Abstract
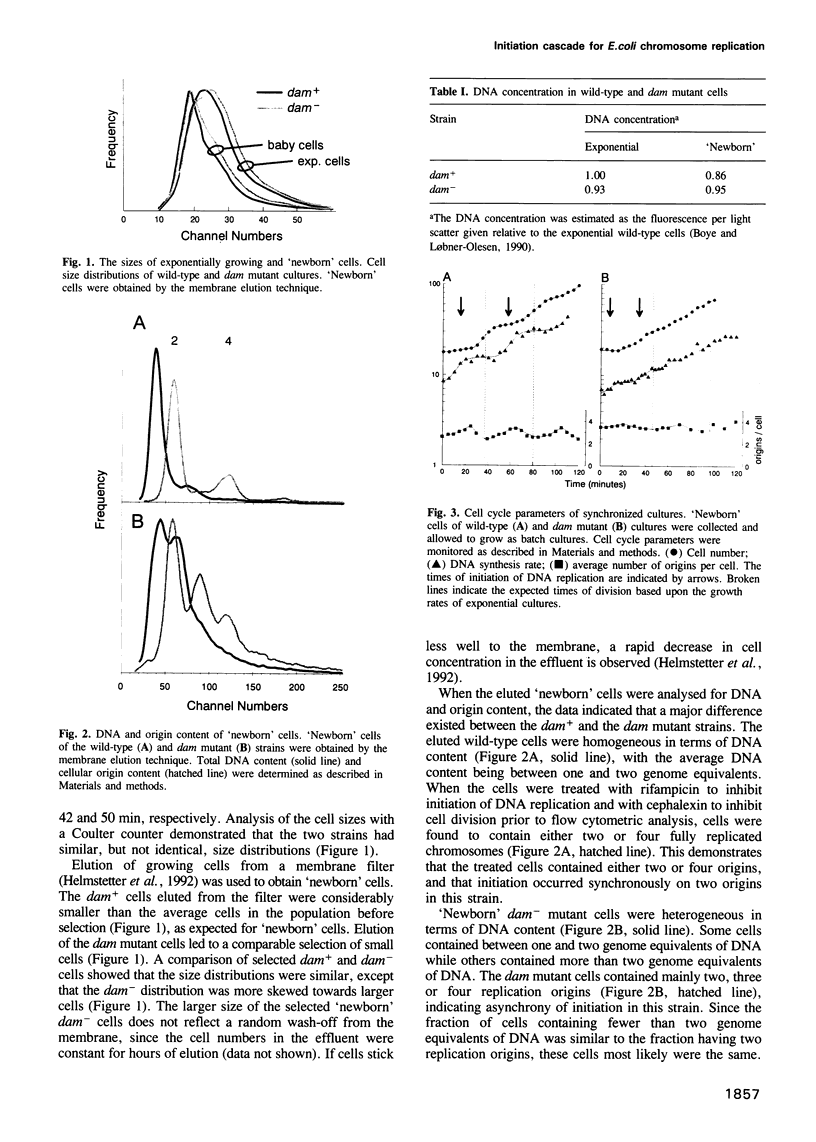
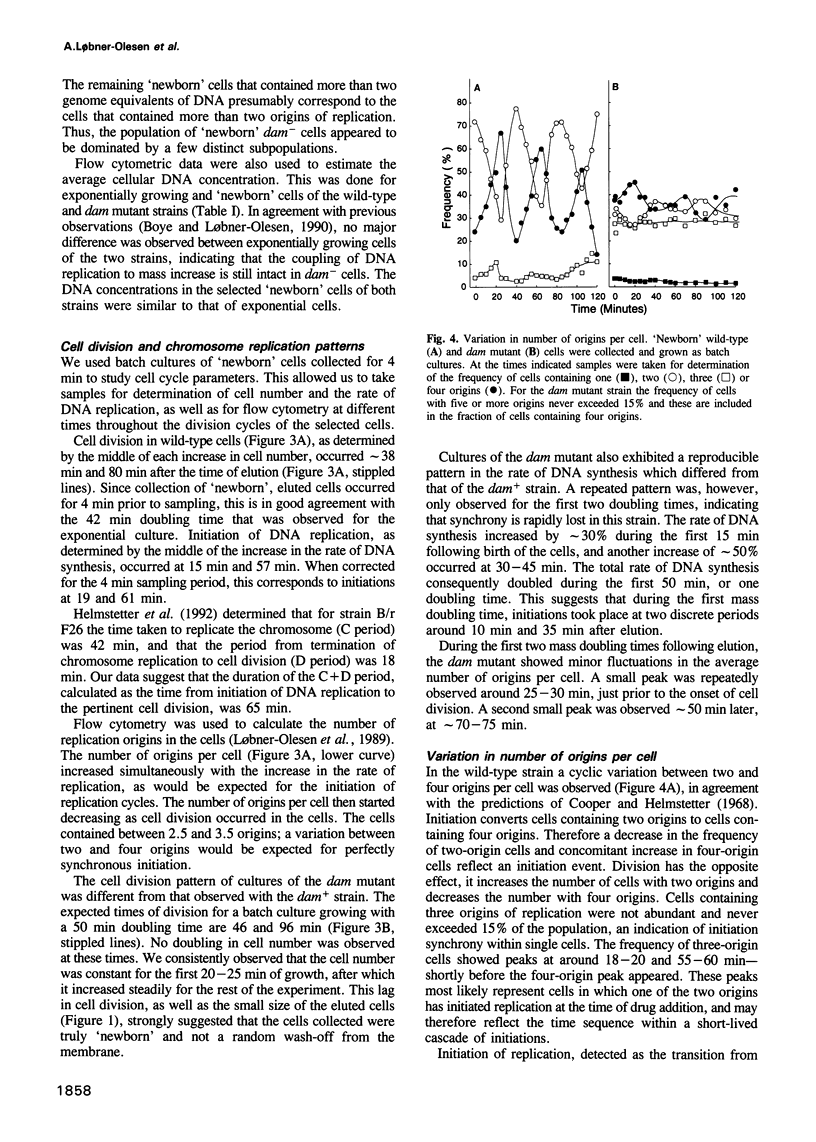
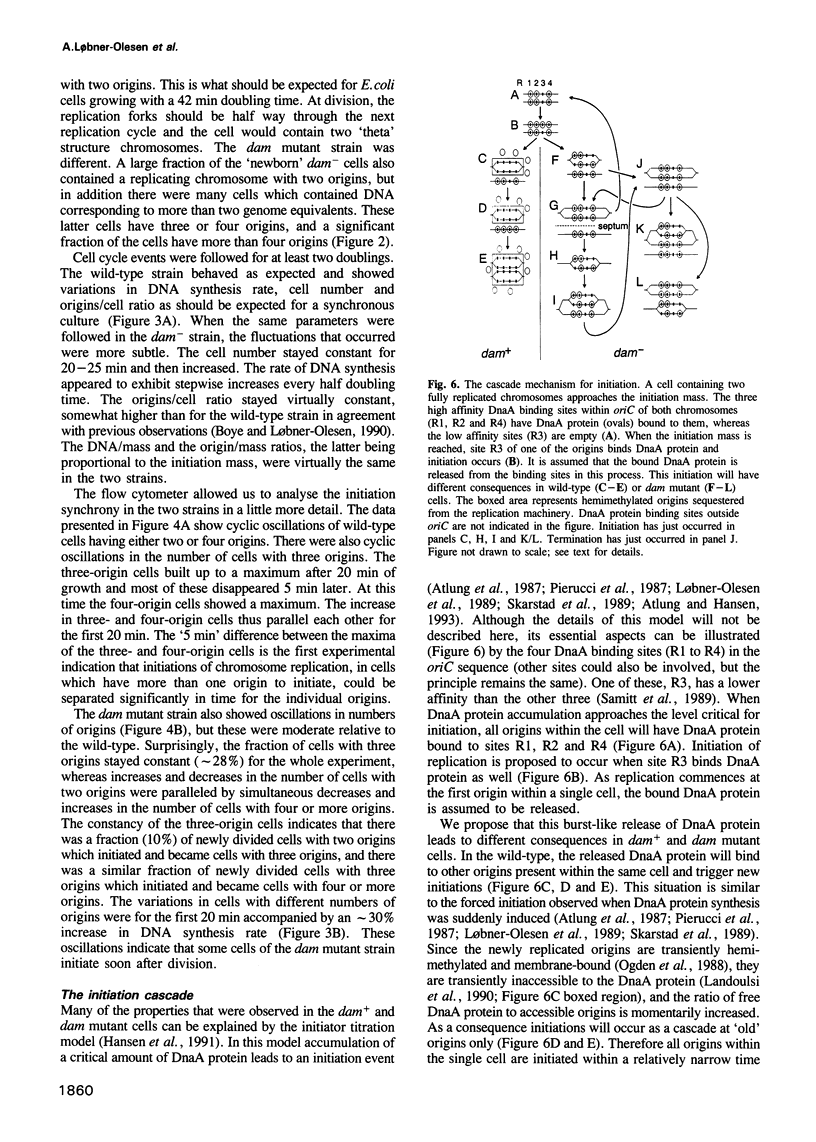
'Newborn' Escherichia coli B/r cells, obtained by membrane elution, were used to study the cell cycles of wild-type and Dam methyltransferase mutants. In wild-type cells, initiation of chromosome replication was synchronous and tightly controlled. In dam mutants, initiation was altered, but not random. We propose that this is due to the absence of an initiation cascade caused by liberated DnaA molecules, and that this cascade normally synchronizes initiation. The dam- cells contained mainly two, three or four replication origins, and this affected nucleoid partitioning as well as cell division. In cultures growing with a 50 min doubling time, a variety of cell cycles were present and half the origins were used every 25 min. Some cells had a 25 min interdivision time, whereas others had an interdivision time longer than the generation time. Partitioning of nucleoids containing unequal numbers of replication origins could also be readily observed by fluorescence microscopy in the dam mutant. Based upon these observations we propose that the dam mutant is also an initiation cascade mutant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlung T., Hansen F. G. Three distinct chromosome replication states are induced by increasing concentrations of DnaA protein in Escherichia coli. J Bacteriol. 1993 Oct;175(20):6537–6545. doi: 10.1128/jb.175.20.6537-6545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlung T., Løbner-Olesen A., Hansen F. G. Overproduction of DnaA protein stimulates initiation of chromosome and minichromosome replication in Escherichia coli. Mol Gen Genet. 1987 Jan;206(1):51–59. doi: 10.1007/BF00326535. [DOI] [PubMed] [Google Scholar]
- Bakker A., Smith D. W. Methylation of GATC sites is required for precise timing between rounds of DNA replication in Escherichia coli. J Bacteriol. 1989 Oct;171(10):5738–5742. doi: 10.1128/jb.171.10.5738-5742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye E., Løbner-Olesen A. Bacterial growth control studied by flow cytometry. Res Microbiol. 1991 Feb-Apr;142(2-3):131–135. doi: 10.1016/0923-2508(91)90020-b. [DOI] [PubMed] [Google Scholar]
