Abstract
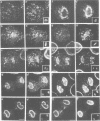
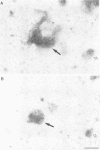
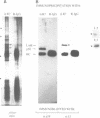
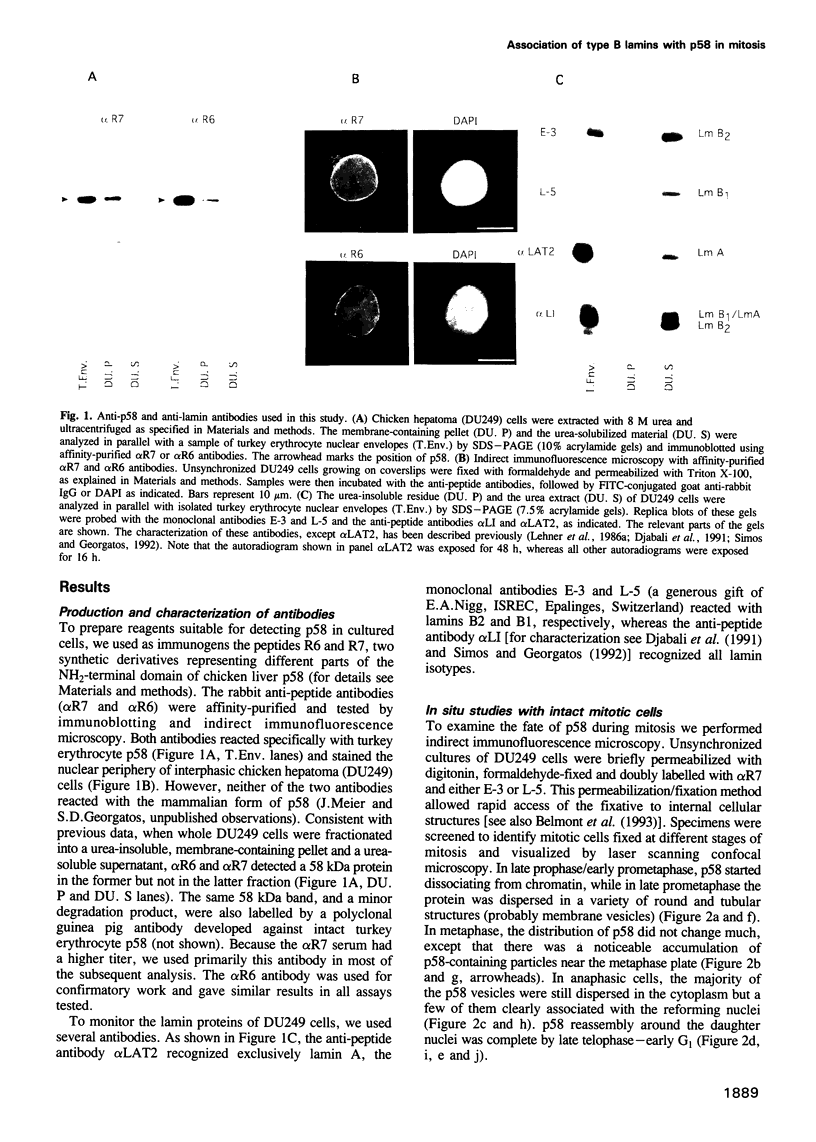
p58 (also referred to as the lamin B receptor) is an integral membrane protein of the nuclear envelope known to form a multimeric complex with the lamins and other nuclear proteins during interphase. To examine the fate of this complex during mitosis, we have investigated the partitioning and the molecular interactions of p58 in dividing chicken hepatoma (DU249) cells. Using confocal microscopy and double immunolabelling, we show here that lamins B1 and B2 co-localize with p58 during all phases of mitosis and co-assemble around reforming nuclei. A close juxtaposition of p58/lamin B-containing vesicles and chromosomes is already detectable in metaphase; however, p58 and lamin reassembly proceeds slowly and is completed in late telophase--G1. Flotation of mitotic membranes in sucrose density gradients and analysis of mitotic vesicles by immunoelectron microscopy confirms that p58 and most of the type B lamins reside in the same compartment. Co-immunoprecipitation of both proteins by affinity-purified anti-p58 antibodies shows that they are physically associated in the context of a mitotic p58 'sub-complex'. This sub-assembly does not include the type A lamins which are fully solubilized during mitosis. Our data provide direct, in vivo and in vitro evidence that the majority of type B lamins remain connected to nuclear membrane 'receptors' during mitosis. The implications of these findings in nuclear envelope reassembly are discussed below.
