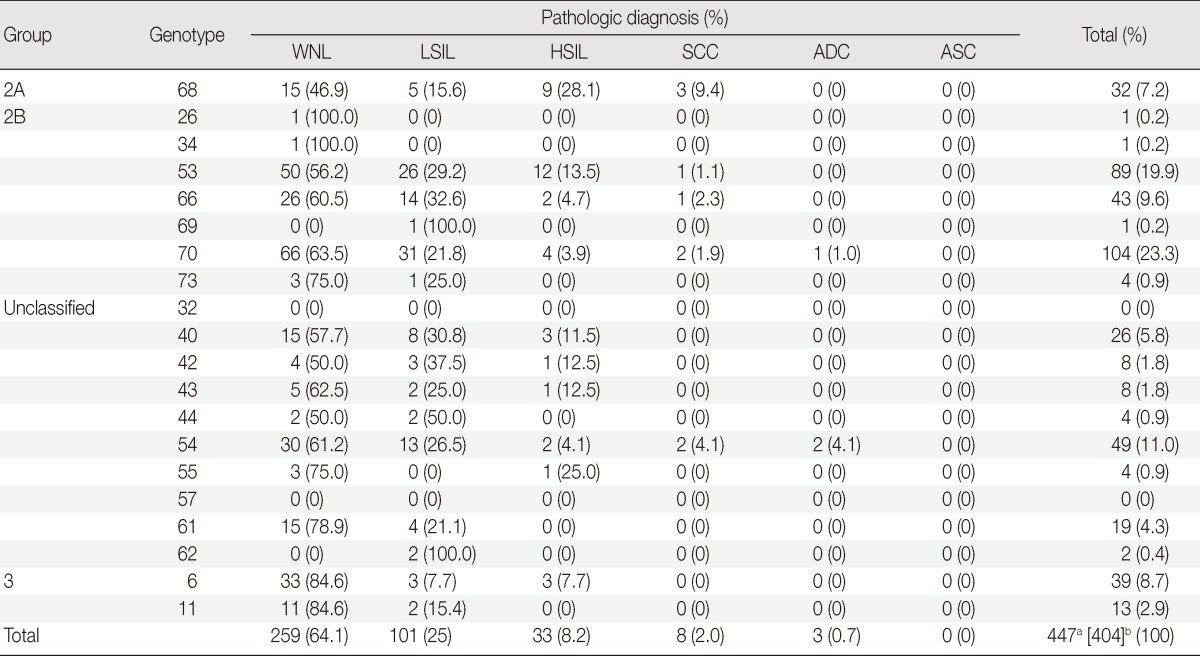
HPV, human papillomavirus; WNL, within normal limit; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous cell carcinoma; ADC, adenocarcinoma; ASC, adenosquamous carcinoma.
aThe total number of extracted HPV genotypes after addition of each genotype of multiple infections; bThe number of examined pathologic specimens.
Comparison of the groups and cytologic or histologic diagnosis
In a comparison of the normal group versus abnormal groups (LSIL, HSIL, and IC) between group 2, unclassified group, and HPV16/HPV6, 11 cases were statistically significant (relative risk [RR], 1.397; p<.001 and RR, 2.616; p<.001, respectively), irrespective of group 1-coinfections. WNL and LSIL versus cases of higher grade than LSIL between group 2, unclassified group, and HPV16 were statistically significant (RR, 3.191; p<.001), while differences were not significant between group 2, unclassified group, and HPV6 and 11 (p=.19) (Table 2). There were three HPV6-infected cases, which were shown to be HSIL. At the time of diagnosis, we re-tested the HPV typing using the same samples and got concordant results. One case involved a 46-year-old female who was diagnosed as having atypical squamous cells, which could not be excluded as being HSIL on Papanicolaou cervicovaginal smears, and moderate dysplasia on punch biopsy. She underwent a hysterectomy, and the diagnosis was severe dysplasia on the cervix. During 14 months of follow-up, there was no evidence of disease recurrence. The other 2 cases were from the same patient, a 32-year-old female. The first time, she was diagnosed as having HSIL by Papanicolaou cervicovaginal smears, and chronic cervicitis on punch biopsy. One month later, the Papanicolaou cervicovaginal smears and HPV typing were repeated. The results of these repeated examinations were that there was HSIL by Papanicolaou cervicovaginal smears and a persistent HPV6 infection. She underwent conization, and the diagnosis was squamous cell carcinoma in situ with clear resection margin. During 3 months of follow-up, the results of Papanicolaou cervicovaginal smears and HPV typing were all negative.