- Boye E., Løbner-Olesen A., Skarstad K. Timing of chromosomal replication in Escherichia coli. Biochim Biophys Acta. 1988 Dec 20;951(2-3):359–364. doi: 10.1016/0167-4781(88)90107-8. [DOI] [PubMed] [Google Scholar]
- Boye E., Løbner-Olesen A. The role of dam methyltransferase in the control of DNA replication in E. coli. Cell. 1990 Sep 7;62(5):981–989. doi: 10.1016/0092-8674(90)90272-g. [DOI] [PubMed] [Google Scholar]
- Boye E. The hemimethylated replication origin of Escherichia coli can be initiated in vitro. J Bacteriol. 1991 Jul;173(14):4537–4539. doi: 10.1128/jb.173.14.4537-4539.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell. 1990 Sep 7;62(5):967–979. doi: 10.1016/0092-8674(90)90271-f. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Chromosome partition in Escherichia coli requires postreplication protein synthesis. J Bacteriol. 1989 Oct;171(10):5405–5409. doi: 10.1128/jb.171.10.5405-5409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968 Sep 7;219(5158):1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., Christensen B. B., Atlung T. The initiator titration model: computer simulation of chromosome and minichromosome control. Res Microbiol. 1991 Feb-Apr;142(2-3):161–167. doi: 10.1016/0923-2508(91)90025-6. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., Koefoed S., Sørensen L., Atlung T. Titration of DnaA protein by oriC DnaA-boxes increases dnaA gene expression in Escherichia coli. EMBO J. 1987 Jan;6(1):255–258. doi: 10.1002/j.1460-2075.1987.tb04747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E., Eenhuis C., Theisen P., Grimwade J., Leonard A. C. Improved bacterial baby machine: application to Escherichia coli K-12. J Bacteriol. 1992 Jun;174(11):3445–3449. doi: 10.1128/jb.174.11.3445-3449.