Abstract
We evaluated the efficacy of a peer-educator network intervention as a strategy to reduce HIV acquisition among injection drug users (IDUs) and their drug and/or sexual networks. A randomized controlled trial was conducted in St. Petersburg, Russia among IDU index participants and their risk network participants. Network units were randomized to the control or experimental intervention. Only the experimental index participants received training sessions to communicate risk reduction techniques to their network members. Analysis includes 76 index and 84 network participants who were HIV uninfected. The main outcome measure was HIV sero-conversion. The incidence rates in the control and experimental groups were 19.57 (95 % CI 10.74–35.65) and 7.76 (95 % CI 3.51–17.19) cases per 100 p/y, respectively. The IRR was 0.41 (95 % CI 0.15–1.08) without a statistically significant difference between the two groups (log rank test statistic X2 = 2.73, permutation p value = 0.16). Retention rate was 67 % with a third of the loss due to incarceration or death. The results show a promising trend that this strategy would be successful in reducing the acquisition of HIV among IDUs.
Keywords: Injection drug users, Russia, HIV prevention, Network
Introduction
HIV Epidemic in Russia
While the HIV-epidemic is stabilizing worldwide, the number of people living with HIV continues to grow in Eastern Europe and Central Asia. According to a UNAIDS 2011 report, there has been a 250 %increase in the number of people living with HIV in Eastern Europe and Central Asia in the past decade [1]. The estimated number of adults and children living with HIV in the region grew from 410,000 [95 %CI 340,000–490,000] in 2001 to 1.5 million [95 %CI 1.3–1.7 million] in 2010. Russia and the Ukraine account for nearly 90 % of the regional epidemic. Injecting drug use remains the leading cause of HIV infection in this region. St. Petersburg is the second largest city in Russia with a population of more than 4 million people with an estimated 30,000–83,000 injection drug users (IDUs) [2, 3]. HIV prevalence and incidence among IDUs in St. Petersburg has remained high; in 2003 HIV prevalence and incidence in IDUs were 30 % and 4.5 per 100 p/y respectively [4, 5]; in 2008 the incidence was 14 per 100 p/y [6]; and in 2010 the prevalence and incidence were 35 % and 7.2 per 100 p/y respectively [7].
This Russian population of IDUs is marginalized and criminalized. IDUs have limited access to syringes through needle exchange. Although syringes are legal at pharmacies, IDUs are often stigmatized and refused service [8, 9]. They also have limited access to World Health Organization (WHO) recommended effective drug treatment [10], due in part to the laws that have criminalized drug replacement therapies such as methadone maintenance. HIV treatment, including antiretroviral therapy for IDUs is also difficult to obtain in many areas of the country [11].
IDUs in Russia tend to interact in small networks where members buy and use drugs communally. The dynamics of these small networks, including their composition, risky sexual practices, injection related risk behaviors, and network turnover has been hypothesized to be important drivers for the epidemic in Russia [8, 12]. Thus, interventions within these risk networks may be an efficient venue to diffuse behavior change in injection and sexual practices with the objective to reduce HIV transmission.
IDU Peer Network HIV Prevention Intervention
Network interventions use a social influence approach based on theories of diffusion of innovations, social learning, social identity, cognitive dissonance, and social norms to diffuse behavior change from peer educators through their social networks [13–21]. Experimental studies also suggest that information presented by members of a network group is likely to be viewed as credible and to be more actively processed than information received from other individuals [19]. In addition, there is an intervention premise that the presence of a peer educator advocating HIV-related health behaviors in one’s network would increase the salience of risk reduction norms [20], and behaviors would change in the direction of these norms. It is also hypothesized that networks which are relatively small and dense, such as IDU networks in Russia, are especially conducive to this approach of behavior change.
Aims of the Study
The purpose of this study was to conduct a randomized controlled trial to assess the efficacy of a peer-educator intervention focused on IDUs and their drug and/or sexual network members. We hypothesized that the intervention would result in a reduction in the incidence of HIV infection and HIV risk behaviors among both the peer-educators and members of their risk network compared to those in a control group. The intervention was designed to have index participants discuss and model injection and sexual risk reduction behaviors with their risk network members. It was anticipated that within small networks the social interaction by peer educators promoting risk reduction through modeling and verbal endorsement would lead network members to view risk reduction as an important set of behaviors practiced and endorsed by their peers. Moreover, the endorsing of the behavior would lead to behavior change among the peer educators and heightening the social norms of risk reduction would then be internalized by network members, leading to a reduction in risk behaviors. It was also anticipated that the change in risk behaviors would help to perpetuate new social norms of safer injection and sexual practices.
Methods
Trial Design
The study was a parallel design randomized trial including control and intervention arms with an enrollment ratio of 1:1. Each eligible index participant was asked to bring one to three network participants for enrollment. Networks clusters consisting of an index participant and his/her network participants were randomized to the control or experimental intervention groups. Only index participants received the intervention with the network participants educated by the index participants. Index participants and their network participants were to have follow-up visits every 6 months for 1 year after randomization. However, due to low return rates, participants were followed for up to 2 years. There was an attempt to contact by phone or home visit all participants 12 months after their enrollment date. At that time they were offered one final follow-up visit to occur up to 24 months following each participant’s enrollment date.
Enrollment of Participants
Prior to enrollment, the protocol was approved by the ethical committees at the Johns Hopkins School of Public Health and the Biomedical Center in St. Petersburg. HIV-negative and HIV-positive IDUs and persons they identified as members of their social network who were either an IDU, a sexual partner, or both were eligible for enrollment. All participants were required to be ≥18 years old. To be eligible for enrollment, index participants had to report having injected drugs in the prior 3 months and to be willing and able to recruit at least one HIV risk network member (network participant) who was eligible for study enrollment. Only network participants who were recruited for the study by an index participant were enrolled. Only network participants who had injected drugs with and/or had sex with the relevant index participant within the 3 months prior to screening were eligible to enroll.