Full text
PDF
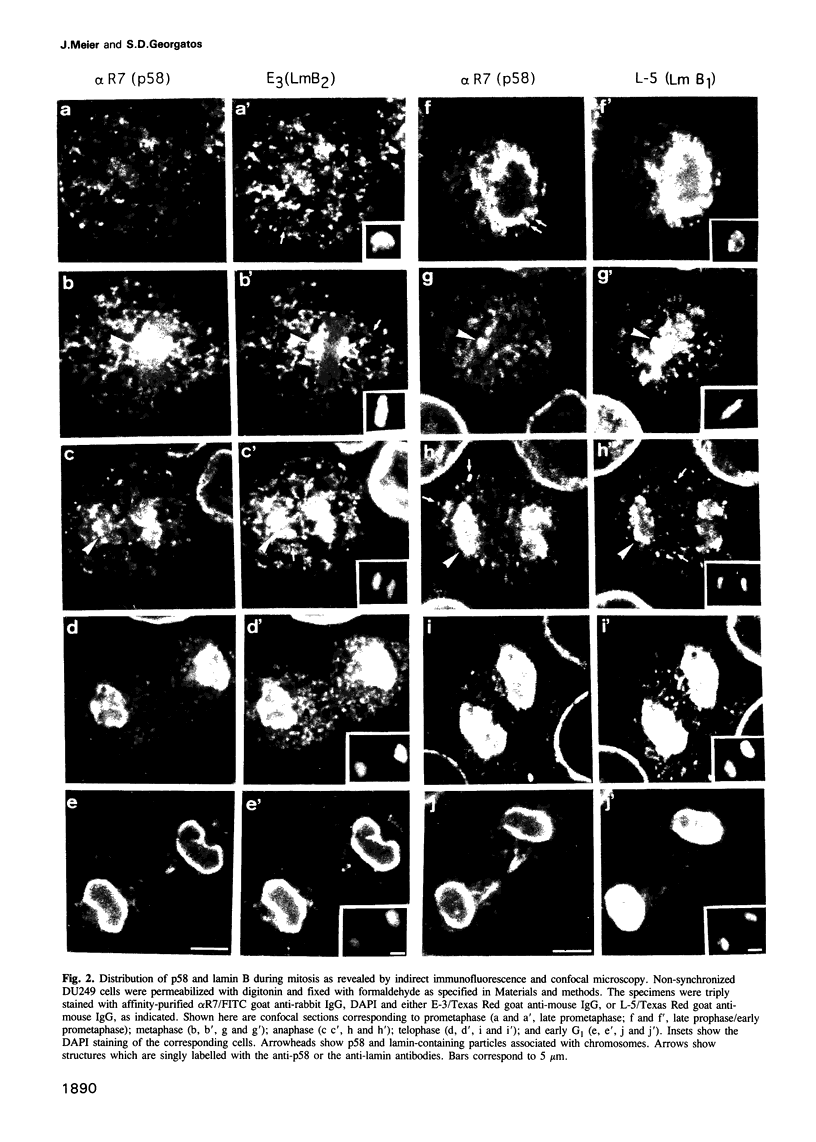
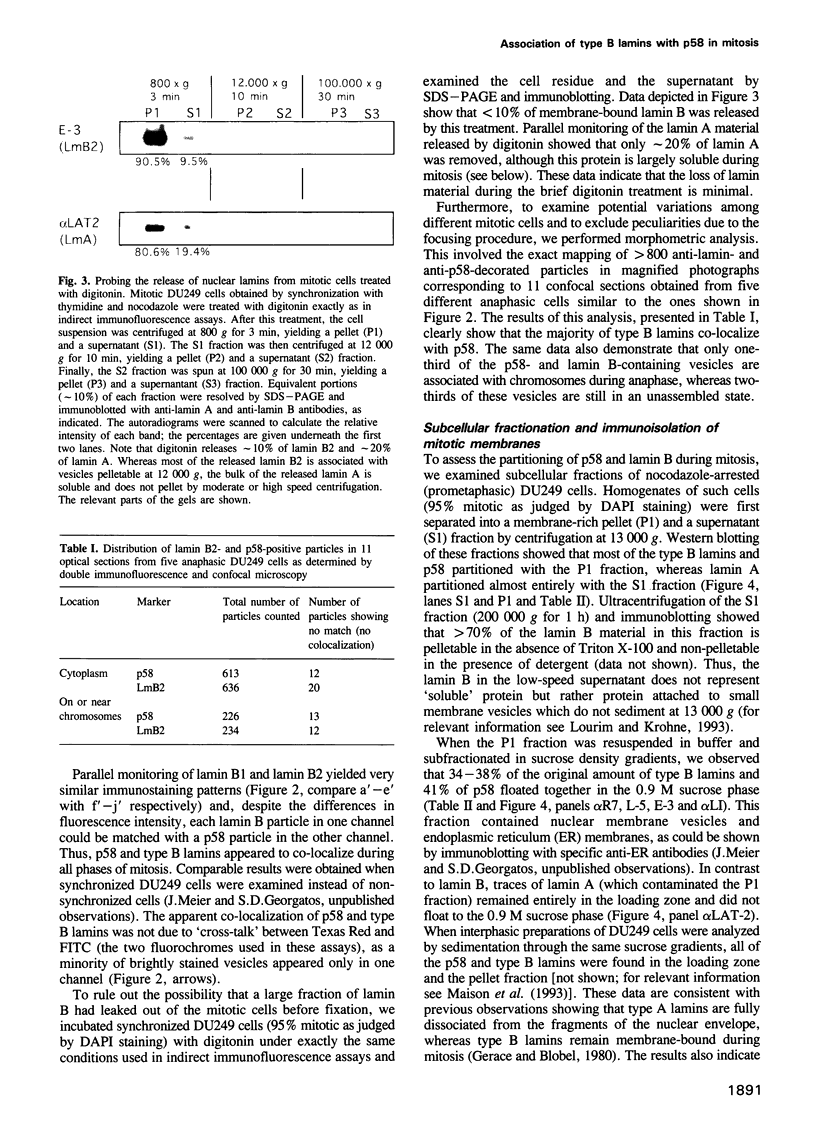







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum J., Blobel G., Georgatos S. D. In vivo phosphorylation of the lamin B receptor. Binding of lamin B to its nuclear membrane receptor is affected by phosphorylation. J Biol Chem. 1990 Mar 15;265(8):4181–4184. [PubMed] [Google Scholar]
- Bailer S. M., Eppenberger H. M., Griffiths G., Nigg E. A. Characterization of A 54-kD protein of the inner nuclear membrane: evidence for cell cycle-dependent interaction with the nuclear lamina. J Cell Biol. 1991 Aug;114(3):389–400. doi: 10.1083/jcb.114.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont A. S., Zhai Y., Thilenius A. Lamin B distribution and association with peripheral chromatin revealed by optical sectioning and electron microscopy tomography. J Cell Biol. 1993 Dec;123(6 Pt 2):1671–1685. doi: 10.1083/jcb.123.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Calakos N., Kreiner T., Scheller R. H. Synaptic vesicle membrane proteins interact to form a multimeric complex. J Cell Biol. 1992 Feb;116(3):761–775. doi: 10.1083/jcb.116.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Gerace L. A cell free system to study reassembly of the nuclear envelope at the end of mitosis. Cell. 1986 Feb 28;44(4):639–652. doi: 10.1016/0092-8674(86)90273-4. [DOI] [PubMed] [Google Scholar]
- Chaudhary N., Courvalin J. C. Stepwise reassembly of the nuclear envelope at the end of mitosis. J Cell Biol. 1993 Jul;122(2):295–306. doi: 10.1083/jcb.122.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin J. C., Segil N., Blobel G., Worman H. J. The lamin B receptor of the inner nuclear membrane undergoes mitosis-specific phosphorylation and is a substrate for p34cdc2-type protein kinase. J Biol Chem. 1992 Sep 25;267(27):19035–19038. [PubMed] [Google Scholar]
- Dabauvalle M. C., Loos K., Merkert H., Scheer U. Spontaneous assembly of pore complex-containing membranes ("annulate lamellae") in Xenopus egg extract in the absence of chromatin. J Cell Biol. 1991 Mar;112(6):1073–1082. doi: 10.1083/jcb.112.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djabali K., Portier M. M., Gros F., Blobel G., Georgatos S. D. Network antibodies identify nuclear lamin B as a physiological attachment site for peripherin intermediate filaments. Cell. 1991 Jan 11;64(1):109–121. doi: 10.1016/0092-8674(91)90213-i. [DOI] [PubMed] [Google Scholar]
