Abstract
Background
Children with myocarditis have multiple risk factors for thrombotic events, yet the role of antithrombotic therapy is unclear in this population. We hypothesized that thrombotic events in critically ill children with myocarditis are common, and that children with myocarditis are at higher risk for thrombotic events than children with non-inflammatory dilated cardiomyopathy.
Methods
Retrospective chart review of all children presenting to a single center cardiac intensive care unit with myocarditis from 1995–2008. A comparison group of children with dilated cardiomyopathy was also examined. Antithrombotic regimens were recorded. The primary outcome of thrombotic events included intracardiac clots and any thromboembolic events.
Results
Out of 45 subjects with myocarditis, 40% were biopsy-proven, 24% viral polymerase chain reaction-supported, and 36% diagnosed based upon high clinical suspicion. There were 2 (4.4%) thrombotic events in the myocarditis group and 3 (6.7%) in the dilated cardiomyopathy group (p=1.0). Neither use of any antiplatelet or anticoagulation therapy, use of intravenous immune globulin, presence of any arrhythmia, nor need for mechanical circulatory support were predictive of thrombotic events in the myocarditis, dilated cardiomyopathy, or combined groups.
Conclusions
Thrombotic events in critically ill children with myocarditis and dilated cardiomyopathy occurred in 6% of the combined cohort. There was no difference in thrombotic events between inflammatory and non-inflammatory cardiomyopathy groups, suggesting that the decision to use antithrombotic prophylaxis should be based upon factors other than the underlying etiology of a child’s acute decompensated heart failure.
Keywords: Anticoagulation, Cardiomyopathy, Myocarditis, Stroke
Introduction
The risk of thrombotic events in children with myocarditis is currently unknown, leading to uncertainty regarding the need for antithrombotic therapy. Some studies in children with dilated cardiomyopathy suggest that those with severely diminished or rapidly deteriorating ventricular function are at increased risk of thrombus formation 1, 2. Kuhn et al published a retrospective study of 28 pediatric patients with myocarditis and reported that 10.7% experienced intracardiac thrombosis 3. Embolic arterial ischemic stroke has also been reported as a complication of myocarditis 4. No large prospective pediatric cohort studies have been published which examine the risk for thrombotic events in myocarditis. Several factors place children with myocarditis at increased risk of thrombus formation, including stasis of blood in a poorly functioning heart, and inflammation of the myocardium and surrounding structures. Myocarditis mouse models and human ex vivo studies have demonstrated a hypercoagulable state secondary to increased myocardial expression of tissue factor that leads to activation of coagulation 5.
The risk of thrombus formation in adults with myocarditis is likewise unknown. There is evidence that anticoagulant or antiplatelet therapy reduces the risk of stroke in adults with ventricular dysfunction after myocardial infarction 6. In particular, the first two weeks after myocardial infarction, presumably a time of increased myocardial inflammation, carry a stroke risk of up to 4.7% 7. In children, inflammation of the heart that occurs in the setting of myocarditis could add to the risk of in situ thrombus formation in much the same way as it does in adults after myocardial infarction.
We therefore hypothesized that thrombotic events in critically ill children with myocarditis are common and that children with myocarditis are at higher risk for thrombotic events than children with non-inflammatory dilated cardiomyopathy. From a single institution we compared the incidence of thrombotic events in critically ill children with myocarditis versus critically ill children with a noninflammatory dilated cardiomyopathy.
Materials and Methods
The study protocol was approved by the Institutional Review Board
We performed a retrospective cohort study of all patients admitted to a single tertiary care center pediatric cardiac intensive care unit with newly diagnosed myocarditis between January 1, 1995 and December 31, 2008, and compared them to a group with newly diagnosed non-inflammatory, dilated cardiomyopathy. The dilated cardiomyopathy comparison group was identified in reverse chronological order, starting from an admission date at or before December 31, 2008 and continuing until the same number of subjects was reviewed for each study group. For subjects with multiple cardiac intensive care unit admissions, we only included data from the first myocarditis or dilated cardiomyopathy admission, and excluded data from subsequent admissions. All subjects were initially identified by searching for “myocarditis” or “dilated cardiomyopathy” in the primary diagnosis field of our center’s cardiac intensive care unit patient database, which captures all admissions to the cardiac intensive care unit with basic demographic, diagnostic, and outcome information during the study time frame.
Our study defined the myocarditis cohort as those subjects who had an admission diagnosis of myocarditis and were subsequently treated as having myocarditis. At our center, myocarditis is routinely treated with intravenous immune globulin unless otherwise contraindicated. Supportive measures are provided as needed including mechanical ventilation, inotropic support, and mechanical circulatory support including extracorporeal membrane oxygenation and ventricular assist device implantation.