Interventions Design
The experimental intervention was designed as a psychological-communicative behavioral training aimed to prevent transmission of HIV and other sexually transmitted infections (STIs) in the population of IDUs. Index participants assigned to the experimental intervention were scheduled to attend eight training sessions (seven group sessions and one individual training) aimed at understanding the risks associated with injecting drug use and unprotected sex, and development of safe behavior skills. An important component of the training was to develop communication skills and encourage participants to share HIV prevention information with their social network members, primarily with sexual and injection partners. These sessions followed a detailed manual with specific role plays, problem-solving scenarios, and informational components. After completing the training, index participants from the experimental group were invited to repeat (booster) meetings once a month for 4 months to discuss their strategies, successes and failures in implementing information transfer to their network members. In the control group there were also eight sessions (all group sessions) of equivalent length among the index participants devoted to discussing issues of interest, viewing lifestyle videos, practicing non-specific exercises aimed at personal development and social adaptation. No booster sessions were offered to the controls. Informational booklets about HIV and STI prevention were available to participants in both groups.
Primary Outcome and Other Collected Data
Prior to enrollment, all participants provided written informed consent. The study followed standard operating procedures for the collection, handling and the testing of all biological materials. During the baseline and at all follow-up visits participants provided blood samples for HIV, syphilis, herpes, hepatitis B and C serology testing. The primary outcome of interest was the HIV status of the participants. The primary endpoint was time from randomization to HIV sero-conversion for individuals who were HIV negative at baseline. HIV status was determined with an ELISA (GENSCREEN HIV1/2) and confirmed with a Western Blot (NEW LAV BLOT 1). HIV RNA testing (AMPLICOR HIV-1 MONITOR Test, version 1.5) to determine acute infection was performed retrospectively on the serum samples. Sera from all HIV antibody negative participants were pooled and RNA tested at baseline and at each subsequent visit to more accurately determine sero-conversion. Any participant found to have acute HIV at baseline was excluded from the HIV incidence analysis.
During the baseline and all follow-up visits the participants were administered a study staff led behavioral questionnaire to determine their injecting and sexual behavior.
Sample Size
To achieve 90 % power to detect a 40 % reduction in the rate of HIV infection, from 6 to 3.6 % per year, using a two-sided alpha of 0.05 and assuming an intra-class correlation of 0.2 and a lost to follow-up rate of 10 % per year, the required sample size was estimated at 2,640, based on a total of 660 index participants and 1,980 network participants (an average of three network participants per index participant). With a predicted HIV prevalence of 50 % at baseline, the anticipated sample size for the sub-set of participants HIV uninfected at baseline used for this HIV incidence analysis was set at 1,320:330 index participants and 990 network participants.
Randomization and Blinding
Network groups (which included an index participant and their network participants) were divided randomly into two groups––experimental and control with a 1:1 ratio. After initial screening, consent and enrollment visits by both the index and network participants, an office manager scheduled the date for the first sessions for 12–20 index participants. At the beginning of this visit the office manager entered the name and gender of the participant in a simple randomization program. Then the program generated the randomization assignment. The two randomization groups then started the sessions in different rooms. Throughout the study, the participants, physicians, counselors, social workers and assessors were blinded to the intervention assignments. Participants in both groups as well as staff members were informed that we were assessing two different HIV prevention interventions. To reduce biases we did not inform staff or participants about the hypothesis or which intervention we anticipated would be more effective. Only office managers and session facilitators were aware of the randomization assignments. The information about the randomization assignments were kept in special files available only to the office managers and facilitators.
Data Analysis
Baseline demographic, behavior and biological characteristics for each group were described using proportions for categorical variables and median, 25th and 75th percentiles for continuous variables. Baseline characteristics were compared between study arms using generalized estimation equations (GEE) to account for network correlation. The incidence rate of HIV sero-conversion in the control and experimental groups and the incidence rate ratios (IRRs) were estimated using GEE Poisson regression.
The cumulative risk of HIV sero-conversion was estimated using the Kaplan–Meier method. For both Poisson regression and the Kaplan–Meier estimator, HIV sero-conversion was assumed to occur at the midpoint between the last negative and first positive antibody test. A permutation test based on re-randomization of networks was used to compare the time to sero-conversion between the experimental and control groups using a generalization of the log rank statistic to allow for interval censoring [22]. Parametric accelerated failure time models were fit to the data allowing for interval censored sero-conversion times. The models assumed the sero-conversion time followed a Weibull distribution and included a random effect to allow for correlation among individuals in the same network. A likelihood ratio test was used to determine if the intra-class correlation was significantly different from zero.
GEE logistic regression models were used to assess if any of the baseline variables were significant predictors of having at least one follow up visit for participants HIV-negative at baseline. All baseline characteristics included in Table 1, as well as randomization assignment were used as potential predictors. All statistical analyses were conducted using SAS version 9.3.
Table 1.