Table 2.
The relationship between pathologic diagnoses and group 2 and unclassified HPV, compared to HPV16, HPV6, and 11 without group 1-coinfection
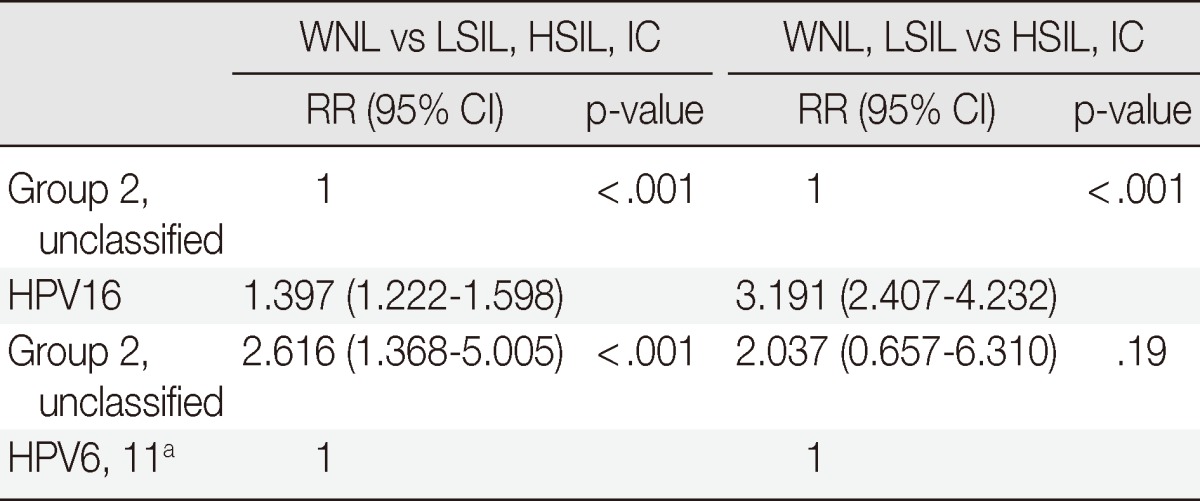
HPV, human papillomavirus; WNL, within normal limit; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; IC, invasive carcinoma; RR, relative risk; CI, confidence interval.
aHPV6, 11 with multiple coinfections by group 1 are not shown in the Table.
Rare and uncommon HPV genotypes compared to HPV6 and 11, excluding group 1-coinfection in abnormal findings
In the groups 2A, 2B, and unclassified, 35.6% (159/447) of uncommon HPV genotypes-HPV68, 26, 34, 53, 66, 69, 70, 73, 32, 40, 42, 43, 44, 54, 55, 57, 61, and 62-infected cases showed LSILs, HSILs, and ICs; 2.7% (12/447) showed an association with ICs, 7.8% (35/447) showed an association with HSILs, and 25.1% (112/447) showed an association with LSILs. LSILs, HSILs, and ICs were detected in cases involving HPV68 (17/32, 53.1%), HPV42 (4/8, 50.0%), HPV53 (39/89, 43.8%), HPV40 (11/26, 42.3%), HPV66 (17/43, 40.0%), HPV54 (19/49, 38.8%), HPV43 (3/8, 37.5%), and HPV70 (38/104, 36.5%).
The rare and uncommon types showing a statistical relationship with LSILs, HSILs, and ICs compared to HPV6 and 11 were as follows: HPV68 (p<.001), HPV53 (p=.001), HPV40 (p=.01), HPV66 (p=.01), HPV54 (p=.01), and HPV70 (p=.01). All of these types were significantly different compared to HPV6 and 11. HPV68 (RR, 3.453) showed the highest the value of relative risk followed by HPV53 (RR, 2.848), HPV40 (RR, 2.750), HPV66 (RR, 2.570), HPV54 (RR, 2.520), and HPV70 (RR, 2.375) (Table 3).