- Feitelson M. A., Millman I., Blumberg B. S. Tree squirrel hepatitis B virus: antigenic and structural characterization. Proc Natl Acad Sci U S A. 1986 May;83(9):2994–2997. doi: 10.1073/pnas.83.9.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Foisner R., Gerace L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell. 1993 Jul 2;73(7):1267–1279. doi: 10.1016/0092-8674(93)90355-t. [DOI] [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Two distinct attachment sites for vimentin along the plasma membrane and the nuclear envelope in avian erythrocytes: a basis for a vectorial assembly of intermediate filaments. J Cell Biol. 1987 Jul;105(1):105–115. doi: 10.1083/jcb.105.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Maroulakou I., Blobel G. Lamin A, lamin B, and lamin B receptor analogues in yeast. J Cell Biol. 1989 Jun;108(6):2069–2082. doi: 10.1083/jcb.108.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Stournaras C., Blobel G. Heterotypic and homotypic associations between the nuclear lamins: site-specificity and control by phosphorylation. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4325–4329. doi: 10.1073/pnas.85.12.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerace L., Comeau C., Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. doi: 10.1242/jcs.1984.supplement_1.10. [DOI] [PubMed] [Google Scholar]
- Höger T. H., Krohne G., Kleinschmidt J. A. Interaction of Xenopus lamins A and LII with chromatin in vitro mediated by a sequence element in the carboxyterminal domain. Exp Cell Res. 1991 Dec;197(2):280–289. doi: 10.1016/0014-4827(91)90434-v. [DOI] [PubMed] [Google Scholar]
- Kitten G. T., Nigg E. A. The CaaX motif is required for isoprenylation, carboxyl methylation, and nuclear membrane association of lamin B2. J Cell Biol. 1991 Apr;113(1):13–23. doi: 10.1083/jcb.113.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouklis P. D., Merdes A., Papamarcaki T., Georgatos S. D. Transient arrest of 3T3 cells in mitosis and inhibition of nuclear lamin reassembly around chromatin induced by anti-vimentin antibodies. Eur J Cell Biol. 1993 Dec;62(2):224–236. [PubMed] [Google Scholar]