Those in the myocarditis group were classified into one of three categories:
Biopsy-proven: Pathologic confirmation of myocarditis obtained by autopsy, explants, or endomyocardial biopsy. Those with “borderline myocarditis” on a pathology report alone did not qualify as “biopsy proven.”
Viral polymerase chain reaction-supported: One or more positive viral polymerase chain reactions that were considered to be a plausible cause for their myocarditis by the treating clinicians. These subjects did not also meet criteria for biopsy-proven myocarditis.
High clinical suspicion: Subject was admitted with suspicion for myocarditis, treated as such, and was discharged with the same presumed diagnosis, despite lack of biopsy or viral polymerase chain reaction evidence.
Dilated cardiomyopathy subjects were those with symptomatic left ventricular dysfunction and dilation who did not meet criteria for myocarditis as defined above. Left ventricular dysfunction was determined by a depressed ejection fraction (<55%) or shortening fraction (<29%) by echocardiogram. Left ventricular dilation was determined by a left ventricular end diastolic dimension z score > 2 8. The cases with primary hypertrophic, primary restrictive, or left ventricular noncompaction cardiomyopathy were excluded, even if they had dilated features, since these conditions may have a different thrombotic risk profile than dilated cardiomyopathy alone 9–11.
Subjects were excluded if their initial cardiac intensive care unit admission for myocarditis or dilated cardiomyopathy was outside of the study period or if they were ≥ 18 years of age at the time of admission. Those with congenital heart disease were excluded. These cases included, but were not limited to, coronary anomalies, unrepaired atrial septal defect, unrepaired ventricular septal defect, hypoplastic left heart syndrome, transposition of the great arteries, tetralogy of Fallot, and atrioventricular canal defect. All charts were systematically reviewed by two physicians, including relevant admission and discharge summaries, progress notes, laboratory studies, pathology reports, echocardiogram reports, operative reports, medication orders, brain magnetic resonance imaging and computed tomography scan reports, and ultrasound reports. Of note, as this was a retrospective study, the use of each imaging modality was prompted by clinical concerns and was thus not uniform for every subject.
Our primary outcome of thrombotic event included identification of intracardiac clots or embolic ischemic injury to the brain, kidneys, spleen, liver, or distal extremities. Any thrombotic events were confirmed by review by a cardiologist (for intracardiac clots), neurologist (for cardioembolic strokes), or hematologist (for any other embolic phenomena). Cardioembolic stroke was defined as an acute neurologic deficit conforming to a vascular territory and confirmed by the presence of a corresponding arterial-distribution acute infarct on neuroimaging (head computed tomography or brain magnetic resonance imaging). In infants < 6 months, where acute focal deficits are unreliable as the presenting symptom, stroke was defined as an acute neurologic syndrome manifest as new onset of depressed mental status and/or seizures associated with arterial-distribution acute infarct on neuroimaging (head computed tomography or brain magnetic resonance imaging). Line-associated thrombi were not included as thrombotic events for purposes of this study. Thrombotic events were only included for analysis if they occurred before initiation of any mechanical circulatory support (i.e. extracorporeal membrane oxygenation or ventricular assist device) due to increased thrombotic risks related to the device. In those who went on to heart transplantation, thrombotic events were only included if they occurred before transplantation.
Statistical analysis was performed using Microsoft Excel 2007 and SAS version 9.2 (Cary, NC). Continuous variables were compared using t-tests for normally distributed data, and Wilcoxon Rank Sum tests for non-parametric data. Categorical variables were compared using the non-parametric Fisher’s exact test. A two-sided probability value of ≤ 0.05 was considered statistically significant.
Results
We identified 45 subjects with myocarditis within the study period. A summary of subject characteristics is shown in Table 1. The myocarditis group consisted of 40% biopsy-proven, 24% supported by viral polymerase chain reaction, and 36% high clinical suspicion cases. Of those with biopsy-proven or viral polymerase chain reaction-supported myocarditis (n=29), the most common viruses identified were parvovirus B19 (n=8) and enterovirus (n=3). In addition, there were single cases of adenovirus, herpes simplex virus, influenza B, and rhinovirus. One child had both parvovirus and human herpesvirus 6 identified in her serum; another had cytomegalovirus (serum and respiratory), parvovirus B19 (respiratory), and rhinovirus (respiratory). The comparison group included 45 newly diagnosed dilated cardiomyopathy cases during the study period. These subjects were admitted between July 10, 1995 and December 31, 2008. Arrhythmias that occurred at any time during the hospitalization were identified on review of the discharge summary and electrocardiograms. These included ventricular tachycardia or fibrillation (n=19 myocarditis and n=13 dilated cardiomyopathy), atrial fibrillation (n=3 myocarditis and n=1 dilated cardiomyopathy), atrial flutter (n=0 myocarditis and n=3 dilated cardiomyopathy), and supraventricular tachycardia (n=5 myocarditis and n=3 dilated cardiomyopathy).
