Abstract
Sartans (Angiotensin II AT1 Receptor Blockers, ARBs) are powerful neuroprotective agents in vivo and protect against IL-1β neurotoxicity in vitro. The purpose of this research was to determine the extent of sartan neuroprotection against glutamate excitotoxicity, a common cause of neuronal injury and apoptosis. The results show that sartans are neuroprotective, significantly reducing glutamate-induced neuronal injury and apoptosis in cultured rat primary cerebellar granule cells (CGCs). Telmisartan was the most potent sartan studied, with an order of potency telmisartan > candesartan > losartan > valsartan. Mechanisms involved reduction of pro-apoptotic caspase-3 activation, protection of the survival PI3K/Akt/GSK-3β pathway, and prevention of glutamate-induced ERK1/2 activation. NMDA receptor stimulation was essential for glutamate-induced cell injury and apoptosis. Participation of AT1A receptor was supported by glutamate-induced upregulation of AT1A gene expression and AT1 receptor binding. Conversely, AT1B or AT2 receptor played no role. Glutamate-induced neuronal injury and the neuroprotective effect of telmisartan were decreased, but not abolished, in CGCs obtained from AT1A knock-out mice. This indicates that although AT1 receptors are necessary for glutamate to exert its full neurotoxic potential, part of the neuroprotective effect of telmisartan is independent of AT1 receptor blockade. PPARγ activation was also involved in the neuroprotective effects of telmisartan, as telmisartan enhanced PPARγ nuclear translocation, and the PPARγ antagonist GW9662 partially reversed the neuroprotective effects of telmisartan. The present results substantiate the therapeutic use of sartans, in particular telmisartan, in neurodegenerative diseases and traumatic brain disorders where glutamate neurotoxicity plays a significant role.
Keywords: Angiotensin II AT1 receptor blockers, Telmisartan, PPARγ, Neuroprotection, Glutamate neurotoxicity, Apoptosis
1. Introduction
Glutamate plays important roles as the predominant excitatory neurotransmitter in the mammalian brain (Coyle and Puttfarcken, 1993). However, excessive release of glutamate leading to excitotoxicity is a major factor in neuronal injury associated with many acute and chronic brain disorders such as brain ischemia, traumatic brain disorder, HIV and neurodegenerative disorders (Chamoun et al., 2010; Coyle and Puttfarcken, 1993; Lau and Tymianski, 2010; Tian et al., 2008). At present, there are no pharmacological treatments to ameliorate glutamate excitotoxicity and provide neuroprotection for these conditions (Lau and Tymianski, 2010). This indicates an urgent need to search for novel compounds with neuroprotective effects.
One of such emerging therapeutic targets is a class of compounds commonly used for the treatment of cardiovascular and metabolic disorders. These compounds, collectively called Angiotensin Receptor Blockers (ARBs) or sartans, effectively block the physiological AT1 receptor (AT1R) and therefore the effects of Angiotensin II, the main active factor of the Renin-Angiotensin Sytem (Timmermans et al., 1993).
Excessive peripheral AT1R activity associates with hypertension, heart and kidney failure, peripheral vascular and tissue inflammation, and metabolic abnormalities such as insulin resistance (Chrysant et al., 2010; Konstam et al., 2009; Savoia and Schiffrin, 2007). Sartans protect end organs not only because they ameliorate hypertension, but also as a consequence of beneficial effects on inflammatory and metabolic alterations beyond their effect on blood pressure control (Bakris, 2010). For these reasons sartans are commonly used for the treatment of cardiovascular and renal disease and diabetes (Chrysant et al., 2010; Konstam et al., 2009; Savoia and Schiffrin, 2007).
Increased brain AT1R stimulation also associates with brain ischemia, abnormal stress responses, blood–brain barrier breakdown, β-amyloid production and toxicity and brain inflammation (Armando et al., 2001; Fleegal-DeMotta et al., 2009; Jezova et al., 1998; Kaiser et al., 1992; Nishimura et al., 2000; Phillips and de Oliveira, 2008; Saavedra, 2012; Saavedra et al., 2011; Tsukuda et al., 2009; Zhu et al., 2011). These are risk factors leading to neuronal injury, the incidence and progression of neurodegenerative disease, mood and traumatic brain disorders, and cognitive decline (Saavedra, 2012).
There is increasing evidence that sartans are effective neuroprotective compounds (Anderson, 2010; Anderson et al., 2011; Saavedra, 2012). In preclinical experiments, sartans ameliorate stress-induced disorders, anxiety and depression, protect cerebral blood flow and cognition during stroke, decrease brain inflammation and β-amyloid neurotoxicity, and reduce traumatic brain injury (Ando et al., 2004; Armando et al., 2001; Benicky et al., 2011; Danielyan et al., 2010; Ito et al., 2002; Jezova et al., 1998; Kaiser et al., 1992; Nishimura et al., 2000; Phillips and de Oliveira, 2008; Saavedra, 2012; Saavedra et al., 2011; Timaru-Kast et al., 2012; Tsukuda et al., 2009; Villapol et al., 2012; Wang et al., 2007; Zhou et al., 2005; Zhu et al., 2011). Direct sartan anti-inflammatory and neuroprotective effects against bacterial endotoxin (lipopolysaccharide, LPS) and interleukin-1β (IL-1β) have been demonstrated in cultured microglia, cerebrovascular endothelial cells, human circulating monocytes, and neurons (Benicky et al., 2011; Dandona et al., 2003; Larrayoz et al., 2009; Miyoshi et al., 2008; Pang et al., 2012a, 2012b). Controlled clinical studies indicate that ARBs protect cognition after stroke and during aging (Chrysant et al., 2010; Fogari et al., 2004), and cohort analyses reveal that these compounds significantly reduce the incidence and progression of Alzheimer’s disease (Davies et al., 2011; Li et al., 2010).
Individual sartans have very diverse pharmacological profiles, leading to marked differences in neuroprotective potency (Benson et al., 2004; Erbe et al., 2006). Telmisartan is considered to be the most potent member of the sartan group because, in addition to AT1R blockade, strongly activates the anti-inflammatory nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) (Benson et al., 2004; Erbe et al., 2006). PPARγ regulates multiple pathways involved in inflammation and carbohydrate and lipid metabolism (Rotman and Wahli, 2010). Full PPARγ agonists reduce inflammation and metabolic alterations associated with cardiovascular disease (Duan et al., 2009). In addition, activation of the anti-inflammatory nuclear receptor PPARγ plays an important role in neuroprotection (Kapadia et al., 2008; Min et al., 2012; Pang et al., 2012a; Tsukuda et al., 2009; Villapol et al., 2012). These observations prompted us to determine to what extent sartans, and in particular telmisartan, may directly ameliorate glutamate excitotoxicity. In the present study, we selected to study cerebellar granule cells (CGCs) in vitro, a well-characterized and reliable model to analyze mechanisms and excitotoxic neuronal damage and neuroprotection (Contestabile et al., 2002; Krämer and Minichiello, 2010).
2. Materials and methods
2.1. Animals
All animal care and experimental procedures in the present study were approved by the National Institute of Mental Health Animal Care and Use Committee (Bethesda, MD). All efforts were made to minimize the number of animals used and their suffering (National Institutes of Health Guide for the Care and Use of Laboratory Animals, Publication number 80–23, revised 1996). Eight-day old Sprague-Dawley male and female pups and their mothers were purchased from Charles Rivers Laboratories (Wilmington, MA).
For experiments with AT1A knock-out mice, male and female wild-type C57BL6/J mice, and AT1A knock-out B6.129P2-Agtratm1Unc/J mice without detectable functional protein (Ito et al., 1995) were obtained from The Jackson Laboratory (Bar Harbor, MA, USA). The AT1A mice have been bred for more than 10 generations to the parental inbred strain. Viable and fertile wild type mice and AT1A knock-out mice of both sexes were used to breed 6-day old wild-type and AT1A knock-out pups, respectively. One male and one female were kept per cage with free access to water and breeder chow at 22°C under a 12:12 h dark-light cycle, at the NIMH Animal Care Facility (Bethesda, MD) according to breeding procedures outlined in “NIH Guidelines for the Establishment and Use of Mouse Breeding Groups”.
2.2. Cerebellar granule cells culture
Primary CGCs were isolated from 8-day old Sprague Dawley rat pups (Charles Rivers Laboratories, Wilmington, MA USA) or 6-day old wild type and AT1A knockout mouse pups as described previously (Gao et al., 1995; Lee et al., 2009). Rat and mouse pups were euthanized by decapitation; the brains were dissected immediately and the cerebella were collected and placed in ice-cold Hank’s balanced salt solution (HBSS) (Invitrogen, Carlsbad, CA). After removal of the meninges, the cerebella were dispersed into the same buffer containing 0.025% trypsin (Invitrogen) and digested for 15 min at 37°C. Trypsin digestion was stopped by adding a same volume of Dulbecco’s Modified Eagle medium (DMEM) (Invitrogen), supplemented with 10% FBS (Invitrogen) and 0.1 mg/ml DNase I (Sigma-Aldrich, St. Louis, MO). After gentle trituration, digested tissues were centrifuged at 1000 × rpm for 5 min. The cell pellets were resuspended in the complete Neurobasal culture medium supplemented with 2% B27 (Invitrogen) and 0.5 mM GlutaMax (Invitrogen). After filtration through a 70 µm cell restrainer (BD Falcon, Vernon Hills, IL), cells were plated at a density of 1×106 cells/ml onto poly-L-lysine coated plates (Becton Dickinson and Company, Franklin Lakes, NJ) or chamber glass slides (Nalge Nunc International, Naperville, IL). Cultures were incubated in a humidified atmosphere of 5% CO2-95% air at 37°C. Cytosine arabinofuranoside (Invitrogen) (10 µM) was added to the cultures 24 h after plating to arrest the growth of non-neuronal cells. Cultures 6–7 days in vitro were used in this study. Immunocytochemical validation with anti-microtubule-associated protein-2 (MAP-2) antibody (EMD Millipore, Billerica, MA) and 4–6-diamino-2-phenylindole (DAPI) (Invitrogen) revealed that more than 95% of the cells in our cultures system were neurons at the time of experiment (results not shown).
2.3. Cell culture treatments
Excitotoxicity was induced by exposing cultures with different concentrations of glutamate (10–200 µM) (Sigma-Aldrich) at different time points. To determine which Angiotensin II receptor type was involved in glutamate-induced cell death, cells were pre-treated with vehicle, or with either the AT1 receptor blockers telmisartan (Sigma-Aldrich), candesartan (a gift from Astra-Zeneca, Mölndal, Sweden), losartan (Sigma-Aldrich) and valsartan (Sigma-Aldrich) (0, 1 to 20 µM) for 2 h, the AT2 receptor agonist CGP42112 (10 µM) (Sigma-Aldrich) or the AT2 receptor antagonist PD123319 (10 µM) (Sigma-Aldrich) for 1 h. To determine whether PPARγ was involved in telmisartan neuroprotective effect, the PPARγ agonist pioglitazone (10 µM) (Sigma-Aldrich) was added 2 h before glutamate treatment; the PPARγ antagonist GW9662 (20 µM) (Sigma-Aldrich) was used 2 h before pioglitazone or telmisartan treatment. All drugs were dissolved in DMSO (Sigma-Aldrich). DMSO was present in all samples at a final 0.1% concentration in the culture medium.
2.4. Measurement of lactate dehydrogenase (LDH) activity
Cell viability was quantified with LDH activity using LDH Cytotoxicity Assay Kit (Cayman Chemical) according to the manufacturer's instructions. The data were normalized to the activity of LDH released from control untreated cells (100%) and expressed as a percent of the control.