Table 3.
The relationship of abnormal findings in the evaluation of rare and uncommon HPV genotypes compared to HPV6 and 11 without group 1-coinfection
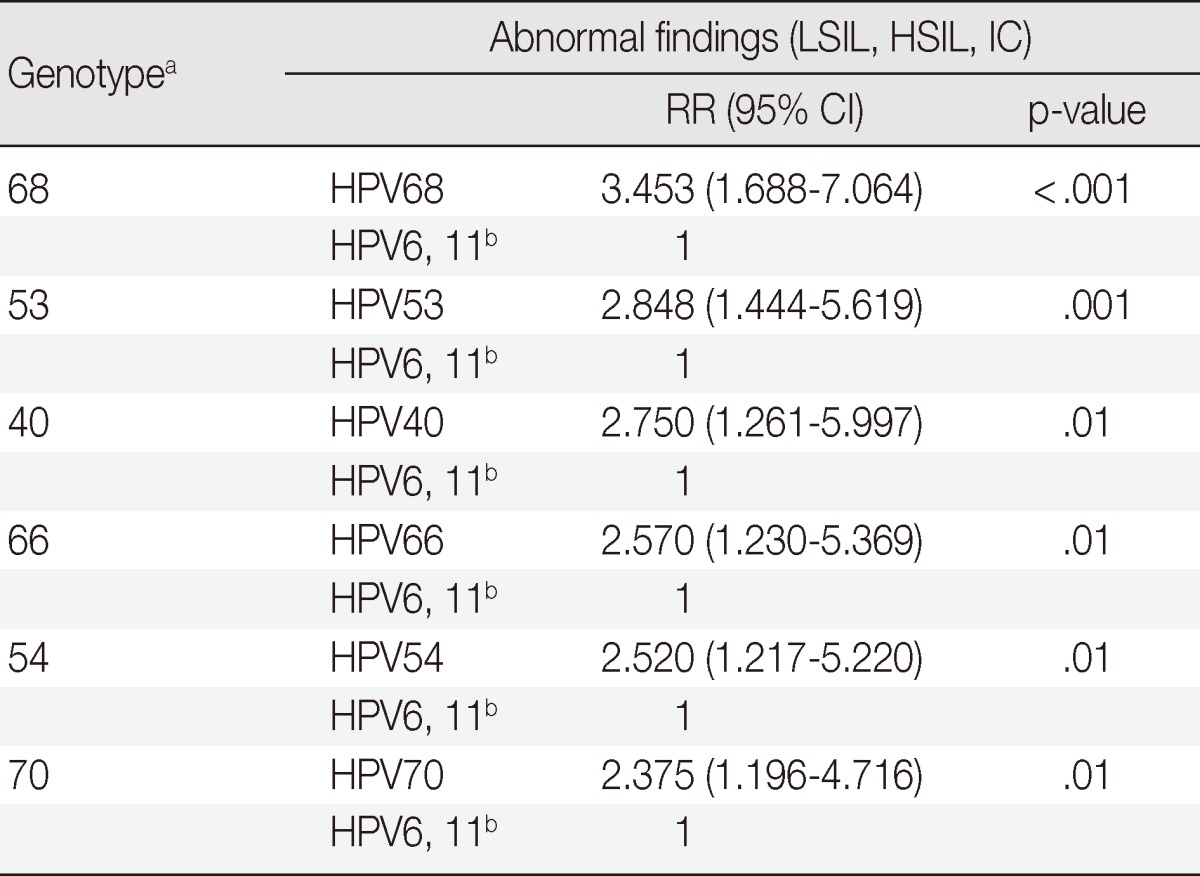
HPV, human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; IC, invasive carcinoma; RR, relative risk; CI, confidence interval.
aHPV type count of less than 20 in total after excluding group 1-coinfection are not shown in the Table; bHPV6 and 11 infected cases that are coinfected by group 1 are not shown in the Table.