Table 1.
Comparison of myocarditis and dilated cardiomyopathy (DCM) groups
| Demographics | Myocarditis (n=45) | DCM (n=45) | p value |
|---|---|---|---|
| Sex | Male 18 (40%) | Male 27 (60%) | 0.09 |
| Age (years) | 1.7 (IQR 0.9–11.5) | 5.9 (IQR 0.5–14.0) | 0.57 |
| ECMO | 12 (27%) | 5 (11%) | 0.10 |
| VAD | 1 (2%) | 9 (20%) | 0.02 |
| CPR | 13 (29%) | 4 (9%) | 0.03 |
| IVIG | 43 (96%) | 14 (31%) | <0.01 |
| Any arrhythmia | 26 (58%) | 18 (40%) | 0.14 |
| SF by TTE on admission | 17.9 ± 9 | 13.1 ± 6 | <0.01 |
| LVEDD z score on admission | 2.58 ± 2.2 | 5.05 ± 2.4 | <0.01 |
| Length of ICU stay (days) | 10 (IQR 5–19) | 11 (IQR 5–51) | 0.23 |
DCM=dilated cardiomyopathy; IQR=interquartile range; ECMO=extracorporeal membrane oxygenation; VAD=ventricular assist device; CPR=cardiopulmonary resuscitation; IVIG=intravenous immune globulin; SF=shortening fraction; TTE=transthoracic echocardiogram; LVEDD=left ventricular end diastolic dimension; ICU=intensive care unit
The anticoagulation and antiplatelet regimens utilized in both groups of patients are summarized in Table 2. Just over half of the myocarditis and dilated cardiomyopathy subjects who never required mechanical circulatory support were treated with either antiplatelet or anticoagulant therapy at some point during their hospitalization. Our cardiac intensive care unit used a strategy of “low-dose heparin” (heparin dosed empirically at 10 units per kilogram per hour without a target prolongation of partial thromboplastin time) in over one third of the subjects in our study.
Table 2.
Antithrombotic regimens employed in myocarditis and dilated cardiomyopathy (DCM) groups
| N(%) | Time to initiation (days) | |||||
|---|---|---|---|---|---|---|
| Therapy | Myocarditis (n=45 total, n=33 non-MCS) | DCM (n=45 total, n=34 non-MCS) | p value | Myocarditis | DCM | p value |
| Aspirin | 15 (33%) | 9 (20%) | 0.23 | 15.60 ± 10.4 | 19.78 ± 20.8 | 0.23 |
| Therapeutic heparin (goal PTT 60–80) | 18 (40%) | 17 (38%) | 1.00 | 2.94 ± 4.1 | 26.24 ± 34.5 | <0.01 |
| 7 (21%) non-MCS | 6 (18%) non-MCS | 1.00 | 4.43 ± 3.2 | 18.00 ± 18.3 | 0.00 | |
| Therapeutic enoxaparin | 2 (4%) | 2 (4%) | 1.00 | 11.00 ± 11.3 | 28.50 ± 40.3 | 0.01 |
| Low-dose heparin (10 units/kg/hour) | 15 (33%) | 20 (44%) | 0.39 | 3.76 ± 6.1 | 4.30 ± 14.4 | 0.82 |
| Warfarin | 2 (4%) | 4 (9%) | 0.43 | 18.00 ± 11.3 | 35.00 ± 34.8 | 0.00 |
| No anticoagulation during hospitalization | 16 (36%) | 15 (33%) | 0.83 | |||
| 16 (48%) non-MCS | 15 (44%) non- MCS | 0.80 | ||||
DCM=dilated cardiomyopathy; MCS=mechanical circulatory support; PTT=partial thromboplastin time
Table 3 summarizes the outcomes (death and thrombotic events) seen in both groups. Two of 45 subjects (4.4%, 95% binomial exact confidence interval 0.5% to 15.1%) in the myocarditis group and 3 of 45 subjects (6.7%, 95% binomial exact confidence interval 1.4% to 18.3%) in the dilated cardiomyopathy group had thrombotic complications. Of the two thrombotic events before mechanical circulatory support in the myocarditis group, one was a left ventricular thrombus with ultrasound evidence of embolism to the kidneys, and the other was an embolic arterial ischemic stroke. Of the three thrombotic events in the dilated cardiomyopathy group, two were left ventricular thrombi and the third was an embolic arterial ischemic stroke. Further details regarding these thrombotic events will be discussed below.