Baseline demographic, biological and behavioral characteristics for each group based on data from 432 randomized participants
| Characteristics | Participants | Network members | Overall | ||||
|---|---|---|---|---|---|---|---|
| Control (N = 92) |
Experimental (N = 99) |
Control (N = 114) |
Experimental (N = 127) |
Control (N = 206) |
Experimental (N = 226) |
Wald statistic z, p value | |
| Age (years) | 28 (25–33) | 29 (25–31) | 28 (25–33) | 28 (25–32) | 28 (25–33) | 29 (25–32) | z = 1.00, p = 0.32 |
| Age at first injection | 18 (16–21) | 18 (16–22) | 18 (16–21) | 19 (17–22) | 18 (16–21) | 18 (17–22) | z = −0.09, p = 0.92 |
| Sexually active (past 3 months) | 75 (82 %) | 85 (89 %) | 96 (86 %) | 104 (83 %) | 171 (84 %) | 189 (86 %) | z = 0.31, p = 0.76 |
| Number of sex partners (past 3 months) | 1 (1–1) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | z = −0.22, p = 0.83 |
| Using condoms (past 30 days) | z = −0.94, p = 0.35 | ||||||
| Never | 35 (55 %) | 32 (46 %) | 55 (68 %) | 45 (52 %) | 90 (62 %) | 77 (50 %) | |
| Sometimes | 11 (17 %) | 14 (20 %) | 15 (19 %) | 18(21 %) | 26 (18 %) | 32 (21 %) | |
| Always | 18 (28 %) | 23 (33 %) | 11 (14 %) | 23 (27 %) | 29 (20 %) | 46 (30 %) | |
| Number of roommates | 2 (1–3) | 3 (2–4) | 2 (1–3) | 3 (2–4) | 2 (1–3) | 3 (2–4) | z = −3.00, p = 0.003 |
| Male gender | 61 (66 %) | 68 (69 %) | 77 (68 %) | 78 (61 %) | 138 (67 %) | 146 (65 %) | z = −0.52, p = 0.60 |
| HIV-positive | 45 (49 %) | 41 (41 %) | 52 (47 %) | 49 (39 %) | 97 (48 %) | 90 (40 %) | z = −1.39, p = 0.16 |
| Educational level | z = 0.06, p = 0.95 | ||||||
| <Completed secondary | 9 (10 %) | 19 (19 %) | 15 (13 %) | 21 (17 %) | 24 (12 %) | 40 (18 %) | |
| Completed secondary | 33 (36 %) | 24 (24 %) | 43 (38 %) | 44 (35 %) | 76 (37 %) | 68 (30 %) | |
| Vocational or trade | 37 (40 %) | 45 (45 %) | 39 (35 %) | 51 (40 %) | 76 (37 %) | 96 (42 %) | |
| Some higher | 13 (14 %) | 11 (11 %) | 16 (14 %) | 11 (9 %) | 29 (14 %) | 22 (10 %) | |
| Source of income | |||||||
| Wages | 32 (35 %) | 34 (35 %) | 54 (48 %) | 61 (48 %) | 86 (42 %) | 95 (42 %) | z = 0.10, p = 0.92 |
| Social assistance | 15 (16 %) | 12 (13 %) | 14 (12 %) | 15 (12 %) | 29 (14 %) | 27 (12 %) | z = 0.59, p = 0.56 |
| No legal income | 42 (47 %) | 47 (48 %) | 50 (45 %) | 56 (45 %) | 92 (46 %) | 103 (46 %) | z = 0.13, p = 0.89 |
| Friends and family | 66 (73 %) | 72 (74 %) | 76 (68 %) | 92 (72 %) | 142 (70 %) | 164 (73 %) | z = 0.81, p = 0.42 |
| Occupation level | z = −0.30, p = 0.77 | ||||||
| Full-time | 10 (11 %) | 12 (12 %) | 25 (22 %) | 21 (17 %) | 35 (17 %) | 33 (15 %) | |
| Part-time | 19 (21 %) | 18 (18 %) | 21 (19 %) | 38 (30 %) | 40 (20 %) | 56 (25 %) | |
| Unemployed | 63 (68 %) | 69 (70 %) | 67 (59 %) | 68 (54 %) | 130 (63 %) | 137 (61 %) | |
| Married or cohabitation | 35 (38 %) | 44 (44 %) | 48 (42 %) | 50 (39 %) | 83 (40 %) | 94 (42 %) | z = −0.22, p = 0.82 |
| Live with parents | 49 (53 %) | 55 (57 %) | 57 (50 %) | 69 (55 %) | 106 (52 %) | 124 (56 %) | z = 0.70, p = 0.49 |
| Commercial sex (F) | 4 (14 %) | 7 (24 %) | 2 (6 %) | 5 (11 %) | 6 (10 %) | 12 (16 %) | z = −1.03, p = 0.30 |
| Ever been Homeless | 6 (7 %) | 7 (7 %) | 4 (4 %) | 10 (8 %) | 10 (5 %) | 17 (8 %) | z = 1.11, p = 0.27 |
| Ever been in prison | 5 (6 %) | 2 (2 %) | 5 (4 %) | 6 (5 %) | 10 (5 %) | 8 (4 %) | z = −0.72, p = 0.47 |
| Alcohol use (past 30 days) | z = −0.14, p = 0.89 | ||||||
| Less than every week | 3 (37 %) | 32 (32 %) | 42 (37 %) | 49 (39 %) | 75 (37 %) | 81 (36 %) | |
| Every week | 35 (39 %) | 39 (39 %) | 39 (35 %) | 46 (37 %) | 74 (37 %) | 85 (38 %) | |
| Almost every day | 21 (24 %) | 28 (28 %) | 32 (28 %) | 31 (25 %) | 53 (26 %) | 59 (26 %) | |
| Injecting any drugs (past 30 days) | 92 (100 %) | 99 (100 %) | 109 (96 %) | 117 (92 %) | 201 (98 %) | 216 (96 %) | z = −1.43, p = 0.15 |
| Heroin injecting | 90 (98 %) | 96 (98 %) | 101 (93 %) | 110 (94 %) | 191 (95 %) | 206 (96 %) | z = 0.37, p = 0.71 |
| Stimulants injecting | 26 (28 %) | 38 (38 %) | 33 (30 %) | 28 (24 %) | 59 (29 %) | 66 (31 %) | z = 0.23, p = 0.82 |
| Mixture | 8 (9 %) | 6 (6 %) | 7 (6 %) | 6 (5 %) | 15 (7 %) | 12 (6 %) | z = −0.79, p = 0.43 |
| Unsafe injection practicesa (3 months) | 75 (82 %) | 82 (83 %) | 79 (72 %) | 94 (80 %) | 154 (77 %) | 176 (81 %) | z = 1.16, p = 0.25 |