- Krohne G., Waizenegger I., Höger T. H. The conserved carboxy-terminal cysteine of nuclear lamins is essential for lamin association with the nuclear envelope. J Cell Biol. 1989 Nov;109(5):2003–2011. doi: 10.1083/jcb.109.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G., Wolin S. L., McKeon F. D., Franke W. W., Kirschner M. W. Nuclear lamin LI of Xenopus laevis: cDNA cloning, amino acid sequence and binding specificity of a member of the lamin B subfamily. EMBO J. 1987 Dec 1;6(12):3801–3808. doi: 10.1002/j.1460-2075.1987.tb02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langlois A. J., Lapis K., Ishizaki R., Beard J. W., Bolognesi D. P. Isolation of a transplantable cell line induced by the MC29 avian leukosis virus. Cancer Res. 1974 Jun;34(6):1457–1464. [PubMed] [Google Scholar]
- Lehner C. F., Fürstenberger G., Eppenberger H. M., Nigg E. A. Biogenesis of the nuclear lamina: in vivo synthesis and processing of nuclear protein precursors. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2096–2099. doi: 10.1073/pnas.83.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., Kurer V., Eppenberger H. M., Nigg E. A. The nuclear lamin protein family in higher vertebrates. Identification of quantitatively minor lamin proteins by monoclonal antibodies. J Biol Chem. 1986 Oct 5;261(28):13293–13301. [PubMed] [Google Scholar]
- Lehner C. F., Stick R., Eppenberger H. M., Nigg E. A. Differential expression of nuclear lamin proteins during chicken development. J Cell Biol. 1987 Jul;105(1):577–587. doi: 10.1083/jcb.105.1.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourim D., Krohne G. Membrane-associated lamins in Xenopus egg extracts: identification of two vesicle populations. J Cell Biol. 1993 Nov;123(3):501–512. doi: 10.1083/jcb.123.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison C., Horstmann H., Georgatos S. D. Regulated docking of nuclear membrane vesicles to vimentin filaments during mitosis. J Cell Biol. 1993 Dec;123(6 Pt 1):1491–1505. doi: 10.1083/jcb.123.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J., Campbell K. H., Ford C. C., Stick R., Hutchison C. J. The role of lamin LIII in nuclear assembly and DNA replication, in cell-free extracts of Xenopus eggs. J Cell Sci. 1991 Mar;98(Pt 3):271–279. doi: 10.1242/jcs.98.3.271. [DOI] [PubMed] [Google Scholar]
- Morein B., Simons K. Subunit vaccines against enveloped viruses: virosomes, micelles and other protein complexes. Vaccine. 1985 Jun;3(2):83–93. doi: 10.1016/0264-410x(85)90055-6. [DOI] [PubMed] [Google Scholar]
- Nakagawa J., Kitten G. T., Nigg E. A. A somatic cell-derived system for studying both early and late mitotic events in vitro. J Cell Sci. 1989 Nov;94(Pt 3):449–462. doi: 10.1242/jcs.94.3.449. [DOI] [PubMed] [Google Scholar]