Table 3.
Outcomes in myocarditis and dilated cardiomyopathy (DCM) groups
| Outcomes | Myocarditis (n=45) | DCM (n=45) | p value |
|---|---|---|---|
|
| |||
| Death | 2 (4%) | 7 (16%) | 0.16 |
|
| |||
| Any thrombotic event (pre-MCS) | 2 (4%) | 3 (7%) | 1.00 |
| Intracardiac thrombus | 1 (2%) | 2 (4%) | |
| Renal infarction | 1 (2%) | 0 (0%) | |
| Splenic/hepatic infarction | 0 (0%) | 0 (0%) | |
| Distal extremity infarction | 0 (0%) | 0 (0%) | |
| Thromboembolic stroke | 1 (2%) | 1 (2%) | |
DCM=dilated cardiomyopathy; MCS=mechanical circulatory support
In the myocarditis group, the left ventricular thrombus occurred in a 20 month old girl with parvovirus myocarditis (Figure 1). Her initial shortening fraction was 18%, and she did not require extracorporeal membrane oxygenation, cardiopulmonary resuscitation, or ventricular assist device. She developed a left ventricular thrombus detected by follow-up surface echocardiogram on hospital day #10 while on low-dose heparin (10 units per kilogram per hour). The thrombus was subsequently confirmed by direct visualization when she was taken to the operating room for thrombectomy on hospital day #12 due to concern for secondary fungal infection and renal infarction. Her shortening fraction had improved to 21% at the time of detection but did not normalize until after hospital discharge. A standard prothrombotic work-up was negative.
Figure 1.
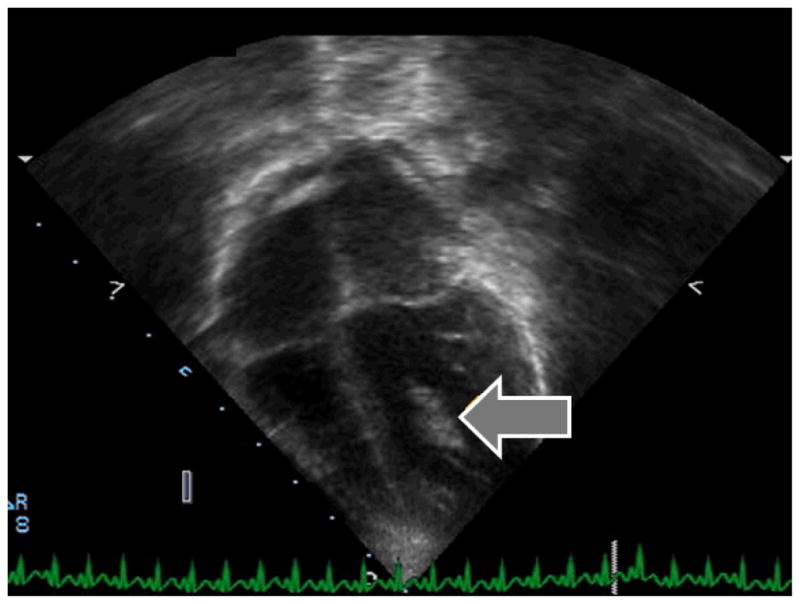
Left ventricular thrombus (see arrow) identified by echocardiogram in a myocarditis subject
An embolic arterial ischemic stroke occurred in an 11 year old girl with myocarditis by high clinical suspicion. Her initial shortening fraction was 14%, and she did not require extracorporeal membrane oxygenation, cardiopulmonary resuscitation, or ventricular assist device. Her stroke presented clinically with acute onset of left-sided weakness, dysarthria, and neglect on hospital day #3 while on no anticoagulation or antiplatelet therapies, and was subsequently confirmed by magnetic resonance imaging (Figure 2). Her shortening fraction had improved to 19% on the day of the stroke but did not normalize during the hospitalization. No thrombus was visualized by ultrasound of her heart and lower extremity veins. A standard prothrombotic work-up was negative.
Figure 2.