Rare and uncommon HPV genotypes compared to HPV16 excluding group 1-coinfection in abnormal findings
The rare and uncommon types showing a statistical relationship with LSILs, HSILs, and ICs compared to HPV16 were as follows: HPV68 (RR, 1.059; p=.73), HPV53 (RR, 1.284; p=.03), HPV40 (RR, 1.330; p=.16), HPV66 (RR, 1.423; p=.03), HPV54 (RR, 1.451; p=.02), and HPV70 (RR, 1.539; p<.001). Differences between HPV16 and HPV53, 66, 54, and 70 genotypes were statistically significant, however, the value of the relative risk of HPV66 showed no significance (Table 4).
Table 4.
The relationship of abnormal findings in the evaluation of rare and uncommon HPV genotypes compared to HPV16 without group 1-coinfection
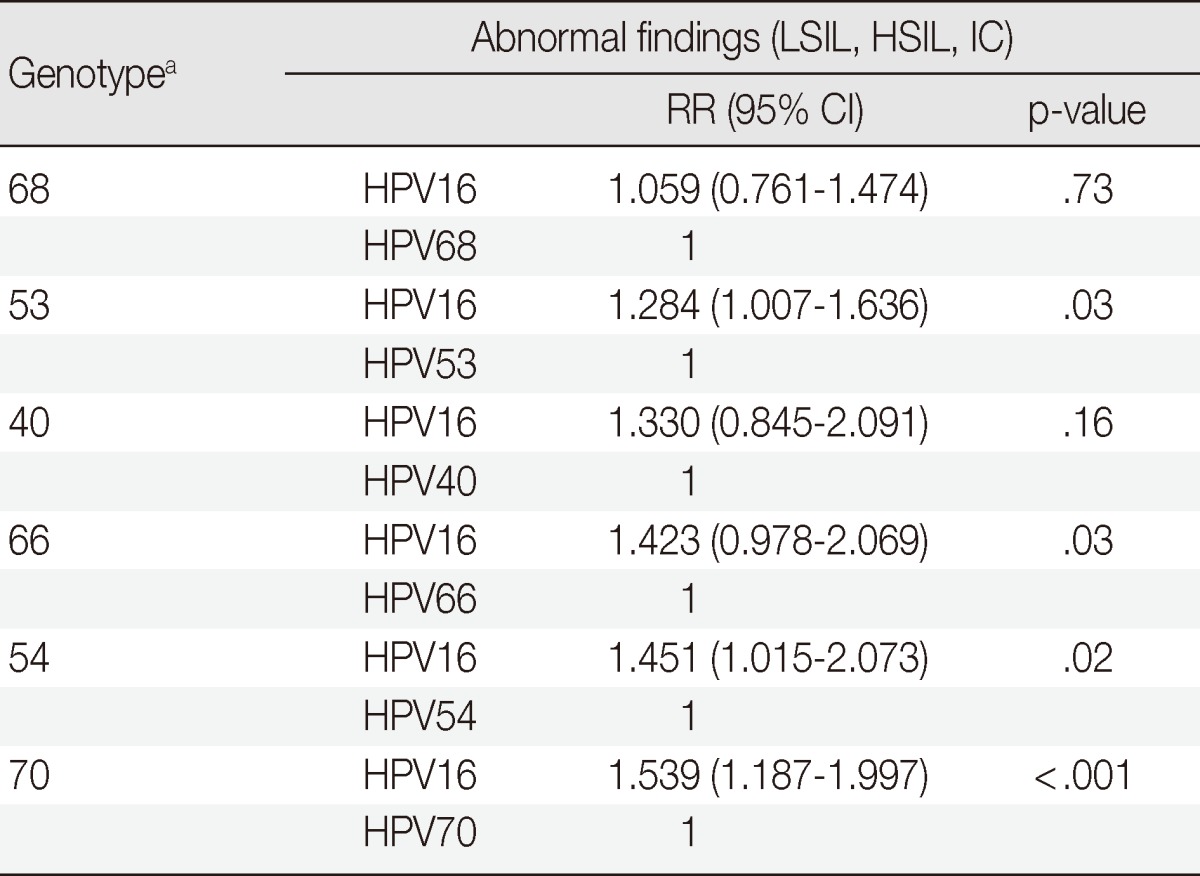
HPV, human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; IC, invasive carcinoma; RR, relative risk; CI, confidence interval.
aHPV type count of less than 20 in total after excluding group 1-coinfection are not shown in the Table.