Values expressed as count (percentage) or median (IQR). p values computed based on Wald tests from linear, logistic, or Poisson regression GEE models with the characteristic (row variable) as the dependent variable and randomization arm (control or experimental) as the independent variable
Unsafe injection practices is a combined variable including any of the following: needle or syringe sharing, shared syringe water, common dish or common drug source
Results
Flow of Participants
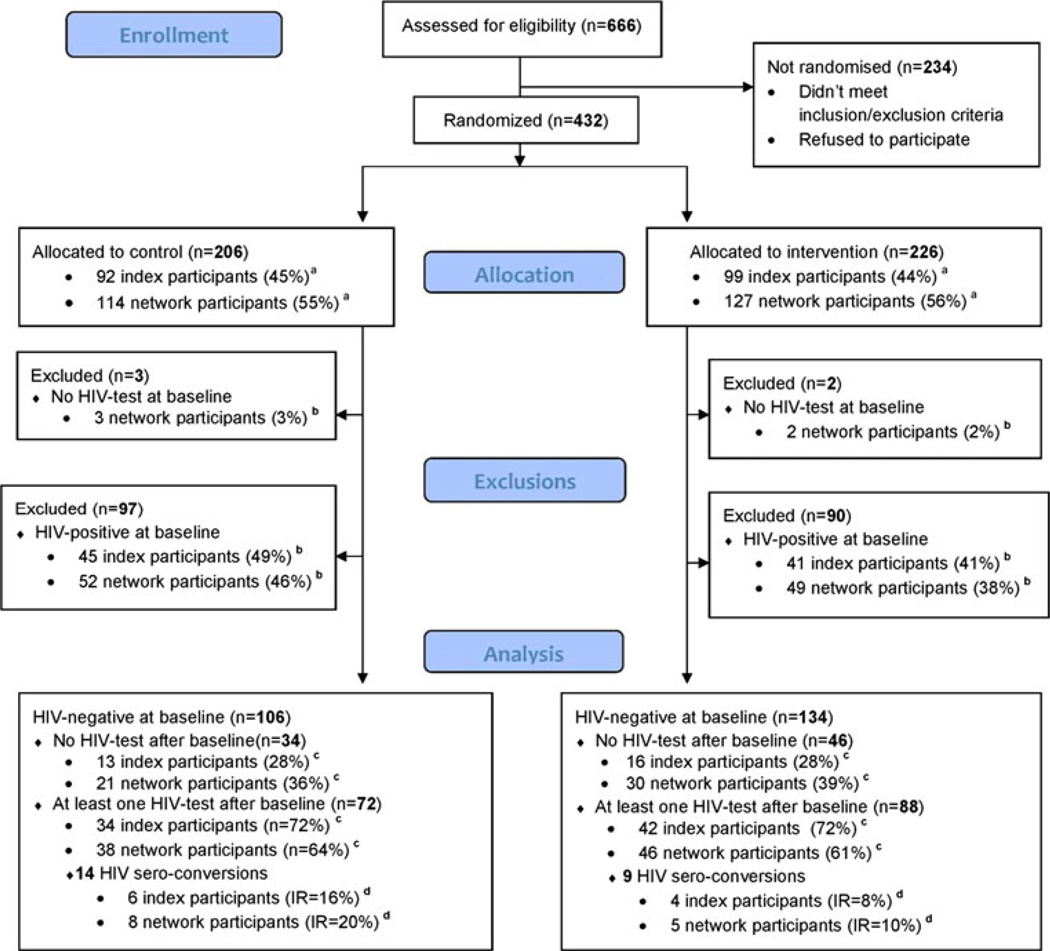
Participants were recruited and enrolled from December 2004 to November 2007 at an IDU research site established at the Biomedical Center. We recruited index participants from a variety of sources including needle exchange sites and other venues where injection drug users congregated. A total of 666 individuals were assessed for eligibility (Fig. 1). The majority of the index participants who were screened but did not enroll failed to identify a network participant who would co-enroll. Four-hundred and thirty two were enrolled and randomized, 191 index participants and 241 network participants. Each index participant brought a mean of 1.3 network participants into the study with a range of 1–4. Of the 432 participants, five were excluded because no HIV test result was available at baseline. An additional 43 % of participants were HIV positive at baseline (86/191 (45 %) index participants and 101/241 (42 %) network participants) and thus excluded from the incidence analysis. One participant who was HIV sero-negative at baseline was later determined to be HIV acutely infected at baseline and was excluded from the incidence analysis.
Fig. 1.
Participants flow diagram. a Percentage of individuals allocated to specified group (control or intervention). b Percentage of individuals belonging to specified subgroup (network cases of index cases). c Percentage of HIV-negative individuals belonging to specified subgroup (network cases of index cases). d Incidence rate (per 100 p/y)
Baseline Characteristics
The baseline characteristics of the individuals assigned to the control and experimental groups are presented in Tables 1 and 2. No important differences were found between groups at baseline (see Tables 1, 2). In general, the majority of the total cohort was in their 20′s and 30′s, two-thirds were male and single, over half lived with their parents and were underemployed. In the prior month, 95 % reported a history of injecting heroin with 30 % also injecting stimulants, (i.e. primarily amphetamines or ephedrine-based stimulants). Approximately 80 % reported a recent history of unsafe injection practices defined as any of the following sharing practices including needle or syringe sharing, shared syringe water, cotton, dish, or common liquid drug source. Ninety-four percent of the network participants were IDUs and 85 % gave a history of being sexually active in the past 3 months. Condom use was very low.
Table 2.
Baseline demographic, clinical and behavioral characteristics for each group based on data from 240 randomized and HIV negative participants
| Characteristics | Participants | Network members | Overall | ||||
|---|---|---|---|---|---|---|---|
| Control (N = 47) |
Experimental (N = 58) |
Control (N = 59) |
Experimental (N = 76) |
Control (N = 106) |
Experimental (N = 134) |
Wald statistic z, p value |
|
| Age (years) | 30 (24–33) | 29 (25–31) | 28 (25–33) | 28 (25–31) | 29 (25–33) | 29 (25–31) | z = 0.93, p = 0.35 |
| Age at first injection | 18 (17–21) | 19 (16–23) | 20 (18–22) | 19 (17–23) | 20 (17–22) | 19 (17–23) | z = 0.19, p = 0.85 |
| Sexually active (past 3 months) | 38 (81 %) | 50 (89 %) | 53 (90 %) | 64 (85 %) | 91 (86 %) | 114 (87 %) | z = 0.25, p = 0.81 |
| Number of sex partners (past 3 months) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | z = 1.09, p = 0.28 |
| Using condoms (past 30 days) | z = −1.11, p = 0.27 | ||||||
| Never | 15 (47 %) | 20 (49 %) | 31 (67 %) | 29 (60 %) | 46 (59 %) | 49 (55 %) | |
| Sometimes | 8(25 %) | 10 (24 %) | 9 (20 %) | 6 (13 %) | 17 (22 %) | 16 (18 %) | |
| Always | 9(28 %) | 11 (27 %) | 6 (13 %) | 13 (27 %) | 15 (19 %) | 24 (27 %) | |
| Number of roommates | 2 (1–4) | 3 (2–4) | 2 (1–3) | 3 (2–4) | 2 (1–3) | 3 (2–4) | z = −2.47, p = 0.014 |
| Male gender | 34 (72 %) | 40 (69 %) | 34 (58 %) | 48 (63 %) | 68 (64 %) | 88 (66 %) | z = 0.26, p = 0.80 |
| Educational level | z = −0.86, p = 0.39 | ||||||
| <Completed secondary | 4 (9 %) | 12 (21 %) | 7 (12 %) | 12 (16 %) | 11 (10 %) | 24 (18 %) | |