2.5. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and DAPI staining
To determine apoptotic morphology of CGCs, TUNEL was applied using the In Situ Cell Death Detection Kit Fluorescein (Roche Diagnostic) according to the manufacturer’s protocol. Neuronal cells were cultured on poly-L-lysine-coated chamber glass slides, and after 6 or 7 DIV were pre-treated with 1 µM telmisartan for 2 hours, followed by 24 hours of 100 µM glutamate exposure. The cells were then fixed with 4% paraformaldehyde. Subsequently, the cells were treated with 0.1% sodium citrate/0.1% Triton X-100 for 2 min on ice, and incubated with TUNEL reaction mixture for 60 min at 37°C. After TUNEL, cerebellar granule cells were incubated with blocking buffer (PBS with 10% goat serum and 0.1% Triton X-100) at RT for 1 h. Cells were incubated with anti-MAP2 antibody at 4°C overnight. Cells then were washed and incubated with Texas Red goat anti-rabbit secondary antibody (Invitrogen) at RT for 2 h. After washing, cells were incubated with 0.5 mg/ml DAPI (Invitrogen) at RT for 2 min. Cells were coverslipped with mounting medium. The cells were observed under inverted fluorescence microscope (AxioObserver, Carl Zeiss). TUNEL-labeled nuclei (green points) and total cells in five areas (0.152 mm2) were randomly selected from each slide and counted under a 40× objective by an observer blind to the protocol and who could not identify the slides. The ratio of number of TUNEL-positive cells to the total cell number was calculated.
2.6. Apoptotic DNA fragmentation assay
CGCs were pretreated with 1 µM telmisartan or 10 µM candesartan for 2 h, followed by 24 h of 100 µM glutamate incubation. The cells were pelleted and DNA fragmentation was detected by Apoptotic DNA Ladder Detection Kit (Millipore) according to the manufacturer's instruction. The cells were lysed by Tris-EDTA (TE) buffer, incubated with RNase A at 37°C for 10 min and Proteinase K at 55°C for 30 min, respectively. After ammonium acetate was added to the sample, DNA was precipitated at −20°C for 2 h with isopropanol and samples were centrifuged for 10 minutes at 16,000× g. Pellets were washed by ethanol, dried and dissolved in DNA suspension buffer. The DNA ladder was visualized under UV light with ethidium bromide staining.
2.7. Measurement of caspase-3 activity
Cells pretreated with 1 µM telmisartan for 2 h, and then were exposed to 100 µM glutamate for 24 h. The activity of caspase-3 was measured by Caspase-3/CPP32 Colorimetric Protease Assay (Invitrogen). Cells were lysed in 50 µl chilled lysis buffer on ice for 10 min. Cell lysates containing 100 µg of proteins were diluted in 50 µl lysis buffer and 50 µl reaction buffer containing 10 mM dithiothreitol (DTT). Caspase-3 DEVD-pNA substrate (200 µM final concentration) was added to react for 2 h at 37°C in the dark. The results were recorded in a microplate reader at 405 nm.
2.8. Angiotensin II receptor binding
Cells were exposed to 100 µM glutamate for indicated times and used for the isolation of cell membrane proteins. Attached CGCs (15 million) were harvested and homogenized in ice-cold buffer containing 10 mM Tris-HCl pH 7.5. Crude membrane fractions were pelleted by centrifugation at 1,000× g for 20 min at 4°C. Supernatants were then centrifugated at 20,000× g for 20 min at 4°C and the pellets were resuspended in ice cold buffer containing 50 mM Tris-HCl and 1 mM EDTA following by centrifugation at 20,000× g for 20 min at 4°C. After next washing step (50 mM Tris-HCl and 1 mM EDTA) and centrifugation (at 20,000× g for 20 min at 4°C), pellets were resuspended in a small volume of binding incubation buffer containing 1 mM KH2PO4, 5 mM Na2HPO4, 120 mM NaCl and 5 mM EDTA. Protein content was assessed by the Bradford reagent.
The binding assay was performed as previously described (Heemskerk et al., 1999). Binding to Angiotensin II receptors was carried out in Eppendorf tubes at 22°C for 120 min in a volume of 0.3 ml with 0.075 nM [125I]Sar1Ile8-Angiotensin II (ARC, St Louis, MO) in incubation buffer (same as described above) supplemented by 50 mg/L bacitracin (Sigma Aldrich) and 2 g/L albumin (protease free) (Sigma Aldrich) with 70–100 µg of membrane protein. Non-specific binding of [125I]Sar1Ile8-Angiotensin II was determined in the presence of 10 µM unlabeled Angiotensin II (Sigma Aldrich). Binding to AT1 receptors was the binding displaced in membrane aliquots incubated as above in the presence of the AT1 receptor blocker losartan (10 µM). The binding was terminated by rapid chilling to 4°C, centrifugation for 10 min at 16,000× g and immediate aspiration of the supernatant. Subsequently the bottom part of the tube was cut and counted in a γ-counter (Clinigamma, LKB, Piscataway, NJ).
2.9. Quantitative real-time PCR
To determine gene expression, total RNA was isolated at indicated using 1 ml TRIzol (Invitrogen), followed by purification using an RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer instructions. Synthesis of complementary DNA (cDNA) was performed by using 0.6 µg of total RNA and Super-Script III first-Strand Synthesis kit (Invitrogen). Quantitative real-time polymerase chain reaction (qPCR) was performed on DNA Engine Opticon™ (MJ Research, Waltham, MA) with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). qPCR was performed in a 20 µl reaction mixture containing 10 µl SYBR Green PCR Master Mix, 4 µl cDNA and 0.3 µM of each primer for a specific target. Primers for qPCR were synthesized by BioServe (Beltsville, MD). The specific primers are listed in Table 1. The remaining reagents for RNA isolation and reverse transcription were from Invitrogen. The amplification conditions consisted of one denaturation/activation cycle at 95°C for 10 min, followed by 45 cycles at 95°C for 15 sec and 60°C for 60 sec. Serial dilutions of cDNA from the same source as samples were used to obtain a standard curve. The individual targets for each sample were quantified by determining the cycle threshold (Ct) and by comparison with the standard curve. The relative amount of the target mRNA was normalized with the housekeeping gene GAPDH.
Table 1.
List of primers used for qPCR
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| AT1Aa | AGCCTGCGTCTTGTTTTGAG | GCTGCCCTGGCTTCTGTC |
| AT1Bb | CACCTCGCCAAGGGAGAC | CACTTGCAGGCTTTGAACC |
| AT2c | AACCGGCAGATAAGCATTTG | CAGCCACAGCCAGATTGAAG |
| PPARγd | ACCACGGTTGATTTCTCCAG | CAACCATTGGGTCAGCTCTT |
| ABCG1e | GAAGGTTGCCACAGCTTCTC | CATGGTCTTGGCCAGGTAGT |
| COX-2f | CGGAGGAGAAGTGGGGTTTAGGAT | TGGGAGGCACTTGCGTTGATGG |
| IL-1βg | CCTCTGCCAAGTCAGGTCTC | GAATGTGCCACGGTTTTCTT |
| GAPDHh | ATGACTCTACCCACGGCAAG | TGGAAGATGGTGATGGGTTT |
Angiotensin II receptor type 1A;
Angiotensin II receptor type 1B;
Angiotensin II receptor type 2;
Peroxisome proliferator-activated receptor gamma;
ATP-binding cassette sub-family G member 1;
Cyclooxygenase isoform 2;
Interleukin 1 beta;
Glyceraldehyde-3-phosphate dehydrogenase
2.10. Electrophoretic mobility shift assay
Rat cerebellar granule cells were incubated for 4 h with DMSO (Sigma-Aldrich), 1 µM telmisartan and 10 µM pioglitazone. Nuclear protein extracts were prepared using Nuclear Extraction kit (Pierce, Rockford, IL), according to the manufacturer’s instructions. Electrophoretic mobility shift assay (EMSA) was carried out using Light-Shift Chemiluminescent EMSA kit (Pierce) with double-stranded DNA probe 5′-GGTAAAGGTCA AAGGTCAATCGGC-3′ labeled with biotin at the 5′-end. Nuclear proteins (4 µg) were incubated for 30 min at room temperature in binding buffer containing 2.5 mM MgCl2, 5% glycerol, 0.05% NP-40, 0.25 µg poly dI:dC and 0.2 µg acetylated bovine serum albumin with 1 nM biotin-labeled probe. For competition assays, 100-fold excess of non-labeled probe was added 10 min prior to biotin-labeled probe. To determine the DNA-binding specificity, 2 µg of rabbit polyclonal antibody against PPARγ or PPARα (ABR-Affinity BioReagents, Golden, CO) was added to binding reaction 30 min before the probe addition. Protein-DNA complexes were separated by electrophoresis on 6% DNA Retardation gels (Invitrogen), transferred onto Hybond-N+ nylon membrane (GE healthcare, Piscataway, NJ) and cross-linked with UV light. The bands were visualized by chemiluminescence.
2.11. Western blotting
Cells were pre-incubated with the PPARγ antagonist or/and telmisartan for 2 h, followed by addition of 100 µM glutamate or saline for another 24 h. To determine phospho-proteins blots, cells were lysed in Tris-Glycine SDS lysis buffer (Invitrogen) in 1 h after glutamate addition, and the lysate was boiled for 10 min. The extracted proteins were separated by electrophoresis on 10% SDS-PAGE gels and transferred onto PVDF membranes. The membranes were blocked for 1 h in blocking buffer containing 5% BSA (Sigma-Aldrich) and incubated overnight at 4°C with the primary antibody followed by washing and exposure to the secondary antibody for 30 min at room temperature. The membranes were exposed to SuperSignal West Dura Substrate (Thermo Scientific) for chemiluminescent detection. After detection of phospho-proteins, the membranes were stripped for 15 min at room temperature in Restore Western Blot Stripping Buffer (Thermo Scientific), blocked for 1 h in casein-based blocking buffer (Sigma-Aldrich), exposed to total proteins or β-actin antibody and chemiluminescence was detected as above. Primary antibodies used for Western blotting analysis were: mouse monoclonal antibody to β-Actin (1:10,000) (Sigma-Aldrich,St. Louis, MO); phospho-Akt (Ser473, 1:2000), Akt (1:1000), phospho-GSK-3β (Ser9, 1:1000), GSK-3β (1:1000), phospho-ERK1/2 (1:1000), ERK1/2 (1:1000) (Cell Signaling Technology, Danvers, MA); rabbit antibody to microtubule-associated protein 2 (MAP-2) (1:200) (EMD Millipore, Billerica, MA); donkey anti-rabbit IgG (1:5000) (Amersham BioSciences Piscataway, NJ); goat anti-mouse IgG (1:5000) (Jackson ImmunoResearch, West Grove, PA); and Texas Red goat anti-rabbit IgG (1:500) (Vector Laboratories, Burlingame, CA). Protease inhibitor cocktail and SuperSignal West Dura Substrate for chemiluminescent detection were purchased from Thermo Fisher Scientific (Pittsburg, PA). All other reagents were obtained from Sigma-Aldrich unless indicated otherwise.
2.12. Statistics
Statistical significance was determined using GraphPad Prism 5 Software (GraphPad Software, San Diego, CA). Multiple group comparisons were performed by one-way ANOVA followed by Newman-Keuls posttest. Comparison data of sartan effects on rat CGCs and telmisartan effects on CGCs from AT1A knock-out mice were analyzed by two-way ANOVA, followed by Bonferroni’s post hoc test. Significance between approximate IC50s was assessed using one-way ANOVA followed by Bonferroni’s multiple comparisons test after log transformation. Differences were considered statistically significant at P < 0.05.