So called 'other' types not specified by HPV DNA chip
Out of 659 cases of 'other' HPV-positive cases, 22.6% (149/659) were accompanied by HSIL and IC. These other types showed statistical significances with HSILs and ICs, compared to HPV6 and 11 (p=.01).
Infection by multiple genotypes
Coinfection with multiple HPV genotypes was observed in 15.7% (497/3,164) of HPV-infected patients. Cases infected with two types, three types, four types, and more than five types were 13.3% (421/3,164), 1.9% (59/3,164), 0.4% (14/3,164), and 0.1% (3/3,164), respectively.
After exclusion of group 1 genotypes, the HPV genotypes affected by multiple infections were 8.2% (37/447). The most common multiple infected type was HPV40. There were 6 cases in HSILs or ICs: HPV40/53 (n=2), HPV54/68 (n=2), HPV40/55 (n=1), and HPV53/66 (n=1).
DISCUSSION
HPVs are epitheliotrophic viruses that predominantly infect mucocutaneous tissue, including the uterine cervix.4 Despite screening based on a national cervical cancer screening program and recent introduction of HPV vaccination, uterine cervical cancer is the second most common female malignancy worldwide.1,13 Cervical cancer remains a major health burden in Korea.2,5
According to the carcinogenic potential, HPV types were classified as high risk, low risk, and probably high risk. Among the HPV genotypes, only 12 are categorized as carcinogens of the uterine cervix by the Working Group of the World Health Organization (WHO) International Agency for Research on Cancer (IARC).12 HPV genotypes are classified as carcinogens (group 1; HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59), probable carcinogens (group 2A; HPV68), possible carcinogens (group 2B, HPV26, 30, 34, 53, 66, 67, 69, 70, 73, 82, 85, and 97), and not classifiable as to its carcinogenicity to humans (group 3; HPV6 and 11). However, a recent worldwide assessment of HPV genotypes revealed that the previously unknown uncommon HPV types, which were identified as types 26, 30, 61, 67, 69, 82, and 91, were identified in 1% of all studied ICs, although the biological behaviors of these rare genotypes have not yet been fully clarified due to insufficient epidemiological research.14,15
In this study, we focused on the correlation of dysplasia or IC with rare and uncommon HPV genotypes to clarify the importance of these uncommon genotypes associated with uterine cervical dysplasia and carcinogenesis.
In our study of the previously unnoticed rare and uncommon HPV genotypes, significant differences were observed between HPV16 and group 2/ unclassified group, except for group 1-coinfection. Significant differences were observed between group 2/ unclassified group and group 3 only when compared between the normal group and the cervical intraepithelial neoplasm. HPV 68 (group 2A) is closely related to HPV39, 70, 59, 45, and 18.4,10 These types belong to the alpha-7 species, associated with high-risk mucosal lesions, and all genotypes in these alpha-7 species were categorized as group 1, except for HPV68 and 70.4 According to our results, HPV68 was statistically different from group 3, however, it did not show a difference with group 1 in the pathogenesis of HSIL and ICs. Especially, the possibility of cervical intraepithelial neoplasms was 3.453 times higher with HPV68 than with HPV6 and 11. These results suggest that HPV genotype may take similar carcinogenic significance in cervical carcinogenesis as the previous study and surveys revealed.
HPV70 had the highest prevalence (4.2%) among the rare and uncommon HPVs. HPV70 was statistically different with WNL versus abnormal findings compared to HPV16 or group 3 (p<.001 and p=.01, respectively). According to the previous data, identification of HPV70 is relatively infrequent in normal and cancer patients, with a higher prevalence seen in precancerous lesions.10,16 The relatively high incidence of LSIL in HPV70 infection was seen in this study, as was seen in previous studies.10,16
HPV53 showed a significant increase in prevalence from 1990-1999 to 2006-2010.11,15,17 In this study, HPV53 was the genotype with the second highest prevalence (3.5%) among the rare and uncommon types, and showed the second highest incidence among the rare and uncommon HPV types in HSILs and ICs (13/89, 14.6%), followed by HPV68, excluding group 1-coinfection. A significant difference was observed in group 3 (p=.001), and the value of relative risk was the second highest among uncommon and rare HPVs (RR, 2.848). A difference in abnormal finding was also observed between HPV53 and that of HPV16, however, the value of relative risk in HPV16 was lower than that of group 3 (RR, 1.284). These results suggest a relatively high incidence of LSIL with HPV53, as is seen with HPV70 infection.18 Untreated LSIL could worsen or progress to persistent LSIL, and has been reported to occur in 29.7% of cases.13 For this reason, awareness of LSIL in HPV53 or HPV70 infection is needed.