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Niki H., Ogura T., Ichinose C., Mori H., Ezaki B., Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989 Mar;171(3):1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landoulsi A., Hughes P., Kern R., Kohiyama M. dam methylation and the initiation of DNA replication on oriC plasmids. Mol Gen Genet. 1989 Apr;216(2-3):217–223. doi: 10.1007/BF00334359. [DOI] [PubMed] [Google Scholar]
- Landoulsi A., Malki A., Kern R., Kohiyama M., Hughes P. The E. coli cell surface specifically prevents the initiation of DNA replication at oriC on hemimethylated DNA templates. Cell. 1990 Nov 30;63(5):1053–1060. doi: 10.1016/0092-8674(90)90508-c. [DOI] [PubMed] [Google Scholar]
- Løbner-Olesen A., Skarstad K., Hansen F. G., von Meyenburg K., Boye E. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell. 1989 Jun 2;57(5):881–889. doi: 10.1016/0092-8674(89)90802-7. [DOI] [PubMed] [Google Scholar]
- Ogden G. B., Pratt M. J., Schaechter M. The replicative origin of the E. coli chromosome binds to cell membranes only when hemimethylated. Cell. 1988 Jul 1;54(1):127–135. doi: 10.1016/0092-8674(88)90186-9. [DOI] [PubMed] [Google Scholar]
- Parker B., Marinus M. G. A simple and rapid method to obtain substitution mutations in Escherichia coli: isolation of a dam deletion/insertion mutation. Gene. 1988 Dec 20;73(2):531–535. doi: 10.1016/0378-1119(88)90517-3. [DOI] [PubMed] [Google Scholar]
- Pierucci O., Helmstetter C. E., Rickert M., Weinberger M., Leonard A. C. Overexpression of the dnaA gene in Escherichia coli B/r: chromosome and minichromosome replication in the presence of rifampin. J Bacteriol. 1987 May;169(5):1871–1877. doi: 10.1128/jb.169.5.1871-1877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Zinder N. D. Hemimethylation prevents DNA replication in E. coli. Cell. 1987 Sep 25;50(7):1071–1079. doi: 10.1016/0092-8674(87)90173-5. [DOI] [PubMed] [Google Scholar]