3. Results
3.1. Sartans attenuate glutamate-induced neurotoxicity in primary cultures of rat CGCs
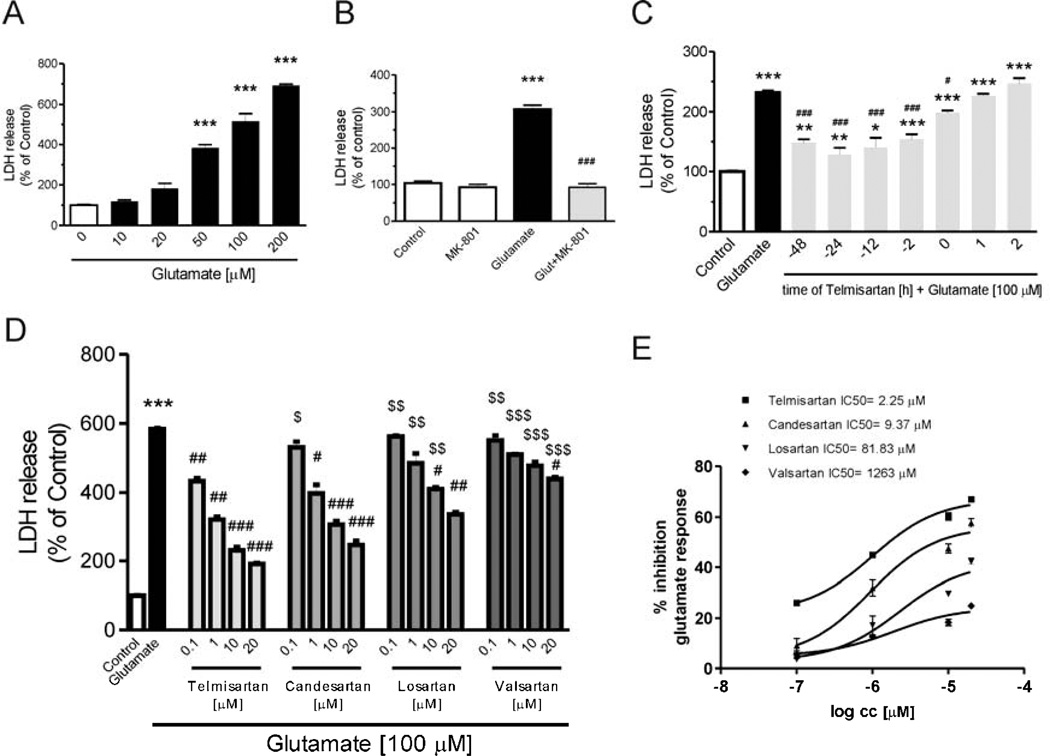
Exposure of primary rat CGC cultures to glutamate concentrations from 10 to 200 µM for 24 h, dose-dependently increased LDH activity in the culture media, indicative of neuronal injury (Fig. 1A). The NMDA receptor antagonist MK801 (10 µM) abolished the glutamate-induced LDH release (Fig. 1B).
Fig. 1.
Angiotensin II AT1 receptor blockers prevent glutamate-induced lactate dehydrogenase (LDH) release in rat CGCs. (A) CGCs were treated for 24 h with different doses of glutamate to determine LDH release. (B) CGCs were treated with 10 µM MK-801 (N-methyl-d-aspartate (NMDA) receptor antagonist) and 100 µM glutamate for 24 h. (C) CGCs were treated with 1 µM telmisartan (Telm) for different times before or after 24 h of glutamate exposure (100 µM). (D and E) CGCs were pretreated with different concentrations of telmisartan (Telm), candesartan (Cand), losartan (Los) or Valsartan for 2 h, followed by treatment with 100 µM glutamate for another 24 h. (D) Comparison of individual doses for each sartan studied. (E) Comparison of approximate IC50s. In all cases, neuronal injury was studied by measurement of LDH release, detected by the LDH Activity Assay kit as described in Material and Methods. Results are means ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Control; #P < 0.05, ###P < 0.001 vs. Glut; $P < 0.05, $$P < 0.01, $$$P < 0.001 vs. the corresponding concentration of Telm. All approximate IC50s are different from each other at the P < 0.01 level, except for Losartan vs. Valsartan that were different at P < 0.05.
Telmisartan, when added 2 to 48 hours before glutamate incubation, significantly decreased glutamate-induced neuronal injury (Fig. 1C). Reduced, but significant neuroprotection was achieved when telmisartan (1 µM) was added together with glutamate (Fig. 1C). Conversely, there was no neuroprotection when telmisartan was added after glutamate exposure (Fig. 1C).
Pretreatment with telmisartan, candesartan, losartan or valsartan (0.1 to 20 µM) for 2 hours prior to glutamate exposure, dose-dependently decreased LDH release, with order of potency: telmisartan > candesartan > losartan > valsartan (Fig. 1D and 1E). Corresponding approximate IC50s were: telmisartan: 2.25, candesartan 9.37, losartan 81.83 and valsartan 1263 µM, respectively”
3.2. Telmisartan reduces glutamate-induced apoptosis in rat CGCs
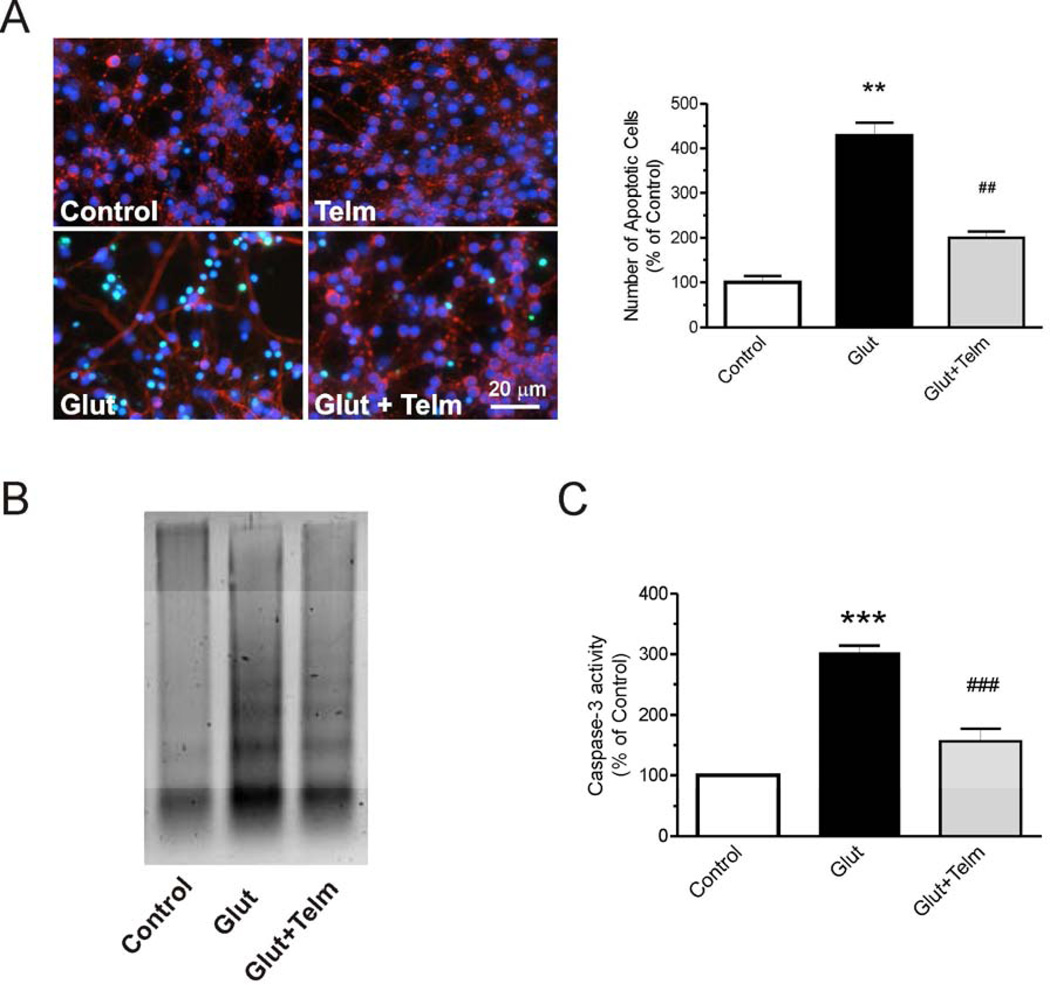
Telmisartan was chosen to determine its effects on glutamate-induced neuronal apoptosis in rat CGC primary cultures. Exposure of CGCs to 100 µM glutamate markedly increased the number of TUNEL stained cells (Fig. 2A). Preincubation with 1 µM telmisartan reduced glutamate-induced increase in TUNEL staining by 75% (Fig. 2A).
Fig. 2.
Telmisartan protects CGCs against glutamate-induced apoptosis. CGCs were pretreated with 1 µM telmisartan (Telm) for 2 h, followed by treatment with 100 µM glutamate for another 24 h. (A) Telmisartan significantly reduced the number of apoptotic cells as determined using the In Situ Cell Death Detection kit. (B) DNA laddering determined by the DNA fragmentation assay. The Figure represents a typical experiment repeated three times. (C) Caspase-3 activity was determined by the Caspase-3/CPP32 Colorimetric Protease Assay and is presented as means ± SEM from three independent experiments. All results are presented as means ± SEM from three independent experiments. **P < 0.01, ***P < 0.001 vs. Control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. Glutamate.
Pretreatment with telmisartan (1 µM) for 2 hours significantly reduced glutamate-induced DNA fragmentation (Fig. 2B).
Exposure of CGCs to 100 µM glutamate induced caspase-3 activity (Fig. 2C). Pretreatment with telmisartan (1 µM) for two hours reduced the glutamate-induced increase in caspase-3 activation (Fig. 2C).
3.3. Telmisartan reduces glutamate-induced inflammation in rat CGCs
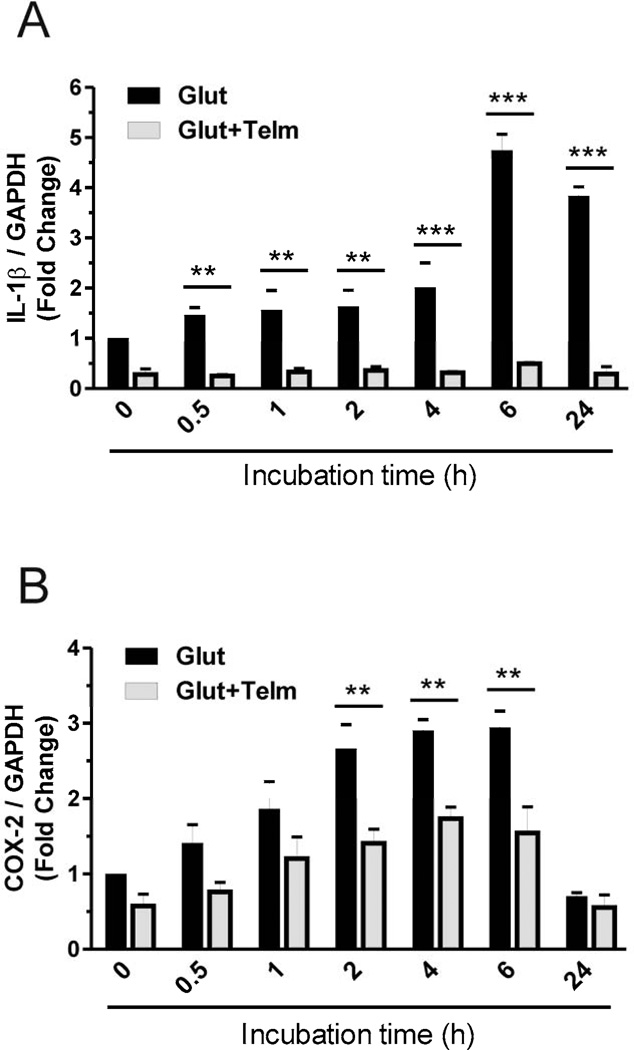
Pretreatment with telmisartan (1 µM), two hours before exposure to 100 µM glutamate, completely prevented the glutamate-induced upregulation of IL-1β (Fig. 3A) and significantly reduced glutamate-induced cyclooxygenase-2 (COX-2) (Fig. 3B) mRNA expression.