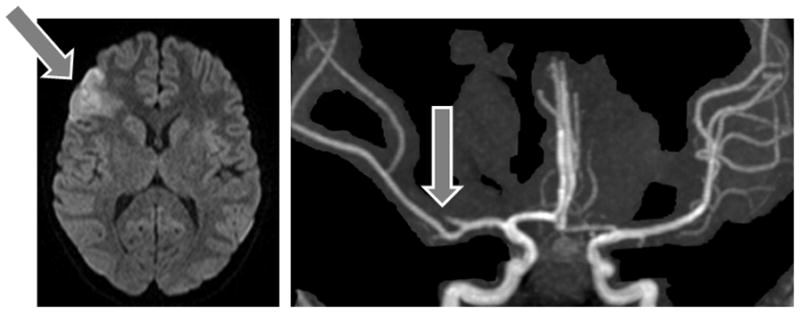
Magnetic resonance imaging from a myocarditis subject who experienced a left middle cerebral artery stroke
In the dilated cardiomyopathy group, the first left ventricular thrombus was identified on hospital day #3 in a 16 year old patient with severe left ventricular dilation and dysfunction (admission shortening fraction 16%, left ventricular end diastolic dimension z score 5.3) of unclear etiology. Endomyocardial biopsy showed no inflammation, and viral studies revealed only respiratory syncytial virus by tracheal aspirate. He had been on low-dose heparin (10 units per kilogram per hour) when the thrombus was identified by surface echocardiogram. A prothrombotic work-up was not performed. The second left ventricular thrombus was incidentally noted by ventriculogram at the time of cardiac catheterization in a 14 year old patient on aspirin with an admission shortening fraction of 12% and a left ventricular end diastolic dimension z score of 3.1. He was subsequently placed on therapeutic heparin and went on to require left ventricular assist device support as a bridge to successful heart transplantation. Finally, the embolic stroke occurred in an 11 year old patient while on low dose heparin (10 units per kilogram per hour) with an initial shortening fraction of 10% and left ventricular end diastolic dimension z score of 6.3. This subject also had a significant history of ventricular tachycardia and pulmonary hypertension. The stroke presented clinically with acute onset left-sided weakness and was confirmed by magnetic resonance imaging; no thrombus was visualized by echocardiogram. The subject was kept on low-dose heparin until months later, when he required left ventricular assist device support as a bridge to heart transplantation. This subject died on hospital day #244 due to complications of transplantation. None of the three dilated cardiomyopathy subjects with a thrombotic event had a documented prothrombotic work-up.
Potential predictors for thrombotic events were explored (Tables 1 and 2). On univariate analysis, neither the use of any antithrombotic medication, use of intravenous immune globulin, presence of any arrhythmia, nor the need for mechanical circulatory support were predictors of thrombotic events in our myocarditis subjects (p=1.00 for all, Fisher’s exact test). These factors were likewise not significant when examining the dilated cardiomyopathy group, and the combined cohort.
Discussion
This retrospective cohort study is novel in its comparison of the incidence of thrombotic events in pediatric patients with myocarditis compared to a similar cohort with dilated cardiomyopathy. We found a similar rate of thrombotic events during hospitalization for acute illness at the time of initial diagnosis in children with myocarditis (4.4%) and dilated cardiomyopathy (6.7%). While there was no statistically significant difference between the incidences of thrombotic events in the two groups, both groups had a thrombotic event rate that should prompt further consideration for prophylactic anticoagulation. We were unable to identify clinical predictors for thrombotic events in either group.
As endomyocardial biopsy is not always performed systematically in children with cardiomyopathy, distinguishing between inflammatory and non-inflammatory causes of left ventricular dysfunction can be difficult. Those with a chronic dilated cardiomyopathy have had more time for their hearts to remodel and dilate and therefore present with larger left ventricular dimensions than those with acute myocarditis who present in heart failure due to an acute inflammatory process. This was observed in a large cohort study (n=1495) of children with myocarditis from the Pediatric Cardiomyopathy Registry 12. The findings in our cohort were consistent with these reports in that those diagnosed with myocarditis did have smaller initial left ventricular end diastolic dimension z scores than did those in the dilated cardiomyopathy cohort. While the Pediatric Cardiomyopathy Registry study and older studies of children with myocarditis focused on comparisons of ventricular dimensions and survival outcomes compared to children with dilated cardiomyopathy 12, 13, relative thrombotic risks in these children were not described.
Our cardiac intensive care unit strategy of administering low-dose heparin (run empirically at 10 units per kilogram per hour without a target prolongation of partial thromboplastin time) is a practice which we use in our congenital heart disease population in the early postoperative period after a Blalock-Taussig shunt has been placed. This has anecdotally decreased the incidence of Blalock-Taussig shunt thrombosis in our center and others, although little published data about thrombotic events exists for patients managed with this strategy 14. This strategy of low-dose heparin was extended to over one third of the subjects in our study. The limited number of events in our cohort precludes definitive statements about the efficacy of this strategy in myocarditis or dilated cardiomyopathy. However, it is interesting to note that three of the five observed thrombotic events were in subjects on low-dose heparin at the time of thrombotic event discovery. The actual degree of anticoagulation provided to each individual at an empiric heparin dose of 10 units per kilogram per hour is unknown, though it was not sufficient to prevent thrombosis from forming in these subjects.