- Samitt C. E., Hansen F. G., Miller J. F., Schaechter M. In vivo studies of DnaA binding to the origin of replication of Escherichia coli. EMBO J. 1989 Mar;8(3):989–993. doi: 10.1002/j.1460-2075.1989.tb03462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Boye E. Degradation of individual chromosomes in recA mutants of Escherichia coli. J Bacteriol. 1993 Sep;175(17):5505–5509. doi: 10.1128/jb.175.17.5505-5509.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Boye E., Steen H. B. Timing of initiation of chromosome replication in individual Escherichia coli cells. EMBO J. 1986 Jul;5(7):1711–1717. doi: 10.1002/j.1460-2075.1986.tb04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Løbner-Olesen A., Atlung T., von Meyenburg K., Boye E. Initiation of DNA replication in Escherichia coli after overproduction of the DnaA protein. Mol Gen Genet. 1989 Jul;218(1):50–56. doi: 10.1007/BF00330564. [DOI] [PubMed] [Google Scholar]
- Skarstad K., Steen H. B., Boye E. Escherichia coli DNA distributions measured by flow cytometry and compared with theoretical computer simulations. J Bacteriol. 1985 Aug;163(2):661–668. doi: 10.1128/jb.163.2.661-668.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., von Meyenburg K., Hansen F. G., Boye E. Coordination of chromosome replication initiation in Escherichia coli: effects of different dnaA alleles. J Bacteriol. 1988 Feb;170(2):852–858. doi: 10.1128/jb.170.2.852-858.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. S., D'Ari R. Genetic and morphological characterization of an Escherichia coli chromosome segregation mutant. J Bacteriol. 1992 Jul;174(13):4513–4516. doi: 10.1128/jb.174.13.4513-4516.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinella D., Jaffé A., D'Ari R., Kohiyama M., Hughes P. Chromosome partitioning in Escherichia coli in the absence of dam-directed methylation. J Bacteriol. 1992 Apr;174(7):2388–2390. doi: 10.1128/jb.174.7.2388-2390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., Mulder E., Huls P. G., Vischer N. Toporegulation of bacterial division according to the nucleoid occlusion model. Res Microbiol. 1991 Feb-Apr;142(2-3):309–320. doi: 10.1016/0923-2508(91)90046-d. [DOI] [PubMed] [Google Scholar]