| Completed secondary | 15 (32 %) | 13 (22 %) | 19 (32 %) | 30 (39 %) | 34 (32 %) | 43 (32 %) | |
| Vocational or Trade | 20 (43 %) | 27 (47 %) | 21 (36 %) | 27 (36 %) | 41 (39 %) | 54 (40 %) | |
| Some higher | 8 (17 %) | 6 (10 %) | 12 (20 %) | 7 (9 %) | 20 (19 %) | 13 (10 %) | |
| Source of income | z = −0.19, p = 0.85 | ||||||
| Wages | 20 (43 %) | 20 (36 %) | 27 (46 %) | 37 (49 %) | 47 (44 %) | 57 (43 %) | |
| Social assistance | 8 (17 %) | 7 (13 %) | 7 (12 %) | 8 (11 %) | 15 (14 %) | 15 (12 %) | z = 0.61, p = 0.54 |
| No legal income | 24 (52 %) | 28 (50 %) | 26 (44 %) | 30 (41 %) | 50 (48 %) | 58 (45 %) | z = −0.47, p = 0.64 |
| Friends and family | 34 (72 %) | 42 (75 %) | 39 (66 %) | 54 (71 %) | 73 (69 %) | 96 (73 %) | z = 0.68, p = 0.50 |
| Occupation level | z = −0.26, p = 0.79 | ||||||
| Full-time | 6 (13 %) | 8 (14 %) | 11 (19 %) | 14 (18 %) | 17 (16 %) | 22 (16 %) | |
| Part-time | 11 (23 %) | 9 (16 %) | 12 (20 %) | 22 (29 %) | 23 (22 %) | 31 (23 %) | |
| Unemployed | 30 (64 %) | 41 (71 %) | 36 (61 %) | 40 (53 %) | 66 (62 %) | 81 (60 %) | |
| Married or cohabitation | 20 (43 %) | 28 (48 %) | 25 (42 %) | 31 (41 %) | 45 (42 %) | 59 (44 %) | z = −0.24, p = 0.81 |
| Live with parents | 24 (51 %) | 29 (51 %) | 28 (47 %) | 41 (55 %) | 52 (49 %) | 70 (53 %) | z = 0.55, p = 0.58 |
| Commercial sex (F) (past 3 months) | 2 (18 %) | 4 (25 %) | 2 (9 %) | 2 (7 %) | 4 (12 %) | 6 (14 %) | z = 0.27, p = 0.79 |
| Ever been Homeless | 5 (11 %) | 5 (9 %) | 1 (2 %) | 6 (8 %) | 6 (6 %) | 11 (8 %) | z = 0.75, p = 0.45 |
| Ever been in prison | 3 (7 %) | 2 (3 %) | 0 (0 %) | 3 (4 %) | 3 (3 %) | 5 (4 %) | z = 0.39, p = 0.70 |
| Alcohol use (past 30 days) | z = −0.75, p = 0.45 | ||||||
| Less than every week | 13 (29 %) | 13 (22 %) | 24 (41 %) | 28 (37 %) | 37 (36 %) | 41 (31 %) | |
| Every week | 21 (47 %) | 26 (45 %) | 20 (34 %) | 28 (37 %) | 41 (39 %) | 54 (41 %) | |
| Almost every day | 11 (24 %) | 19 (33 %) | 15 (25 %) | 19 (25 %) | 26 (25 %) | 38 (29 %) | |
| Injecting any drugs (past 30 days) | 47 (100 %) | 58 (100 %) | 56 (95 %) | 68 (89 %) | 103 (97 %) | 126 (94 %) | z = −1.14, p = 0.26 |
| Heroin injecting | 46 (98 %) | 56 (97 %) | 51 (91 %) | 63 (93 %) | 97 (94 %) | 119 (94 %) | z = 0.08, p = 0.93 |
| Stimulants injecting | 13 (28 %) | 22 (38 %) | 19 (34 %) | 17 (25 %) | 32 (31 %) | 39 (31 %) | z = −0.02, p = 0.99 |
| Mixture of injection drugs | 7 (15 %) | 4 (7 %) | 4 (7 %) | 4 (6 %) | 11 (11 %) | 8 (6 %) | z = −1.16, p = 0.25 |
| Unsafe injection practicesa (3 months) | 38 (81 %) | 46 (79 %) | 37 (66 %) | 51 (75 %) | 75 (73 %) | 97 (77 %) | z = 0.72, p = 0.47 |
Values expressed as count (percentage) or median (IQR). p values computed based on Wald tests from linear, logistic, or Poisson regression GEE models with the characteristic (row variable) as the dependent variable and randomization arm (control or experimental) as the independent variable
Unsafe injection practices is a combined variable including any of the following: needle or syringe sharing, shared syringe water, common dish or common drug source
Attendance at Index Intervention Sessions
There was no difference when we compared the eight scheduled intervention and control training session attendance rates. The median number of training sessions attended was not significantly different (Kruskal–Wallis test = 2.26, p = 0.13) between the intervention and controls respectively, 7 (IQR 3, 8) versus 5 (IQR 2, 7). The booster sessions, which focused on peer outreach, were only offered to the intervention group.
Follow-Up Visits (Fig. 1)
Among the 240 HIV uninfected cohort at baseline, 80 (33.3 %) had no follow-up visit beyond baseline, thus were not included in the HIV incidence analysis. This included 46/134 (34 %) in the intervention group (28 % index participants, 39 % network participants) and 34/106 (32 %) in the control group (28 % index participants, 36 % network participants). Among the participants lost to follow-up, 33 % (26/80) had no follow-up visit due to death 15/80 (18.8 %) or incarceration 11/80 (13.8 %). None of the baseline variables were significant predictors of no visits after baseline (results not shown). Among the 160 participants with a follow up visit, 88 (55 %) had only a single visit. The mean interval between the baseline and the final visit was 292 days with an overall range of 62–728 days.