Fig. 3.
Telmisartan prevents glutamate-induced inflammation in rat CGCs. CGCs were treated with 1 µM telmisartan (Telm) or vehicle, and two hours later were exposed for different time points to 100 µM glutamate (Glut). The gene expressions of IL-1β (A) and COX-2 (B) were determined by qPCR as described in Materials and Methods. Results are means ± SEM of at least three independent experiments. **P < 0.01, ***P < 0.001 Glut vs. Glut+Telm.
3.4. The anti-apoptotic effect of telmisartan is mediated through the PI3K/Akt/GSK-3β and the ERK1/2 pathways
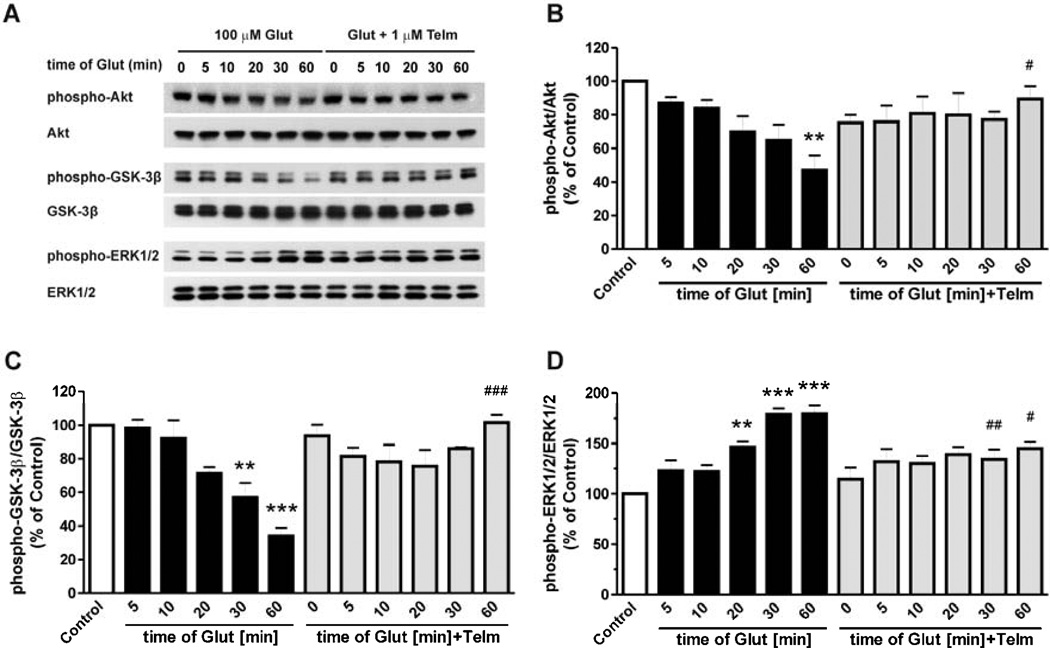
Exposure of CGCs to glutamate produced a time-dependent decrease in Akt and GSK-3β phosphorylation, and a time-dependent increase in ERK1/2 phosphorylation (Fig. 4), indicative of a reduction of Akt activation, and enhanced GSK-3β and ERK1/2 activity. Co-incubation of telmisartan prevented the glutamate-induced alterations in Akt/GSK-3β and ERK1/2 activation (Fig. 4).
Fig. 4.
Telmisartan attenuates the glutamate-induced alterations of Akt, GSK-3β and ERK1/2 phosphorylation. CGCs were pre-treated with 1 µM telmisartan (Telm) for 2 h followed by exposure to 100 µM glutamate (Glut) for the indicated time intervals. Total and phosphorylated Akt, GSK-3β and ERK1/2 were determined by Western blotting (A). Results for Akt (B), GSK-3β (C) and ERK1/2 (D) are shown as a percentage of the control group. All results are presented as means ± SEM from three independent experiments. **P < 0.01, ***P < 0.001 vs. Control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. the corresponding time of glutamate treatment.
3.5. Angiotensin II AT1A receptors, but not AT1B or AT2 receptors, participate in the glutamate-induced neuronal injury and apoptosis
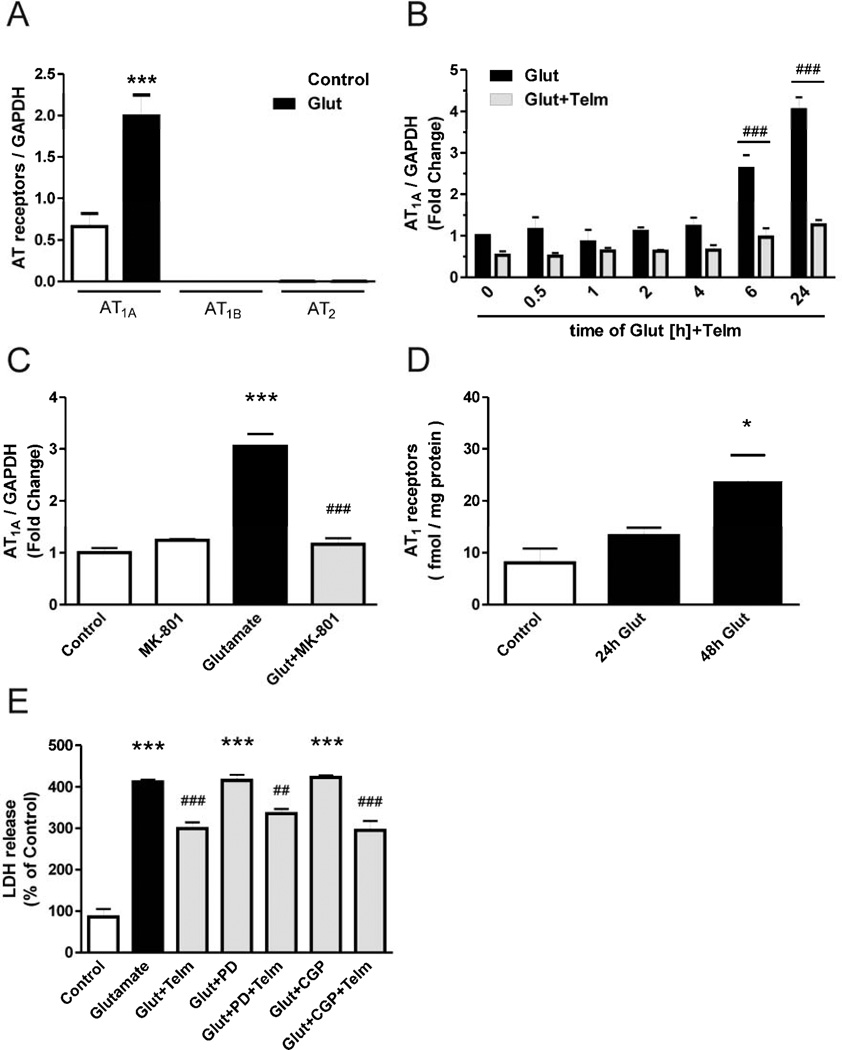
Gene expression of Angiotensin II AT1A, AT1B and AT2 receptors was determined in primary rat CGC cultures exposed to 100 µM glutamate or vehicle for 24 hours. While there was substantial AT1A gene expression, the gene expression of AT1B and AT2 receptors was below the detection limit of our method (Fig. 5A). Exposure to glutamate significantly increased AT1A receptor gene expression (Fig. 5A, 5B, 5C), and the protein expression of AT1 receptors as determined by receptor binding (Fig. 5D) in a time-dependent fashion. The glutamate-induced increase in AT1A receptor gene expression was completely abolished by simultaneous addition of the NMDA receptor antagonist MK801 (10 µM) (Fig. 5C). Conversely, glutamate did not modify either AT1B or AT2 gene expression (Fig. 5A). Furthermore, preincubation with the AT2 receptor antagonist PD123319 or the AT2 receptor agonist CGP42112 did not change glutamate-induced LDH release or the effect of telmisartan on glutamate-induced LDH release (Fig. 5E).
Fig. 5.
AT1A receptors, but not AT1B or AT2 receptors, are involved in telmisartan neuroprotection. (A) Angiotensin II receptor gene expression after exposure to glutamate. AT1A, AT1B and AT2 gene expression were determined in rat CGCs after 24 hour exposure to glutamate. (B) CGCs were pre-treated with 1 µM telmisartan (Telm) for 2 h followed by exposure to 100 µM glutamate (Glut) for the indicated time intervals. (C) CGCs were simultaneously exposed to the NMDA receptor antagonist MK-801 (10 µM) and glutamate, and AT1A gene expression was determined 24 hours later. (D) CGCs were exposed to glutamate and AT1 receptor expression was determined by the Angiotensin II receptor binding assay, 24 and 48 hours later. (E) CGCs were treated with the AT2 receptor antagonist PD123319 (PD, 10 µM) or the AT2 receptor agonist CGP42112A (CGP, 10 µM) for one hour, followed by treatment with vehicle or telmisartan (1 µM) for two hours, followed by exposure to glutamate for 24 hours, followed by determination of LDH activity in the culture supernatant. Data are presented as means ±SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Control; ##P < 0.01, ###P < 0.001 vs. Glutamate.