There has been considerable interest in the use of antithrombotic therapies as primary prophylaxis against thrombotic events in adults, likely because the morbidity and mortality after a thrombotic event can be quite high. A meta-analysis of adults after myocardial infarction found that systemic embolization occurred in 11% of patients with a left ventricular thrombus.15 Two large, randomized trials examining antiplatelet versus warfarin for thrombus prevention in adults with chronic left ventricular systolic dysfunction have recently been published, with neither study demonstrating a significant advantage among agents.16, 17 Adult guidelines still do not conclusively support the use of any antithrombotic therapies in patients with any form of cardiomyopathy unless other risk factors, such as a mechanical valve or atrial fibrillation, are identified.18
The most recently published guidelines for antithrombotic therapy in neonates and children are silent as to whether to anticoagulate children with myocarditis or dilated cardiomyopathy, except to give a grade 2C recommendation for vitamin K antagonists in any cardiomyopathy cases by the time they have deteriorated to the point of listing for cardiac transplantation 19. The same guidelines gave a strong (1C) recommendation for primary thrombophylaxis (with either aspirin, or heparin as a bridge to long-term vitamin K antagonist) in children after Fontan surgery 19. As reviewed in an editorial by Monagle et al, the reported incidence of venous thromboembolism and stroke after Fontan surgery is 1–7% in studies assessing multiple outcomes, and 3–19% in studies in which thromboembolism was the major outcome 20. Other clinical scenarios in which the guidelines recommend full anticoagulation for primary thrombophylaxis include primary pulmonary hypertension by the time of vasodilator therapy initiation (2C), Kawasaki disease with moderate or giant coronary aneurysms (2C), ventricular assist device therapy (2C, with additional antiplatelet therapy), mechanical valves (as per adult guidelines), hemodialysis (2C), and cardiac catheterizations with arterial access (1A) 19. The incidence of thrombotic events in children with those conditions is variable.
In this study we have confirmed that there is a measurable risk of thrombotic events in critically ill children with both myocarditis and dilated cardiomyopathy, with 6% of subjects experiencing a thrombotic event. None of the subjects were on full anticoagulation at the time of the thrombotic event; low-dose heparin or aspirin was in use in four out of five subjects when the thrombotic event was discovered. There are theoretical risks of low-dose heparin, including heparin-induced thrombocytopenia, and aspirin, such as increased inflammation, myocyte necrosis, and mortality as seen in animal myocarditis studies 21. The retrospective nature and small sample size of our study precludes firm conclusions about safety or efficacy of low-dose heparin, aspirin, or full anticoagulation in this patient population.
This study did not have adequate power to determine whether full anticoagulation would decrease the risk of thrombotic events in critically ill children with myocarditis or dilated cardiomyopathy at the time of cardiac intensive care unit admission. From the ventricular assist device literature, in which subjects are commonly on aggressive antithrombotic regimens, inflammation with or without evidence of infection has been implicated as a risk factor for stroke in some single center adult ventricular assist device series.22 However, pediatric and larger adult ventricular assist device studies have not confirmed this relationship.23, 24 Our study likewise does not implicate inflammation alone as a risk factor to prompt consideration for full anticoagulation. A large, randomized controlled trial of full anticoagulation versus placebo or low-dose heparin is necessary to fully evaluate the role of inflammation as a thrombotic risk in pediatric myocarditis. Short of a clinical trial, a larger multicenter investigation of children with myocarditis and dilated cardiomyopathy to confirm our observed incidence of thrombotic events would be valuable for future, non-randomized efficacy studies in order to understand more about the risk factors for clinically significant thromboembolic events.
We were unable to identify any risk factors for thrombotic events in the myocarditis, dilated cardiomyopathy, or the combined cohort, although this is likely because we were underpowered to detect a difference. Interestingly, there was not a higher rate of thrombotic events in the myocarditis group despite a significantly higher rate of intravenous immune globulin administration (Table 1). There was a significantly shorter time to initiation of anticoagulation therapies in myocarditis subjects compared with dilated cardiomyopathy subjects for those in whom full anticoagulation was used (Table 2), which may have mitigated the theoretically pro-thrombotic effect of intravenous immune globulin. The retrospective nature and small sample size of our study precluded a systematic review of several other potential risk factors. For example, when attempting to document presence or absence of a family history of thrombotic events, we found that information in the medical chart was often missing. While all potential variations in clinical care between cohort groups cannot be fully determined from a retrospective study, the cohorts were clinically managed by a small cadre of cardiac intensivists who employed a relatively consistent diagnostic and management style for these subjects.