HIV Incidence
The estimated incidence and incidence ratio for HIV in the control and experimental groups are presented in Table 3. Estimated incidences in control and experimental groups were 19.57 (95 % confidence interval (CI) 10.74–35.65) and 7.76 (95 % CI 3.51–17.19) respectively. The estimated incidence rate ratio (IRR) based on the fitted GEE Poisson regression model was 0.41 (95 % CI 0.15–1.08). Although not statistically significant, the estimated IRR suggests that the experimental intervention may result in a roughly 60 % decline in HIV incidence. The relative incidence between the experimental and control groups was similar when considering only index participants or considering only network participants. The fitted Weibull frailty model yielded results similar to the GEE model. The estimated hazard ratio comparing the experimental group to the control group was 0.19 (95 % CI 0.02–1.65; p = 0.17). The likelihood ratio test for the cluster random effect was significant (test statistic 5.45, p < 0.001), indicating correlation between individuals within a network.
Table 3.
Incidence of HIV in control and experimental groups for 2 years (0–730 days)
| Group | Events | N | Person-years | Incidence (per 100 p/y) |
95 % Confidence intervals |
|
|---|---|---|---|---|---|---|
| Index cases | Control | 6 | 34 | 36.40 | 16.49 | (7.18–37.91) |
| Experimental | 4 | 42 | 48.06 | 8.26 | (3.12–21.87) | |
| Network members | Control | 8 | 38 | 40.57 | 20.01 | (9.78–40.93) |
| Experimental | 5 | 46 | 51.1 | 10.61 | (4.44–25.36) | |
| Total | Control | 14 | 72 | 76.98 | 19.57 | (10.74–35.65) |
| Experimental | 9 | 88 | 99.16 | 7.76 | (3.51–17.19) | |
| IRR | ||||||
| Experimental versus | Control | 0.41 | (0.15–1.08) |
IRR incidence rate ratio
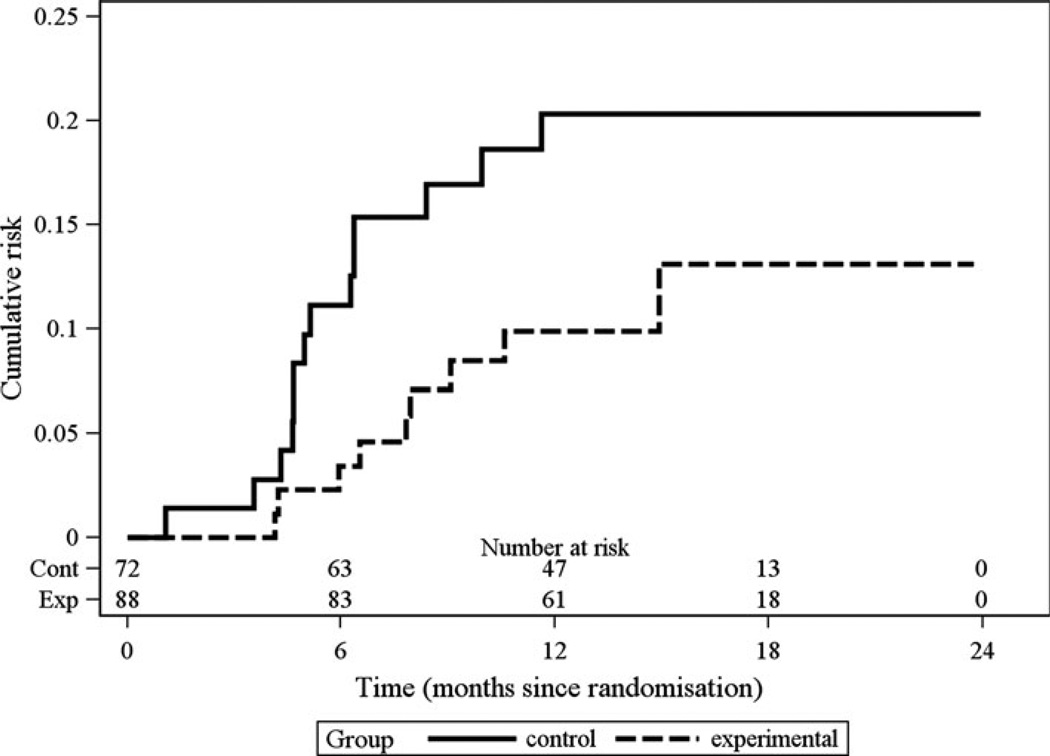
The Kaplan–Meier estimates of the cumulative risk of HIV sero-conversion for the two groups are presented in Fig. 2. The estimated risk of sero-conversion was higher for the control group compared to the experimental group at all time-points, although there was not a statistically significant difference in the rate of sero-conversion between the groups (log rank test statistic X2 = 2.73, permutation p-value = 0.16). At 1 year, the estimated cumulative risk of sero-conversion was 20.0 % in the control group and 9.9 % in the experimental group. Among the 84 network participants, 50 (60 %) were injection partners only to the index participant; 29 (35 %) were both injection and sexual partners; and 5 (6 %) were sexual partners only with the index participant. Among the 50 injection partners only, 9 (18 %) HIV seroconverted while 4/29 (14 %) of the both injection and sexual partners seroconverted. None of the five sexual partners only seroconverted. There were inadequate numbers of sero-conversions to further stratify these risk groups by the experimental and control arms.
Fig. 2.
Estimated cumulative risk of HIV sero-conversion
Sensitivity Analysis
Using HIV RNA testing to detect acute infection, one individual was found to have acute HIV during his second follow-up visit. Adjustments were made to-recalculate the interval over which we believed infection occurred, which resulted in no appreciable difference in the overall results.