3.6. The neuroprotective effect of telmisartan is partially dependent on PPARγ activation
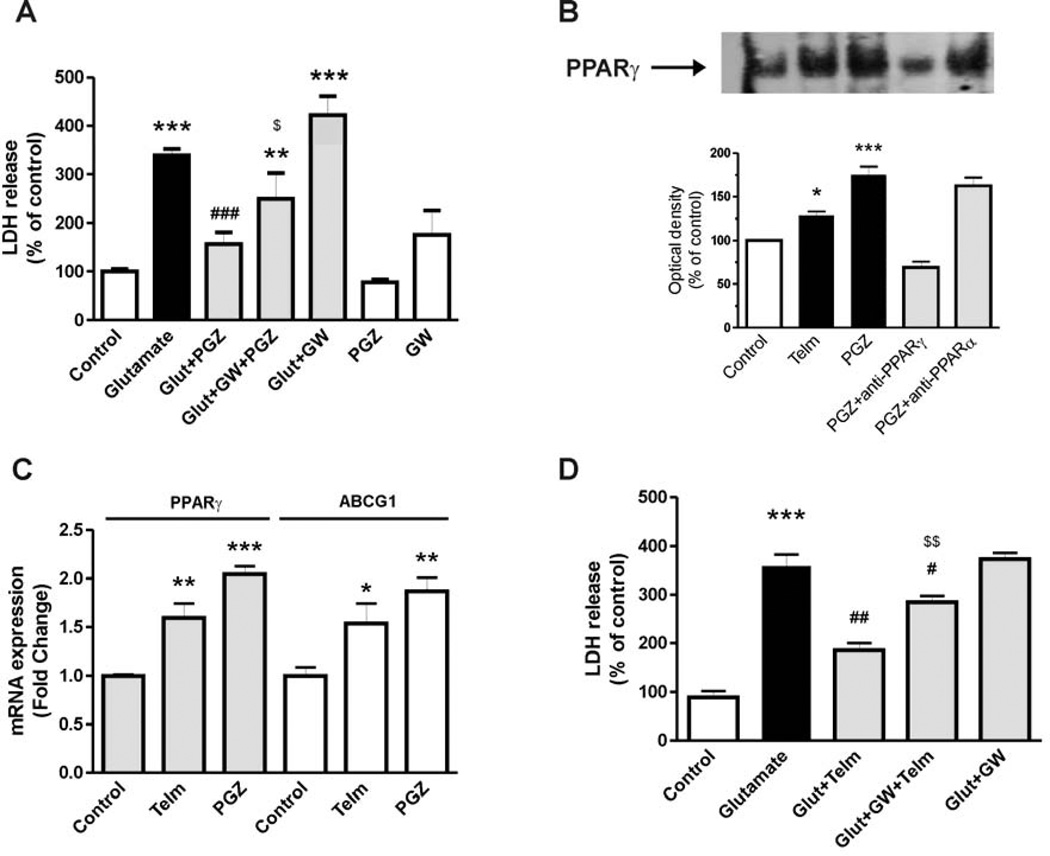
The PPARγ agonist pioglitazone (10 µM) significantly decreased glutamate-induced neuronal injury, as determined by the LDH activity assay (Fig. 6A). The effect of pioglitazone was partially blocked by concomitant incubation with the PPARγ antagonist GW9662 (20 µM) (Fig. 6A).
Fig. 6.
PPARγ activation is partially involved in the neuroprotective effect of telmisartan in rat CGCs. (A) CGCs were pre-treated with the PPARγ agonist pioglitazone (PGZ) (10 µM) for 2 hours followed by exposure to glutamate (Glut) for 24 hours. The PPARγ antagonist GW9662 (GW, 20 µM) was added 2 hours before PGZ treatment. (B) CGCs were treated with 1 µM telmisartan (Telm) or 10 µM pioglitazone (PGZ) for 4 hours to determine nuclear PPARγ activity using the Electrophoretic Mobility Shift Assay. Figure is a representative picture showing PPARγ-DNA binding. Intensity was measured by densitometry for quantitative analysis. Anti-PPARγ and anti-PPARα antibodies (2 µg) were used to determine the specificity of the shift. (C) CGCs were treated with 1 µM telmisartan or 10 µM pioglitazone for 24 hours to determine expression of the PPARγ target gene ABCG1. (D) CGCs were pre-treated with 20 µM GW9662 for 2 hours, followed by 2 hours exposure to telmisartan, followed by exposure to glutamate for 24 hours. Data are presented as means ±SEM of three independent experiments. *P < 0.05, ***P < 0.001 vs. Control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. Glutamate; $P < 0.05 vs. Glut+PGZ; $$P < 0.01 vs. Glut+Telm.
Telmisartan (1 µM) and pioglitazone (10 µM) significantly enhanced PPARγ activation in CGCs (Fig. 6B). Incubation in the presence of a selective PPARγ antibody (2 µg) prevented PPARγ-DNA binding activity, while a PPARα antibody (2 µg) did not (Fig. 6B).
Telmisartan (1 µM) and pioglitazone (10 µM) significantly increased gene expression of PPARγ and the PPARγ target gene, ATP-binding cassette sub-family G member 1 (ABCG1) (Fig. 6C).
The PPARγ antagonist GW9662 (20 µM) partially reversed the reduction of the glutamate-induced LDH release by telmisartan (Fig. 6D).
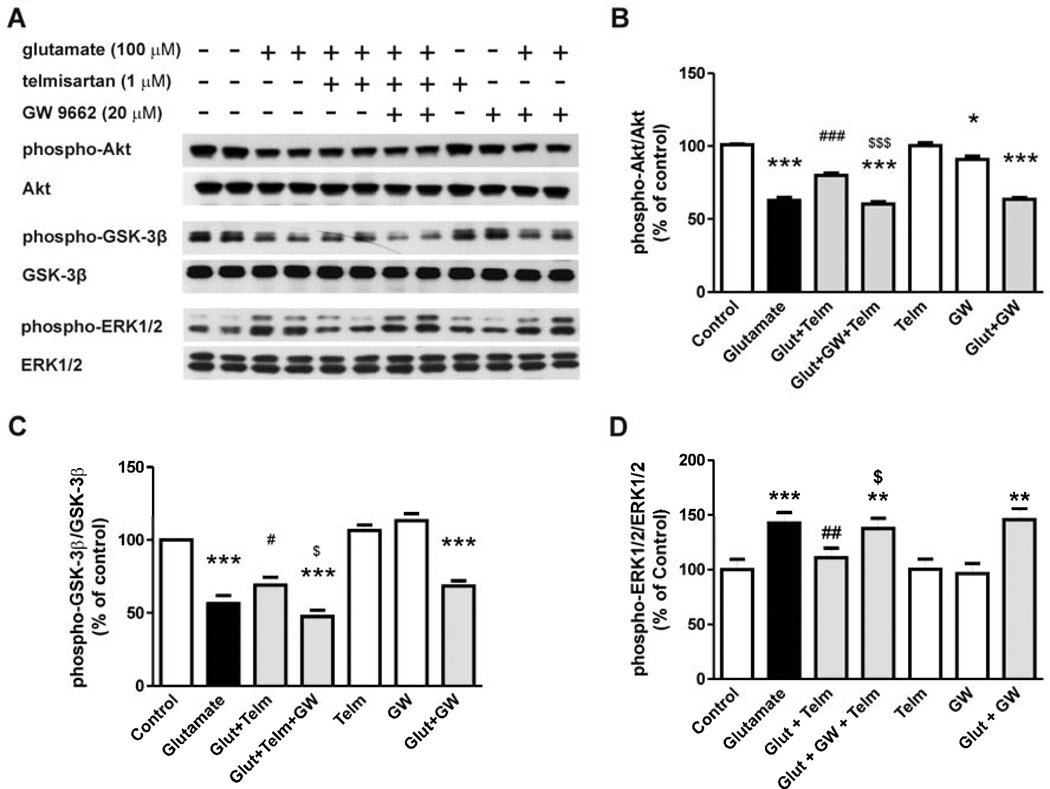
In rat CGCs, the telmisartan (1 µM) preventive effects on glutamate-induced Akt dephosphorylation (Fig. 7A, 7B), GSK-3β (Ser9) dephosphorylation (Fig. 7A, 7C) and ERK1/2 phosphorylation (Fig. 7A, 7D) were significantly reduced by the PPARγ antagonist GW9662 (20 µM) (Fig. 7).
Fig. 7.
Telmisartan prevents the glutamate-induced alterations in Akt/GSK-3β and ERK1/2 in rat CGCs, and the telmisartan effect is reduced by exposure to a PPARγ antagonist. CGCs were pre-treated with 1 µM telmisartan (Telm) followed by 1 hour exposure to glutamate (Glut). GW9662 (GW, 20 µM) was added 2 hours before telmisartan treatment. (A) Shown are representative Western blots for each protein level. Ratios of total and phospho-Akt (B), phospho-GSK-3β (C) and phospho–ERK1/2 (D) are presented as means ± SEM from three independent experiments. All results are shown as a percentage of the control group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. Glut; $P < 0.05, $$$P < 0.001 vs. Glut+Telm.
3.7. Glutamate-induced neuronal injury is only partially dependent on AT1A receptor expression
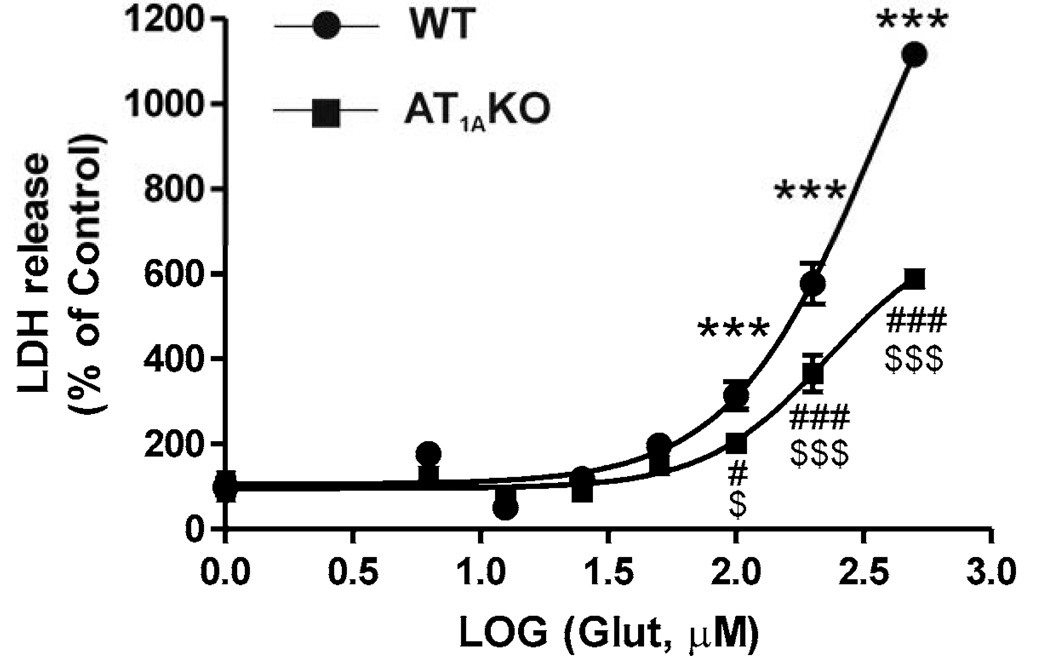
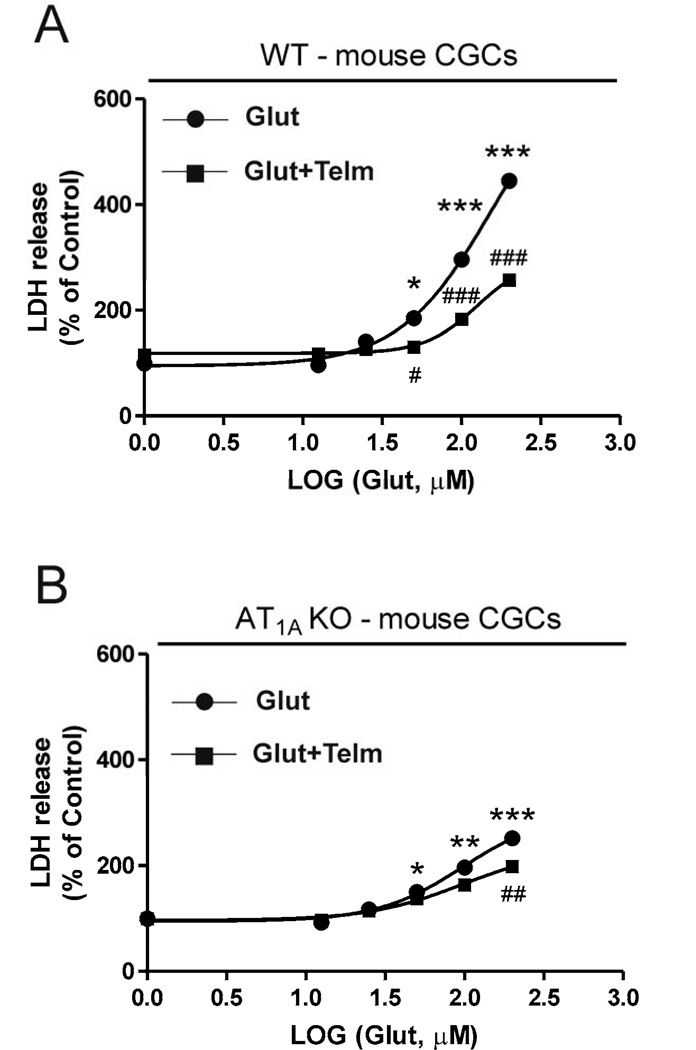
Exposure of primary CGCs obtained from wild-type or AT1A knock-out mice to glutamate (6.25 to 500 µM) induced a dose-dependent neuronal injury as determined by LDH release into the incubation media, with a threshold of 100 µM (Fig. 8). The maximum glutamate-induced LDH release was significantly lower in CGCs obtained from AT1A knock-out mice when compared to wild type mice (Fig. 8).