There were several cases in our cohort that did not meet our prespecified criteria for thrombotic events but nonetheless deserve mention. The first was a 13 year old boy with biopsy-proven myocarditis who clinically recovered and was discharged home on hospital day #10. Upon readmission 17 days later for recurrent heart failure symptoms, he was not on any anticoagulant medications and was found to have biventricular thrombi. He ultimately died due to complications after heart transplantation. In the dilated cardiomyopathy group, there were four additional cases of thrombotic events that occurred in the setting of mechanical circulatory support: three subjects experienced left ventricular assist device-related thrombi (two subjects developed evidence of embolic arterial stroke, one had evidence of splenic and renal infarcts; one died), and one subject on extracorporeal membrane oxygenation had an embolic arterial stroke and evidence of embolism to the spleen and kidneys. The added thrombotic risks when on mechanical circulatory support are well-documented 25–27 and difficult to differentiate from the risk for thrombus formation imparted by the underlying cardiomyopathy.
In critically ill children with heart failure due to either myocarditis or dilated cardiomyopathy, thrombotic events are common and potentially preventable. Current published guidelines recommend antithrombotic prophylaxis in children with comparable risk profiles. As the consequences of a thrombotic event can be catastrophic, resulting in death or significant neurological impairment from stroke, a strategy of antithrombotic prophylaxis should be considered in this population as well. The most effective medication regimen with the lowest risk/benefit ratio for this population remains to be determined. There was no significant difference in our small cohort in thrombotic events between inflammatory and non-inflammatory cardiomyopathy groups, suggesting that the decision to use antithrombotic prophylaxis should not be based solely upon the underlying etiology of the acute decompensated heart failure.
Acknowledgments
The authors wish to thank Drs. Jean Ballweg, Sarah Tabbutt, and Gil Wernovsky for maintaining the Cardiac Intensive Care Unit database at the Children’s Hospital of Philadelphia during the study period.
Funding Sources
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. The authors report the following sources of support for the time they put forward to this and other related research: Hemostasis and Thrombosis Research Society Mentored Research Award (C.W., NIH-K23-HL107455); L. Morton Morley Funds of the Philadelphia Foundation (L.B., NIH-K12-NS049453, NIH-T32-NS007413); June and Steve Wolfson Family Fund for Neurological Research (D.L., R01-NS072338); Other NIH support (R.I., NIH-R01-NS050488, NIH-K23-NS062110).
Footnotes
Potential Conflicts of Interest/Disclosures
R.N. Ichord: Consultant/Advisory Board, Berlin Heart Clinical Event Committee.
References
- 1.Gunthard J, Stocker F, Bolz D, et al. Dilated cardiomyopathy and thrombo-embolism. Eur J Pediatr. 1997;156:3–6. doi: 10.1007/s004310050541. [DOI] [PubMed] [Google Scholar]
- 2.Choi SH, Jeong SI, Yang JH, et al. A single-center experience with intracardiac thrombosis in children with dilated cardiomyopathy. Pediatr Cardiol. 2010;31:264–269. doi: 10.1007/s00246-009-9602-3. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn B, Shapiro ED, Walls TA, Friedman AH. Predictors of outcome of myocarditis. Pediatr Cardiol. 2004;25:379–384. doi: 10.1007/s00246-003-0568-2. [DOI] [PubMed] [Google Scholar]
- 4.Ramanan AS, Pandit N, Yashwant M, Srinivas A. Embolic stroke in myocarditis. Indian Pediatr. 1993;30:531–533. [PubMed] [Google Scholar]
- 5.Antoniak S, Boltzen U, Riad A, et al. Viral myocarditis and coagulopathy: increased tissue factor expression and plasma thrombogenicity. J Mol Cell Cardiol. 2008;45:118–126. doi: 10.1016/j.yjmcc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Loh E, Sutton MS, Wun CC, et al. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med. 1997;336:251–257. doi: 10.1056/NEJM199701233360403. [DOI] [PubMed] [Google Scholar]