- Newport J. W., Wilson K. L., Dunphy W. G. A lamin-independent pathway for nuclear envelope assembly. J Cell Biol. 1990 Dec;111(6 Pt 1):2247–2259. doi: 10.1083/jcb.111.6.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A. Assembly-disassembly of the nuclear lamina. Curr Opin Cell Biol. 1992 Feb;4(1):105–109. doi: 10.1016/0955-0674(92)90066-l. [DOI] [PubMed] [Google Scholar]
- Peter M., Kitten G. T., Lehner C. F., Vorburger K., Bailer S. M., Maridor G., Nigg E. A. Cloning and sequencing of cDNA clones encoding chicken lamins A and B1 and comparison of the primary structures of vertebrate A- and B-type lamins. J Mol Biol. 1989 Aug 5;208(3):393–404. doi: 10.1016/0022-2836(89)90504-4. [DOI] [PubMed] [Google Scholar]
- Powell L., Burke B. Internuclear exchange of an inner nuclear membrane protein (p55) in heterokaryons: in vivo evidence for the interaction of p55 with the nuclear lamina. J Cell Biol. 1990 Dec;111(6 Pt 1):2225–2234. doi: 10.1083/jcb.111.6.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röber R. A., Weber K., Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989 Feb;105(2):365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- Senior A., Gerace L. Integral membrane proteins specific to the inner nuclear membrane and associated with the nuclear lamina. J Cell Biol. 1988 Dec;107(6 Pt 1):2029–2036. doi: 10.1083/jcb.107.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimanuki M., Goebl M., Yanagida M., Toda T. Fission yeast sts1+ gene encodes a protein similar to the chicken lamin B receptor and is implicated in pleiotropic drug-sensitivity, divalent cation-sensitivity, and osmoregulation. Mol Biol Cell. 1992 Mar;3(3):263–273. doi: 10.1091/mbc.3.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos G., Georgatos S. D. The inner nuclear membrane protein p58 associates in vivo with a p58 kinase and the nuclear lamins. EMBO J. 1992 Nov;11(11):4027–4036. doi: 10.1002/j.1460-2075.1992.tb05496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Blobel G. The first membrane spanning region of the lamin B receptor is sufficient for sorting to the inner nuclear membrane. J Cell Biol. 1993 Feb;120(3):631–637. doi: 10.1083/jcb.120.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soullam B., Worman H. J. The amino-terminal domain of the lamin B receptor is a nuclear envelope targeting signal. J Cell Biol. 1993 Mar;120(5):1093–1100. doi: 10.1083/jcb.120.