Fig. 8.
Glutamate-induced neuronal injury is reduced in CGCs isolated from AT1A receptor knock-out mice (KO) when compared with wild-type controls (WT). Primary CGCs from wild-type or AT1A knock-out mice were exposed to different doses of glutamate (6.25 to 500 µM). The maximum glutamate-induced LDH release was significantly lower in CGCs obtained from AT1A knock-out mice when compared to those from wild-type mice. Results are Means ± SEM for groups of three independent experiments. ***P < 0.001 vs. WT untreated controls ; #P < 0.05, ###P < 0.001 vs. AT1A KO untreated controls; $P < 0.05, $$$P < 0.001 WT vs. AT1A KO.
3.8. Telmisartan neuroprotection against glutamate-induced neurotoxicity is reduced but not eliminated in CGCs isolated from AT1A knock-out mice
Pretreatment with telmisartan (1 µM) significantly reduced glutamate-induced LDH release from CGCs obtained from wild-type (Fig. 9A) or AT1A knock-out mice (Fig. 9B).
Fig. 9.
Glutamate-induced neuronal injury is only partially dependent on AT1A receptors in mouse CGCs. Primary CGCs from wild-type (A) or AT1A knock-out (B) mice were exposed to glutamate (Glut) (100 µM) with or without telmisartan (Telm) (1 µM). Treatment with telmisartan significantly reduced glutamate-induced LDH release from CGCs obtained from wild-type (A) or AT1A knock-out (B) mice. Results are Means ± SEM for groups of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. untreated controls; #P < 0.05, ##P < 0.01, ###P < 0.001 Glut vs. Glut+Telm.
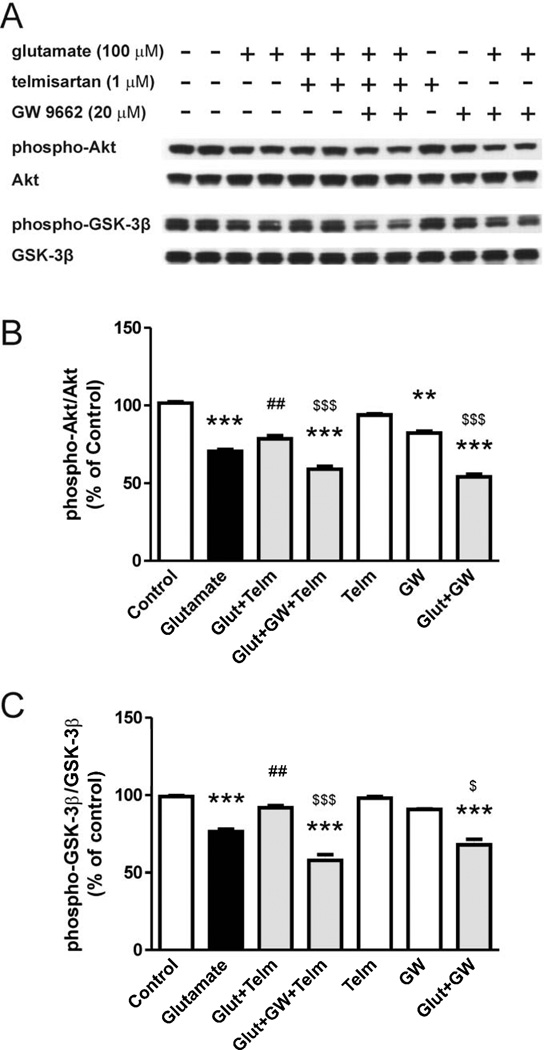
3.9. Telmisartan neuroprotection in CGCs isolated from AT1A knock-out mice is partially dependent on PPARγ activation
In CGCs isolated from AT1A knock-out mice, pretreatment with telmisartan (1 µM) partially reversed glutamate-induced decrease in the phosphorylation of Akt (Fig. 10A, 10B) and GSK-3β (Fig. 10A, 10C). Incubation with the PPARγ antagonist GW9662 partially reversed telmisartan effects.
Fig. 10.
Telmisartan prevents the glutamate-induced alterations in Akt and GSK-3β phosphorylation in AT1A-deficient CGCs isolated from AT1A knock-out mice. The telmisartan effect is reduced by PPARγ inhibition. CGCs were pretreated with 1 µM telmisartan (Telm) followed by 1 hour exposure to glutamate (Glut) (100 µM). GW9662 (GW, 20 µM) was added 2 hours before telmisartan treatment. (A) Shown are representative Western blots for each protein level. Ratios of total and phospho-Akt (B) and phospho-GSK-3β (C) are presented as means ± SEM from three independent experiments. All results are shown as a percentage of the control group. **P < 0.01, ***P < 0.001 vs. control; ##P < 0.01 vs. Glut; $P < 0.05, $$$P < 0.001 vs. Glut+Telm.
4. Discussion
This study was designed to test the hypothesis that sartans, and in particular telmisartan, are directly neuroprotective in primary neuronal cultures. We tested sartan neuroprotection against glutamate-induced excitotoxicity, a major factor leading to neuronal injury in inflammatory and neurodegenerative diseases of the brain (Chamoun et al., 2010; Coyle and Puttfarcken, 1993; Lau and Tymianski, 2010; Tian et al., 2008). The present study demonstrated that telmisartan, the most potent sartan tested, was directly and significantly neuroprotective in our preparations.
The principal observations in this study are: a) in primary neuronal cultures, telmisartan very effectively ameliorates glutamate-induced neuronal injury, apoptosis and inflammation; b) AT1A, but not AT1B or AT2 receptors, are involved in telmisartan effects; c) the beneficial effects of telmisartan are partly the result of PPARγ activation in addition to AT1 receptor blockade; d) telmisartan neuroprotection is associated with prevention of glutamate-induced inhibition of Akt/GSK-3β phosphorylation and stimulation of the ERK1/2 pathway.
We proposed that the neuroprotective effects of telmisartan were associated with its direct AT1R blockade effects. Angiotensin II stimulates two receptor types, the AT1 and AT2 receptors (De Gasparo et al., 2000). Rodents, but not humans, express two AT1 receptor subtypes, the AT1A and AT1B receptors (Chiu et al., 1993; Sasamura et al., 1992), and most of the central effects of AT1 receptor stimulation correspond to AT1A receptor activation (Saavedra, 2012). While excessive AT1 receptor stimulation was associated with brain inflammation and cell injury (Saavedra, 2012), stimulation of AT2 receptors has been proposed to exert balancing neuroprotective effects, particularly when AT1 receptors are blocked by ARB administration (Mogi et al., 2006; Zhao et al., 2005). We found that primary CGCs expressed AT1A receptor mRNA, while AT1B and AT2 mRNAs were undetectable in these cells. Furthermore, exposure of CGCs to PD123319 (an AT2 receptor antagonist) or CGP42112 (an AT2 receptor agonist) did not change the effects of glutamate or modified the neuroprotective effect of telmisartan. These results indicated that the neuroprotective effect of telmisartan in primary CGCs was associated with AT1A receptor blockade, without AT1B or AT2 receptor participation. Further indications of AT1A receptor participation in the neurotoxic effect of glutamate include the NMDA-dependent upregulation of AT1A receptor transcription and expression, and the reduction of glutamate neurotoxicity in CGC preparations from AT1A receptor knock-out mice not expressing AT1 receptors. A similar neuroprotective role for AT1, but not AT2 receptors, has been earlier reported in SK-N-SH human neuroblasts (Pang et al., 2012b). These cells expressed AT1 receptors, but not AT2 receptors, (Pang et al., 2012b). In SK-N-SH human neuroblasts, while telmisartan decreased IL-1β toxicity, PD123319 or CGP42112 were not effective (Pang et al., 2012b).
The neurotoxic effects of glutamate concentration, confirmed in this study, have been well characterized (Leng and Chuang, 2006; Leng et al., 2008). In our preparations, NMDA receptor stimulation by exposure of primary neuronal cultures to glutamate induced apoptosis, as demonstrated by increased DNA fragmentation, caspase-3 activation and characteristic morphological changes, and a significant inflammatory response, with large increases in COX-2 and IL-1β gene expressions. In the brain, COX-2 is an important component of cytotoxicity associated with inflammation (O’Banion et al., 1999). Neuronal COX-2 expression is upregulated following brain insults via glutamatergic and inflammatory mechanisms, and implicated in several neurological diseases including stroke and Alzheimer’s disease (Kaufmann et al., 1997). COX-2 inhibition is neuroprotective (Strauss and Marini, 2002), and COX-2 overexpression accelerates glutamate-mediated apoptotic damage (Mirjany et al., 2002). In turn, IL-1β enhances NMDA receptor-mediated neurotoxicity (Viviani et al., 2003).
Our results demonstrate that telmisartan significantly prevented glutamate-induced apoptosis and inflammation in CGCs, with a potency superior to that of other sartans studied. Individual sartans have very diverse pharmacological profiles, leading to marked differences in neuroprotective potency (Benson et al., 2004; Erbe et al., 2006; Miura et al., 2011). There is evidence that some sartans, in addition to AT1 receptor blockade, activate the anti-inflammatory nuclear receptor PPARγ, an important neuroprotective system (Kapadia et al., 2008; Min et al., 2012; Qi et al., 2010; Sauerbeck et al., 2011; Tsukuda et al., 2009; Yi et al., 2008). Prolonged candesartan administration upregulates PPARγ gene expression in adipose tissue (Zorad et al., 2006), and the beneficial effect of candesartan in a mouse model of traumatic brain injury (TBI) is in part the result of PPARγ activation (Villapol et al., 2012). Another sartan, losartan, has been reported to activate PPARγ in vivo (Koh et al., 2013) and in vitro (An et al., 2010). However, the PPARγ activating effect of telmisartan has been reported to be higher than that of candesartan and losartan (Benson et al., 2004; Erbe et al., 2006; Min et al., 2012; Tsukuda et al., 2009). When tested in primary cultures of CGCs, we found that telmisartan was a far more potent neuroprotective agent, than other sartans tested, with reduced (candesartan, losartan) PPARγ activating properties (Benson et al., 2004; Erbe et al., 2006), and even more potent than valsartan, a sartan without significant PPARγ activation properties (Fujino et al., 2010; Wang et al., 2007). This suggests that the PPARγ activating effect of telmisartan is a determinant of its higher neuroprotective efficacy.