Coinfection with multiple HPV genotypes is commonly encountered in HPV studies.6,8-11,15 The reported prevalences of multiple genotype infection span a broad range, from 4.4% to 78.3%.15 Furthermore, the carcinogenic effects of multiple infections are still debatable. A high incidence of coinfection by multiple genotypes (15.7%) was observed in this study, which might have been affected by the sensitivity of the HPV DNA array system, the characteristics of the study population, and geographic distribution.19 Watari et al.7 reported that coinfection with HPV34 could prevent tumor progression of invasive squamous cell carcinoma with HPV16. However, according to the study reported by Lee et al.,20 multiple infection showed a 31.8-fold higher risk of cervical cancer, while the single HPV type showed a 19.9-fold increased risk compared to no HPV infection. We found that multiple infections were statistically different from single infections (p<.001), in that they were more prevalent in HSILs and ICs. These results were expected because most HSILs and ICs in multiple infections involved high risk HPV (133/139, 95.7%). However, previous studies on the relationship between infection by multiple HPV genotypes and cervical carcinogenesis have reported inconsistent and controversial results and subsequent possible influence of group 1, the most powerful carcinogens, on evaluation of these uncommon and rare HPV genotypes. Therefore, we excluded those types of cases from this study. Additional studies will be needed in order to determine the association between multiple infections and pathogenesis.
A previous study reported the detection of HPV DNA in up to 89.1% of IC cases when on the basis of formalin-fixed paraffin-embedded blocks,8 and that rate is similar to the one identified in our study (204/245, 85.3%). We also found that 2.4% (92/3,758) of uncommon groups 2A, 2B, 3, and unclassified genotypes showed HSILs and ICs, whereas 7.9% (298/3,758) of group 1 genotypes, except for HPV16, 18, and 58, showed HSILs and ICs, similar to rates found by the survey on the previously unknown HPV types that comprised 1% of all studied ICs, such as HPV26, 30, 61, 67, 69, 82, and 91.14 The DNA microarray (DNA Chip) method that we used is widely available for clinical use due to its relatively simplified and quick method, its use of both fresh and paraffin samples with high sensitivity, and its capacity for detection of single and multiple coinfections of HPV at once.19 Out of 659 cases of other types, 22.6% (149/659) were HSIL and IC, which cannot be detected by this method and may influence the results of this study.
Despite low prevalence and less potency rare and uncommon HPV type, this paper might provide clues for the closeness of the link between rare and uncommon HPV type, carcinogenicity, and invasive cervical carcinomas. Unlike women with HPV infection, the age of patients with ICs related to HPV16, 18, or 58 is not different from that of IC patients infected with rare and uncommon HPV types. Two recently developed vaccines, the recombinant quadrivalent HPV vaccine (Gardasil, Merck), mainly prevents the HPV targeting types 16, 18, 6, 11, and 31, while the bivalent HPV recombinant vaccine (Cervarix, GlaxoSmithKline) mainly prevents the HPV targeting types 16, 18, 45, and 31.21 As shown in the end-of-study analysis of PATRICIA, the HPV16 and 18 vaccine provides cross-protective efficacy against six-month persistent infection and moderate to severe dysplasia or IC associated with HPV31, 33, 45, and 51, although Gardasil showed slightly less potency for cross-protection. Even if clinical trials of prophylactic vaccines targeted for HPV16 and 18 showed drastically preventive effects for HPV infection and precancerous lesions, the possibility of cross-protection against other HPV genotypes,21 especially uncommon and rare HPV genotypes, is extremely important but remains unsolved. Because HPV genotypes share structural similarities, the vaccines may protect against HPV types other than these targeted types. However, detailed and targeted developed vaccine may be required.