Discussion
Prevention of HIV transmission and acquisition among IDUs is extremely challenging in settings where the HIV prevalence is high, there is no or little access to drug replacement therapy or needle exchange, condom use is low and the population of IDUs is marginalized and criminalized with limited access to HIV treatment, including antiretroviral therapy. Although the implementation of this study was challenging and ultimately underpowered, our results suggest this intervention may reduce HIV incidence within IDU networks. We employed parametric (Weibull AFT model), semi-parametric (GEE Poisson regression) and non-parametric (Kaplan–Meier estimates, generalized log rank test) methods to compare HIV incidence rates in the experimental and control groups; all methods gave comparable results, indicating incidence in the control group was roughly twice that in the intervention group. This ratio was the same among the index participants and their network participants, showing a promising trend that the intervention might reduce the risk of HIV infection at the network level. The results of this social network intervention conducted in Russia are encouraging and particularly relevant in settings where such a strategy could be part of a comprehensive package of interventions for IDUs, including biomedical interventions such as pre-exposure prophylaxis and early initiation of antiretroviral therapy [23, 24]. This type of network intervention is also culturally appropriate as most IDUs interact in small networks and this intervention has the characteristics necessary to reach hidden and stigmatized populations of injectors, including sexual partners, and is, by design, much less costly than training each individual at risk.
Similar to past St. Petersburg IDU cohorts, this group of peer IDUs and their network members were at very high risk of HIV acquisition. Eighty percent reported recent injection sharing and 30 % of these heroin injectors also injected stimulants, a known co-factor to HIV sero-conversion [5]. Thus, it is not surprising that the HIV incidence among both the control and intervention groups was so high. It is unfortunate that for over a decade this high prevalence and incidence among IDU in Russia has been widely disseminated to Russian public health policy makers and has resulted in no structural changes regarding access to effective drug use treatment, harm reduction programs or elimination of barriers to ART for IDU.
Several prior studies based on theories of social influence have demonstrated the feasibility of training IDUs to promote risk reduction among peers and thereby changing their own behaviors as well as those of their network members. The SHIELD study in Baltimore found significantly greater reductions in the experimental condition in which participants were trained to be peer educators, as compared to the control group [25]. And the STEP study, which was also a peer education randomized control trial, found significantly lower risk behaviors in the index participants in the experimental arm as well as their network members at the 18 month follow-up [26]. Neither STEP nor SHIELD used biological outcomes. With a similar design as ours, HPTN 037, which was implemented in Chiang Mai, Thailand and Philadelphia, USA, was designed to test a social network intervention with HIV sero-conversion as the outcome [27]. However, the actual HIV incidence in Thailand turned out to be much lower than anticipated. Moreover, the Thai war on drugs seriously disrupted the network of drug users and led to mass incarcerations and killings. While there was no intervention effect in Thailand, the study results in Philadelphia did demonstrate a pattern of statistically significant reductions in injection related risk behaviors for up to 24 months among peer educators and their network members, as compared to those in the control group [27].
There were important limitations to this study. Although our predicted HIV prevalence of 50 % was similar to the 45 % we observed, we over estimated both the feasibility of enrolling over 2,500 IDU at a single site and our ability to retain 90 %of the cohort over the study period. In a previous St. Petersburg longitudinal cohort study we were able to enroll and follow 500 high risk IDU in an observational study [4]. However, in this intervention study we failed to appreciate the difficulty in co-enrolling network members despite an incentivized strategy of network member referrals. Although an adequate sample of index IDU were screened, only 65 % were willing or able to convince their network members to participate, and when they did, were successful in only enrolling a mean of 1.3 network participants per index participant, far short of the predicted 3 per index participant we anticipated. From our experience, future research in this area will require a smaller sample size per site, or a research design where a community effect can be measured instead of following individual HIV incidence. Our predicted retention of 90 % per year was unrealistic. Our previous NIH funded, monitored and managed longitudinal observational cohort study among IDU in St. Petersburg [5] had a 1 year lost to follow up of 20 %with half of the loss to follow-up due to incarceration or death. However, similar to our previous cohorts, there were no socio-demographic or behavioral differences between the groups lost to follow-up and those retained, either among the index or network participants or between the control and intervention groups. So we have some level of confidence that our high lost to follow-up rate did not have an inordinate effect on our reported outcomes. Another limitation is that we only accounted for inside network correlations, not for correlations which might have occurred among index participants who had intervention sessions together, which might have resulted in new IDU relationships and perhaps magnified the effect of the intervention; or the potential contamination between the control and experimental groups, which may have reduce the effect size.
Despite the serious limitations in the implementation of the study, the results of this peer educator network intervention show great promise that this strategy may be successful in reducing the transmission of HIV among severely marginalized IDUs.
Acknowledgments
The authors acknowledge the sponsors of the study: The National Institute on Drug Abuse (NIDA) (R01 DA016142, Latkin PI), the University of North Carolina Center for AIDS Research (UNC CFAR) (NIH P30 AI50410); and our collaborators the John’s Hopkins School of Public Health, the Biomedical Center and St. Petersburg State University, Russia. We would also like to acknowledge the implementing and clinical staff at the Biomedical Center and all the participants.
Footnotes
ClinicalTrials.gov Identifier of this trial is NCT00218673.
Contributor Information
Irving F. Hoffman, Email: hoffmani@med.unc.edu, Division of Infectious Diseases, Department of Medicine, UNC Hospitals, University of North Carolina, CB # 7030, Chapel Hill, NC 27599, USA.
Carl A. Latkin, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA
Polina V. Kukhareva, Biomedical Center, St. Petersburg, Russia Department of Biostatistics, School of Public Health, University of North Carolina (UNC), Chapel Hill, NC, USA.
Sergey V. Malov, St. Petersburg St. University, St. Petersburg, Russia
Julia V. Batluk, Biomedical Center, St. Petersburg, Russia St. Petersburg St. University, St. Petersburg, Russia.
Alla V. Shaboltas, Biomedical Center, St. Petersburg, Russia St. Petersburg St. University, St. Petersburg, Russia.
Roman V. Skochilov, Biomedical Center, St. Petersburg, Russia St. Petersburg St. University, St. Petersburg, Russia.
Nicolay V. Sokolov, Biomedical Center, St. Petersburg, Russia St. Petersburg St. University, St. Petersburg, Russia.
Sergei V. Verevochkin, Biomedical Center, St. Petersburg, Russia
Michael G. Hudgens, Department of Biostatistics, School of Public Health, University of North Carolina (UNC), Chapel Hill, NC, USA
Andrei P. Kozlov, Biomedical Center, St. Petersburg, Russia St. Petersburg St. University, St. Petersburg, Russia.