The present results provide supportive evidence for this hypothesis. Telmisartan, at concentrations in the range of steady-state levels reported in clinical trials (Stangier et al, 2000), increases PPARγ activation, stimulating the gene expression of PPARγ and its target gene ABCG1 (Hodgkinson and Ye, 2003). The neuroprotective effect of telmisartan was partially decreased by exposure to a PPARγ antagonist. Telmisartan neuroprotection compared well with that of the classical PPARγ agonist pioglitazone. Furthermore, incubation with a PPARγ antagonist partially reduced telmisartan neuroprotection in CGCs from AT1A knock-out mice, devoid of AT1 receptors. The present observations support a previous report demonstrating that telmisartan amelioration of inflammatory injury to human monocytes is partially the result of PPARγ activation (Pang et al., 2012a). The higher PPARγ activation of telmisartan is explained by its unique structural characteristics and high lipophilicity, favoring its incorporation into the cell, and by its strong hydrophobic interactions at unique sites within PPARγ ligand domain (Benson et al., 2004; Erbe et al., 2006).
Although we demonstrate that PPARγ activation is important for sartan neuroprotection, our results do not totally exclude an association of PPARγ agonist effects with AT1 receptor blockade. There is cross-talk between AT1 and PPARγ activation; PPARγ agonists reduce AT1-mediated inflammation and hypertension in vivo (Ji et al., 2009), and downregulate AT1 expression (Zhao et al., 2008), whereas Angiotensin II downregulates PPARγ mRNA expression (Tham et al., 2002).
Classical PPARγ agonists are neuroprotective (Kapadia et al., 2008; Qi et al., 2010; Sauerbeck et al., 2011). However, the use of classical PPARγ agonists such as the thioglitazones is limited by their clinical toxicity (Kung and Henry, 2012). Telmisartan is a FDA-approved drug for the treatment of cardiovascular and metabolic disease (Suksomboon et al., 2012), which maybe also have the potential as a neuroprotective agent.
Our results demonstrate that telmisartan amelioration of glutamate-induced cell injury is associated with inhibition of glutamate-induced ERK1/2 phosphorylation and with the reversal of glutamate-induced suppression of phosphorylated Akt and GSK-3β (Hu et al., 2013). This is in agreement with previous reports of a major participation of PI3K/Akt/GSK-3β and the ERK1/2 pathways in glutamate excitotoxicity (Dasari et al., 2008; Liu et al., 2012; Nishimoto et al., 2008) and in inflammation (Pang et al., 2012b). Incubation in the presence of glutamate reduced the Akt phosphorylation, and this is associated with decreased GSK-3β (Ser9) phosphorylation, indicative of GSK-3β activation. We report that telmisartan prevented the glutamate-induced decrease in Akt phosphorylation and the associated GSK-3β phosphorylation. Our results are consistent with the hypothesis that activation of PI3K/Akt is followed by subsequent GSK-3β inhibition and that inactivated GSK-3β attenuates glutamate-induced caspase-3 activation and neurotoxicity (Nishimoto et al., 2008).
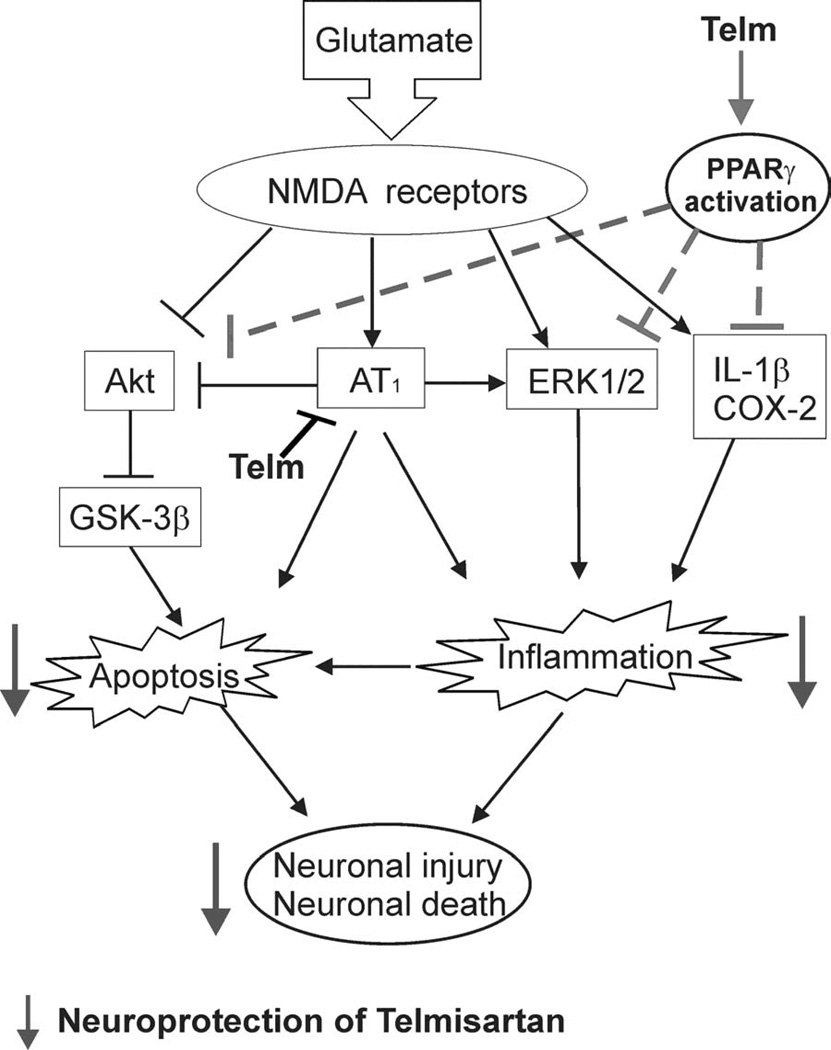
In conclusion, the present study showed that sartans potently and directly ameliorated glutamate-induced neuronal injury and apoptosis in a model of primary neuronal culture. Of the sartans studied, telmisartan was more potent than candesartan, losartan or valsartan. Telmisartan neuroprotection was in part the consequence of AT1 receptor blockade, since both glutamate neurotoxicity and telmisartan neuroprotection were decreased, but not eliminated, in CGCs obtained from mice devoid of AT1A receptor. In turn, part of the glutamate-induced neuronal injury may be the consequence of AT1A receptor upregulation by glutamate NMDA receptor stimulation. In addition to AT1 receptor blockade, telmisartan neuroprotection was partially dependent on PPARγ activation. As illustrated in Figure 11, mechanisms of sartan neuroprotection include suppression of glutamate-induced apoptotic pathways, PI3K/Akt/GSK-3β pathway and ERK1/2 activation.
Fig. 11.
Proposed mechanisms of telmisartan neuroprotection. Glutamate stimulates NMDA receptors and induces neuronal injury by increasing apoptosis and inflammation. Mechanisms include increased AT1 receptor expression, inhibition of the anti-apoptotic Akt pathway, and ERK1/2 stimulation. Telmisartan reduces glutamate-induced apoptosis and inflammation in CGCs. Telmisartan neuroprotection is the result of dual AT1 receptor blockade and PPARγ activation, decreasing apoptosis and inflammation by mechanisms involving a reduction of glutamate-induced alterations in the Akt/GSK-3β and ERK1/2 pathways.
The present results substantiate the use of sartans with dual AT1-blocking and PPARγ-activating properties as potential therapeutic agents in neurodegenerative and traumatic brain disorders, where glutamate-induced neuronal injury plays a significant role.
Significance of our results
Our results contribute to clarify the neuroprotective effects of ARBs, as demonstrated in pre-clinical rodent models of stroke, traumatic brain injury and neurodegenerative diseases such as Alzheimer’s disease, and further suggested by clinical reports (Ando et al., 2004; Benicky et al., 2011; Davies et al., 2011; Ito et al., 2002; Li et al., 2010; Nishimura et al., 2000; Saavedra et al., 2011; Timaru-Kast et al., 2012; Tsukuda et al., 2009; Yamakawa et al., 2003; Zhou et al., 2005). Excessive glutamate production is not only a significant factor in neuronal injury associated with neurodegenerative and traumatic disorders, but also participates in the mechanism of LPS and HIV-induced toxicity (Takaki et al., 2012; Cisneros and Ghorpade, 2012). For this reason, the use of sartans may be beneficial for the treatment of many brain disorders (Saavedra, 2012). Selecting telmisartan, the most potent sartan tested, will allow us, for the first time, to recruit a major but understudied neuroprotective mechanism, the activation of PPARγ. This, combined with the beneficial effects of Angiotensin II receptor blockade, makes telmisartan a very promising neuroprotective compound and substantiates the therapeutic use of this drug in neurodegenerative diseases and traumatic brain disorders where glutamate neurotoxicity plays a significant role.
Sartans (ARBs) protect against glutamate toxicity in cultured primary neurons.
Telmisartan is the most potent neuroprotective sartan.
Telmisartan neuroprotection involves the PI3K/Akt/GSK-3β and ERK1/2 pathways.
Glutamate neurotoxicity partially depends on Angiotensin AT1A receptor activation.
Telmisartan neuroprotection results from AT1 receptor blockade and PPARγ activation.
Acknowledgements
This study was supported by the Division of Intramural Research Programs, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, USA (MH 002762-16), the Natural Science Foundation of Jiangsu Province, P.R.China (BK20130653), the Fundamental Research Funds for the Central Universities, P.R.China (JKZD2013006), and the Initial Fund of China Pharmaceutical University to Dr. Tao Pang. None of the funding sources had any involvement on the study design, collection, analysis and interpretation of data, writing of the report or decision to submit the article for publication.
Abbreviations
- ABCG1
ATP-binding cassette sub-family G member 1
- AT1A
Angiotensin II receptor type 1A
- AT1B
Angiotensin II receptor type 1B
- AT1R
AT1 receptor
- AT2
Angiotensin II receptor type 2
- ARBs
Angiotensin II AT1 Receptor Blockers
- CGCs
cerebellar granule cells
- COX-2
cyclooxygenase 2
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- IL-1β
interleukin-1β
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- PPARγ
peroxisome proliferator-activated receptor γ
- qPCR
quantitative real-time polymerase chain reaction
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors state that none of them has any conflict of interest.