In summary, we found that uncommon and rare HPV genotypes may provide incremental etiologic contributions in cervical carcinogenesis, especially HPV68, 70, and 53. The rare and uncommon HPV genotypes are correlated with cervical carcinogenesis as well as well known, established high-risk HPV genotypes. The results of our study may be helpful in formulating a strategy for further second-generation vaccine development in a particular region. We emphasize that future studies should also focus on these rare uncommon HPV genotypes, especially on the different distributions of these rare HPV genotypes in particular regions.
Acknowledgments
This work was supported by the Gachon University Research Fund of 2013 (GCU-2012-M104).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 2.Oh JK, Lim MK, Yun EH, Lee EH, Shin HR. Awareness of and attitude towards human papillomavirus infection and vaccination for cervical cancer prevention among adult males and females in Korea: a nationwide interview survey. Vaccine. 2010;28:1854–1860. doi: 10.1016/j.vaccine.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 3.Jovanović AM, Dikic SD, Jovanovic V, et al. Correlation of human papilloma virus infection with cytology, colposcopy and histopathological examination of the bioptic tissue in low- and high-grade intraepithelial lesions. Eur J Gynaecol Oncol. 2012;33:512–516. [PubMed] [Google Scholar]
- 4.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Hong SR, Kim IS, Kim DW, et al. Prevalence and genotype distribution of cervical human papillomavirus DNA in Korean women: a multicenter study. Korean J Pathol. 2009;43:342–350. [Google Scholar]
- 6.Bello BD, Spinillo A, Alberizzi P, et al. Cervical infections by multiple human papillomavirus (HPV) genotypes: Prevalence and impact on the risk of precancerous epithelial lesions. J Med Virol. 2009;81:703–712. doi: 10.1002/jmv.21429. [DOI] [PubMed] [Google Scholar]
- 7.Watari H, Michimata R, Yasuda M, et al. High prevalence of multiple human papillomavirus infection in Japanese patients with invasive uterine cervical cancer. Pathobiology. 2011;78:220–226. doi: 10.1159/000326770. [DOI] [PubMed] [Google Scholar]
- 8.Alemany L, Pérez C, Tous S, et al. Human papillomavirus genotype distribution in cervical cancer cases in Spain. Implications for prevention. Gynecol Oncol. 2012;124:512–517. doi: 10.1016/j.ygyno.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 9.de Mattos AT, de Freitas LB, Lima BM, Miranda AE, Spano LC. Diversity and uncommon HPV types in HIV seropositive and seronegative women attending an STI clinic. Braz J Microbiol. 2011;42:786–793. doi: 10.1590/S1517-838220110002000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longuet M, Beaudenon S, Orth G. Two novel genital human papillomavirus (HPV) types, HPV68 and HPV70, related to the potentially oncogenic HPV39. J Clin Microbiol. 1996;34:738–744. doi: 10.1128/jcm.34.3.738-744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik ZA, Hailpern SM, Burk RD. Predictors of seropositivity to human papillomavirus type 53: one of the most prevalent high risk-related cervical human papillomaviruses. Viral Immunol. 2008;21:371–377. doi: 10.1089/vim.2008.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IARC. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2011;100B:1–475. [PMC free article] [PubMed] [Google Scholar]
- 13.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 14.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 15.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–935. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 16.Choi IH, Jin SY, Lee DW, Kim DW, Jeen YM. Cytomorphologic features according to HPV DNA type in histologically proven cases of the uterine cervix. Korean J Pathol. 2011;45:612–620. [Google Scholar]
- 17.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 18.Meyer T, Arndt R, Beckmann ER, Padberg B, Christophers E, Stockfleth E. Distribution of HPV 53, HPV 73, and CP8304 in genital epithelial lesions with different grades of dysplasia. Int J Gynecol Cancer. 2001;11:198–204. doi: 10.1046/j.1525-1438.2001.01009.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee D, Kim S, Park S, et al. Human papillomavirus prevalence in Gangwon Province using reverse blot hybridization assay. Korean J Pathol. 2011;45:348–353. [Google Scholar]
- 20.Lee SA, Kang D, Seo SS, et al. Multiple HPV infection in cervical cancer screened by HPVDNAChip. Cancer Lett. 2003;198:187–192. doi: 10.1016/s0304-3835(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler CM, Castellsagué X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–110. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]