- 7.Thompson PL, Robinson JS. Stroke after acute myocardial infarction: relation to infarct size. Br Med J. 1978;2:457–459. doi: 10.1136/bmj.2.6135.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grenier MA, Osganian SK, Cox GF, et al. Design and implementation of the North American Pediatric Cardiomyopathy Registry. Am Heart J. 2000;139:S86–95. doi: 10.1067/mhj.2000.103933. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, Olivotto I, Bellone P, et al. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;39:301–307. doi: 10.1016/s0735-1097(01)01727-2. [DOI] [PubMed] [Google Scholar]
- 10.Finsterer J. Left ventricular non-compaction and its cardiac and neurologic implications. Heart Fail Rev. 2010;15:589–603. doi: 10.1007/s10741-010-9175-5. [DOI] [PubMed] [Google Scholar]
- 11.Arslan S, Sevimli S, Gundogdu F. Fatal biatrial thrombus in a patient with idiopathic restrictive cardiomyopathy during sinus rhythm. Int J Cardiol. 2007;117:e68–70. doi: 10.1016/j.ijcard.2006.11.141. [DOI] [PubMed] [Google Scholar]
- 12.Foerster SR, Canter CE, Cinar A, et al. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: an outcomes study from the Pediatric Cardiomyopathy Registry. Circ Heart Fail. 2010;3:689–697. doi: 10.1161/CIRCHEARTFAILURE.109.902833. [DOI] [PubMed] [Google Scholar]
- 13.Grogan M, Redfield MM, Bailey KR, et al. Long-term outcome of patients with biopsy-proved myocarditis: comparison with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1995;26:80–84. doi: 10.1016/0735-1097(95)00148-s. [DOI] [PubMed] [Google Scholar]
- 14.Swain SK, Dharmapuram AK, Reddy P, Ramdoss N, Raghavan SS, Kona SM. Neonatal Blalock-Taussig shunt: technical aspects and postoperative management. Asian Cardiovasc Thorac Ann. 2008;16:7–10. doi: 10.1177/021849230801600103. [DOI] [PubMed] [Google Scholar]
- 15.Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol. 1993;22:1004–1009. doi: 10.1016/0735-1097(93)90409-t. [DOI] [PubMed] [Google Scholar]
- 16.Massie BM, Collins JF, Ammon SE, et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119:1616–1624. doi: 10.1161/CIRCULATIONAHA.108.801753. [DOI] [PubMed] [Google Scholar]
- 17.Homma S, Thompson JL, Pullicino PM, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e637S–668S. doi: 10.1378/chest.11-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e737S–801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monagle P, Cochrane A, McCrindle B, Benson L, Williams W, Andrew M. Thromboembolic complications after fontan procedures--the role of prophylactic anticoagulation. J Thorac Cardiovasc Surg. 1998;115:493–498. doi: 10.1016/s0022-5223(98)70310-1. [DOI] [PubMed] [Google Scholar]
- 21.Meune C, Spaulding C, Mahe I, Lebon P, Bergmann JF. Risks versus benefits of NSAIDs including aspirin in myocarditis: a review of the evidence from animal studies. Drug Saf. 2003;26:975–981. doi: 10.2165/00002018-200326130-00005. [DOI] [PubMed] [Google Scholar]
- 22.Tsukui H, Abla A, Teuteberg JJ, et al. Cerebrovascular accidents in patients with a ventricular assist device. J Thorac Cardiovasc Surg. 2007;134:114–123. doi: 10.1016/j.jtcvs.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 23.Polito A, Netto R, Soldati M, et al. Neurological Complications During Pulsatile Ventricular Assistance With the Berlin Heart EXCOR in Children: Incidence and Risk Factors. Artif Organs. 2013 doi: 10.1111/aor.12075. [DOI] [PubMed] [Google Scholar]
- 24.Lazar RM, Shapiro PA, Jaski BE, et al. Neurological events during long-term mechanical circulatory support for heart failure: the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) experience. Circulation. 2004;109:2423–2427. doi: 10.1161/01.CIR.0000129414.95137.CD. [DOI] [PubMed] [Google Scholar]
- 25.Reinhartz O, Keith FM, El-Banayosy A, et al. Multicenter experience with the thoratec ventricular assist device in children and adolescents. J Heart Lung Transplant. 2001;20:439–448. doi: 10.1016/s1053-2498(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 26.Blume ED, Naftel DC, Bastardi HJ, Duncan BW, Kirklin JK, Webber SA. Outcomes of children bridged to heart transplantation with ventricular assist devices: a multi-institutional study. Circulation. 2006;113:2313–2319. doi: 10.1161/CIRCULATIONAHA.105.577601. [DOI] [PubMed] [Google Scholar]
- 27.Duncan BW, Hraska V, Jonas RA, et al. Mechanical circulatory support in children with cardiac disease. J Thorac Cardiovasc Surg. 1999;117:529–542. doi: 10.1016/s0022-5223(99)70333-8. [DOI] [PubMed] [Google Scholar]