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C., Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987 Nov 6;51(3):383–392. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- Stick R., Angres B., Lehner C. F., Nigg E. A. The fates of chicken nuclear lamin proteins during mitosis: evidence for a reversible redistribution of lamin B2 between inner nuclear membrane and elements of the endoplasmic reticulum. J Cell Biol. 1988 Aug;107(2):397–406. doi: 10.1083/jcb.107.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur N., Harel A., Feinstein N., Gruenbaum Y. Lamin activity is essential for nuclear envelope assembly in a Drosophila embryo cell-free extract. J Cell Biol. 1992 Oct;119(1):17–25. doi: 10.1083/jcb.119.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. Membrane partitioning during cell division. Annu Rev Biochem. 1993;62:323–348. doi: 10.1146/annurev.bi.62.070193.001543. [DOI] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Traub P. Maturation of nuclear lamin A involves a specific carboxy-terminal trimming, which removes the polyisoprenylation site from the precursor; implications for the structure of the nuclear lamina. FEBS Lett. 1989 Nov 6;257(2):411–414. doi: 10.1016/0014-5793(89)81584-4. [DOI] [PubMed] [Google Scholar]
- Wilson K. L., Newport J. A trypsin-sensitive receptor on membrane vesicles is required for nuclear envelope formation in vitro. J Cell Biol. 1988 Jul;107(1):57–68. doi: 10.1083/jcb.107.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolda S. L., Glomset J. A. Evidence for modification of lamin B by a product of mevalonic acid. J Biol Chem. 1988 May 5;263(13):5997–6000. [PubMed] [Google Scholar]
- Worman H. J., Evans C. D., Blobel G. The lamin B receptor of the nuclear envelope inner membrane: a polytopic protein with eight potential transmembrane domains. J Cell Biol. 1990 Oct;111(4):1535–1542. doi: 10.1083/jcb.111.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman H. J., Lazaridis I., Georgatos S. D. Nuclear lamina heterogeneity in mammalian cells. Differential expression of the major lamins and variations in lamin B phosphorylation. J Biol Chem. 1988 Aug 25;263(24):12135–12141. [PubMed] [Google Scholar]
- Worman H. J., Yuan J., Blobel G., Georgatos S. D. A lamin B receptor in the nuclear envelope. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8531–8534. doi: 10.1073/pnas.85.22.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]