References
- 1.Geneva: UNAIDS; 2011. Global HIV/AIDS response––progress report 2011. http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20111130_UA_Report_en.pdf. [Google Scholar]
- 2.Aral SO, St Lawrence JS, Dyatlov R, Kozlov A. Commercial sex work, drug use, and sexually transmitted infections in St. Petersburg, Russia. Soc Sci Med. 2005;60(10):2181–2190. doi: 10.1016/j.socscimed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Heimer R, White E. Estimation of the number of injection drug users in St. Petersburg, Russia. Drug Alcohol Depend. 2010;109:79–83. doi: 10.1016/j.drugalcdep.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaboltas AV, Toussova OV, Hoffman IF, et al. HIV prevalence, sociodemographic, and behavioral correlates and recruitment methods among IDUs in St. Petersburg, Russia. J Acquir Immune Defic Syndr. 2006;41(5):657–663. doi: 10.1097/01.qai.0000220166.56866.22. [DOI] [PubMed] [Google Scholar]
- 5.Kozlov AP, Shaboltas AV, Toussova OV, et al. HIV incidence and factors associated with HIV acquisition among IDUs in St Petersburg. Russia Aids. 2006;20(6):901–906. doi: 10.1097/01.aids.0000218555.36661.9c. [DOI] [PubMed] [Google Scholar]
- 6.Niccolai LM, Verevochkin SV, Toussova OV, et al. Estimates of HIV incidence among drug users in St. Petersburg, Russia: continued growth of a rapidly expanding epidemic. Eur J Public Health. 2010;21(5):613–619. doi: 10.1093/eurpub/ckq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verevochkin SV, Gagarina SN, Skochilov RV, et al. High HIV incidence rate in St. Petersburg IDU cohort [TUPE346]. Paper presented at 6th IAS conference on HIV pathogenesis, treatment and prevention, in 6th IAS conference on HIV pathogenesis, treatment and prevention; Rome, Italy. 2011. [Google Scholar]
- 8.Gyarmathy VA, Tobin KE, Hoffman IF, Sokolov N, Levchenko J, Batluk J, Kozlov AA, Kozlov AP, Latkin CA. Correlates of unsafe equipment sharing among injecting drug users in St. Petersburg, Russia. Eur Addict Res. 2009;15(3):163–170. doi: 10.1159/000220344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes T, Mikhailova L, Sarang A, Lowndes CM, Rylkov A, Khutorskoy M, Renton A. Situational factors influencing drug injecting, risk reduction and syringe exchange in Togliatti City, Russian Federation: a qualitative study of micro risk environment. Soc Sci Med. 2003;57(1):39–54. doi: 10.1016/s0277-9536(02)00521-x. [DOI] [PubMed] [Google Scholar]
- 10.WHO/Europe website. [Accessed on 04/15/2012]; http://www.euro.who.int/en/what-we-do/health-topics/communicable-diseases/hivaids/policy/injecting-drug-users-idu.
- 11.Wolfe D. Paradoxes in antiretroviral treatment for injecting drug users: access, adherence and structural barriers in Asia and the former Soviet Union. Int J Drug Policy. 2007;18(4):246–254. doi: 10.1016/j.drugpo.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Latkin C, Knowlton AR. Social network approaches to HIV prevention: implications to community impact and sustainability. In: Trickett EJ, Pequegnat W, editors. Community interventions and AIDS. New York: Oxford University Press; 2005. p. 105. [Google Scholar]
- 13.White KM, Smith JR, Terry DJ, Greenslade JH, McKimmie BM. Social influence in the theory of planned behaviour: the role of descriptive, injunctive, and in-group norms. Br J Soc Psychol. 2009;48(Pt 1):135–158. doi: 10.1348/014466608X295207. [DOI] [PubMed] [Google Scholar]
- 14.Rogers E. Diffusion of innovations. vol. 5th. New York: Free Press; 2003. [Google Scholar]
- 15.Bandura A. Social learning theory. Englewood Cliffs: Prentice Hall; 1977. [Google Scholar]
- 16.Turner JC. Social comparison and social identity: some perspectives for intergroup behavior. Eur J Soc Psychol. 1978;5:5–34. [Google Scholar]
- 17.Festinger L. Conflict, decision and dissonance. Stanford: Stanford University Press; 1964. [Google Scholar]
- 18.Van Knippenberg D. Group norms, prototypicality, and persuasion. In: Terry DJ, Hogg MA, editors. Attitudes, behavior, and social context: the role of norms and group membership. Mahwah: Lawrence Erlbaum Associates; 2000. p. 157. [Google Scholar]
- 19.Jannis LL, Mann L. Effectiveness of emotional role-playing in modifying smoking habits and attitudes. J Exp Res Pers. 1977;1:84–90. [Google Scholar]
- 20.Terry DJ, Hogg MA. Attitudes, behavior, and social context: the role of norms and group membership. Mahwah: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 21.Jonson BT, Redding CA, DiClemente RJ, Mustanski BS, Dodge B, Sheeran P, Warren MR, Zimmerman RS, Fisher WA, Conner MT, Carey MP, Fisher JD, Stall RD, Fishbein M. A network-individual-resource model for HIV prevention. AIDS Behav. 2010;14(Suppl 2):204–221. doi: 10.1007/s10461-010-9803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Zhao Q, Zhao X. Generalized log-rank test for interval-censored failure time data. Scand J Stat. 2005;32:49–57. [Google Scholar]
- 23.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen Myron S, Chen Ying Q, McCauley Marybeth, et al. Prevention of HIV-1 infection with early antiretroviral therapy. NEJM. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: outcome of a network-oriented peer outreach intervention. Health Psychol. 2003;22(4):332–339. doi: 10.1037/0278-6133.22.4.332. [DOI] [PubMed] [Google Scholar]
- 26.Tobin KE, Kuramoto SJ, Davey-Rothwell MA, Latkin CA. The STEP into action study: a peer-based, personal risk network-focused HIV prevention intervention with IDUs in Baltimore. Maryland Addiction. 2011;106(2):366–375. doi: 10.1111/j.1360-0443.2010.03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latkin CA, Donnell D, Metzger D, Sherman S, Aramrattna A, Davis-Vogel A, Quan VM, Gandham S, Vongchak T, Perdue T, Celentano DD. The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia. USA Soc Sci Med. 2009;68(4):740–748. doi: 10.1016/j.socscimed.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]