References
- An J, Nakajima T, Kuba K, Kimura A. Losartan inhibits LPS-induced inflammatory signaling through a PPARgamma-dependent mechanism in human THP-1 macrophages. Hypertens. Res. 2010;33:831–835. doi: 10.1038/hr.2010.79. [DOI] [PubMed] [Google Scholar]
- Anderson C. More indirect evidence of potential neuroprotective benefits of angiotensin receptor blockers. J. Hypertens. 2010;28:429. doi: 10.1097/HJH.0b013e3283371355. [DOI] [PubMed] [Google Scholar]
- Anderson C, Teo K, Gao P, Arima H, Dans A, Unger T, Commerford P, Dyal L, Schumacher H, Pogue J, Paolasso E, Holwerda N, Chazova I, Binbrek A, Young J, Yusuf S ONTARGET and TRANSCEND Investigators. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10:43–53. doi: 10.1016/S1474-4422(10)70250-7. [DOI] [PubMed] [Google Scholar]
- Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT1 receptor blockade reverses pathological remodeling and inflammation in brain microvessels of Spontaneously Hypertensive Rats. Stroke. 2004;35:1726–1731. doi: 10.1161/01.STR.0000129788.26346.18. [DOI] [PubMed] [Google Scholar]
- Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terrón JA, Falcón-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral administration of an angiotensin II AT(1) receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation Stress. Endocrinology. 2001;142:3880–3889. doi: 10.1210/endo.142.9.8366. [DOI] [PubMed] [Google Scholar]
- Bakris G. Are there effects of renin-angiotensin system antagonists beyond blood pressure control? Am. J. Cardiol. 2010;105:21A–29A. doi: 10.1016/j.amjcard.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Benicky J, Sánchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM. Angiotensin II AT(1) Receptor Blockade Ameliorates Brain Inflammation. Neuropsychopharmacology. 2011;36:857–870. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 2010;113:564–570. doi: 10.3171/2009.12.JNS09689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu AT, Dunscomb J, Kosierowski J, Burton CR, Santomenna LD, Corjay MH, Benfield P. The ligand binding signatures of the rat AT1A, AT1B and the human AT1 receptors are essentially identical. Biochem. Biophys. Res. Commun. 1993;197:440–449. doi: 10.1006/bbrc.1993.2499. [DOI] [PubMed] [Google Scholar]
- Chrysant SG, Chrysant GS, Chrysant C, Shiraz M. The treatment of cardiovascular disease continuum: focus on prevention and RAS blockade. Curr. Clin. Pharmacol. 2010;5:89–95. doi: 10.2174/157488410791110742. [DOI] [PubMed] [Google Scholar]
- Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr. HIV Res. 2012;10:392–406. doi: 10.2174/157016212802138832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A. Cerebellar granule cells as a model to study mechanisms of neuronal apoptosis or survival in vivo and in vitro. Cerebellum. 2002;1:41–55. doi: 10.1080/147342202753203087. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Dandona P, Kumar V, Aljada A, Ghanim H, Syed T, Hofmayer D, Mohanty P, Tripathy D, Garg R. Angiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-kappa B, in mononuclear cells of normal subjects: evidence of an anti-inflammatory action. J. Clin. Endocrinol. Metab. 2003;88:4496–4501. doi: 10.1210/jc.2002-021836. [DOI] [PubMed] [Google Scholar]
- Danielyan L, Klein R, Hanson L, Buadze M, Schwab M, Gleiter CH, Frey WH. Protective effects of intranasal losartan in the APP/PS1 transgenic mouse model of Alzheimer disease. Rejuvenation Res. 2010;13:195–201. doi: 10.1089/rej.2009.0944. [DOI] [PubMed] [Google Scholar]
- Dasari VR, Veeravalli KK, Saving KL, Gujrati M, Fassett D, Klopfenstein JD, Dinh DH, Rao JS. Neuroprotection by cord blood stem cells against glutamate-induced apoptosis is mediated by Akt pathway. Neurobiol. Dis. 2008;32:486–498. doi: 10.1016/j.nbd.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM. Associations of Anti-Hypertensive Treatments with Alzheimer’s Disease, Vascular Dementia, and Other Dementias. J. Alzheimers. Dis. 2011;26:699–708. doi: 10.3233/JAD-2011-110347. [DOI] [PubMed] [Google Scholar]
- De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Duan SZ, Usher MG, Mortensen RM. PPARs: the vasculature, inflammation and hypertension. Curr. Opin. Nephrol. Hypertens. 2009;18:128–133. doi: 10.1097/MNH.0b013e328325803b. [DOI] [PubMed] [Google Scholar]
- Erbe DV, Gartrell K, Zhang YL, Suri V, Kirincich SJ, Will S, Perreault M, Wang S, Tobin JF. Molecular activation of PPARgamma by angiotensin II type 1-receptor antagonists. Vascul. Pharmacol. 2006;45:154–162. doi: 10.1016/j.vph.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J. Cereb. Blood Flow Metab. 2009;29:640–647. doi: 10.1038/jcbfm.2008.158. [DOI] [PubMed] [Google Scholar]
- Fogari R, Mugellini A, Zoppi A, Marasi G, Pasotti C, Poletti L, Rinaldi A, Preti P. Effects of valsartan compared with enalapril on blood pressure and cognitive function in elderly patients with essential hypertension. Eur. J. Clin. Pharmacol. 2004;59:863–868. doi: 10.1007/s00228-003-0717-9. [DOI] [PubMed] [Google Scholar]
- Fujino M, Miura S, Kiya Y, Tominaga Y, Matsuo Y, Karnik SS, Saku K. A small difference in the molecular structure of angiotensin II receptor blockers induces AT1 receptor-dependent and-independent beneficial effects. Hypertens. Res. 2010;33:1044–1052. doi: 10.1038/hr.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XM, Margolis RL, Leeds P, Hough C, Post RM, Chuang DM. Carbamazepine induction of apoptosis in cultured cerebellar neurons: effects of N-methyl-D-aspartate, aurintricarboxylic acid and cycloheximide. Brain Res. 1995;703:63–71. doi: 10.1016/0006-8993(95)01066-1. [DOI] [PubMed] [Google Scholar]
- Heemskerk FM, Zorad S, Xu N, Gutkind SJ, Saavedra JM. Characterization of AT2 receptor expression in NIH 3T3 fibroblasts. Cell Mol. Neurobiol. 1999;19:277–288. doi: 10.1023/a:1006985329240. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CP, Ye S. Microarray analysis of peroxisome proliferator activated receptor-gamma induced changes in gene expression in macrophages. Biochem. Biophys. Res. Commun. 2003;308:505–510. doi: 10.1016/s0006-291x(03)01416-5. [DOI] [PubMed] [Google Scholar]
- Hu S, Cui W, Mak S, Tang J, Choi C, Pang Y, Han Y. Bis(propyl)-cognitin protects against glutamate-induced neuro-excitotoxicity via concurrent regulation of NO, MAPK/ERK and PI3-K/Akt/GSK3β pathways. Neurochem. Int. 2013;62:468–477. doi: 10.1016/j.neuint.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yamakawa H, Bregonzio C, Terrón JA, Falcón-Neri A, Saavedra JM. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an Angiotensin II AT1 antagonist. Stroke. 2002;33:2297–2303. doi: 10.1161/01.str.0000027274.03779.f3. [DOI] [PubMed] [Google Scholar]
- Jezova D, Ochedalski T, Kiss A, Aguilera G. Brain angiotensin II modulates sympathoadrenal and hypothalamic pituitary adrenocortical activation during stress. J. Neuroendocrinol. 1998;10:67–72. doi: 10.1046/j.1365-2826.1998.00182.x. [DOI] [PubMed] [Google Scholar]
- Ji Y, Liu J, Wang Z, Liu N, Gou W. PPARgamma agonist, rosiglitazone, regulates angiotensin II-induced vascular inflammation through the TLR4-dependent signaling pathway. Lab. Invest. 2009;89:887–902. doi: 10.1038/labinvest.2009.45. [DOI] [PubMed] [Google Scholar]
- Kaiser FC, Palmer GC, Wallace AV, Carr RD, Fraser-Rae L, Hallam C. Antianxiety properties of the angiotensin II antagonist, DUP 753, in the rat using the elevated plus-maze. Neuroreport. 1992;3:922–924. doi: 10.1097/00001756-199210000-00026. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Andreasson KI, Isakson PC, Worley PF. Cyclooxygenases and the central nervous system. Prostaglandins. 1997;54:601–624. doi: 10.1016/s0090-6980(97)00128-7. [DOI] [PubMed] [Google Scholar]
- Koh EJ, Yoon SJ, Lee SM. Losartan protects liver against ischemia-reperfusion injury through PPARγ activation and receptor for advanced glycation end-products down-regulation. Br. J. Pharmacol. 2013;169:1404–1416. doi: 10.1111/bph.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GA, Malbecq W, Smith RD, Guptha S, Poole-Wilson PA HEAAL Investigators. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840–1848. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- Krämer D, Minichiello L. Cell culture of primary cerebellar granule cells. Methods Mol. Biol. 2010;633:233–239. doi: 10.1007/978-1-59745-019-5_17. [DOI] [PubMed] [Google Scholar]
- Kung J, Henry RR. Thiazolidinedione safety. Expert Opin. Drug Saf. 2012;11:565–579. doi: 10.1517/14740338.2012.691963. [DOI] [PubMed] [Google Scholar]
- Larrayoz IM, Pang T, Benicky J, Pavel J, Sanchez-Lemus E, Saavedra JM. Candesartan reduces the innate immune response to lipopolysaccharide in human monocytes. J. Hypertens. 2009;27:2365–2376. doi: 10.1097/HJH.0b013e3283314bc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers. Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- Lee HY, Greene LA, Mason CA, Manzini MC. Isolation and Culture of Post-Natal Mouse Cerebellar Granule Neuron Progenitor Cells and Neurons. J. Vis. Exp. 2009:e990. doi: 10.3791/990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Chuang DM. Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotocixity. J. Neurosci. 2006;26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J. Neurosci. 2008;28:2576–2588. doi: 10.1523/JNEUROSCI.5467-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Cai H, Zhang P, Li H, Liu H, Li Z. Activation of ERK1/2 and PI3K/Akt by IGF-1 on GAP-43 expression in DRG neurons with excitotoxicity induced by glutamate in vitro. Cell Mol. Neurobiol. 2012;32:191–200. doi: 10.1007/s10571-011-9746-6. [DOI] [PubMed] [Google Scholar]
- Min LJ, Mogi M, Shudou M, Jing F, Tsukuda K, Ohshima K, Iwanami J, Horiuchi M. Peroxisome Proliferator-Activated Receptor-gamma Activation With Angiotensin II Type 1 Receptor Blockade Is Pivotal for the Prevention of Blood-Brain Barrier Impairment and Cognitive Decline in Type 2 Diabetic Mice. Hypertension. 2012;59:1079–1088. doi: 10.1161/HYPERTENSIONAHA.112.192401. [DOI] [PubMed] [Google Scholar]
- Mirjany M, Ho L, Pasinetti GM. Role of cyclooxygenase-2 in neuronal cell cycle activity and glutamate-mediated excitotoxicity. J. Pharmacol. Exp. Ther. 2002;301:494–500. doi: 10.1124/jpet.301.2.494. [DOI] [PubMed] [Google Scholar]
- Miura S, Karnik SS, Saku K. Review: Angiotensin II type 1 receptor blockers: class effects versus molecular effects. J. Renin Angiotensin Aldosterone Syst. 2011;12:1–7. doi: 10.1177/1470320310370852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi M, Miyano K, Moriyama N, Taniguchi M, Watanabe T. Angiotensin type 1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor kB and activator protein-1 activation. Eur. J. Neurosci. 2008;27:343–351. doi: 10.1111/j.1460-9568.2007.06014.x. [DOI] [PubMed] [Google Scholar]
- Mogi M, Li JM, Iwanami J, Min LJ, Tsukuda K, Iwai M, Horiuchi M. Angiotensin II type-2 receptor stimulation prevents neural damage by transcriptional activation of methyl methanesulfonate sensitive 2. Hypertension. 2006;48:141–148. doi: 10.1161/01.HYP.0000229648.67883.f9. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Kihara T, Akaike A, Niidome T, Sugimoto H. Alpha-Amino-3-hydroxy-5-methyl-4-isoxazole propionate attenuates glutamate-induced caspase-3 cleavage via regulation of glycogen synthase kinase 3beta. J. Neurosci. Res. 2008;86:1096–1105. doi: 10.1002/jnr.21567. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Ito T, Saavedra JM. Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke. 2000;31:2478–2486. doi: 10.1161/01.str.31.10.2478. [DOI] [PubMed] [Google Scholar]
- O’Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit. Rev. Neurobiol. 1999;13:45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- Pang T, Benicky J, Wang J, Orecna M, Sanchez-Lemus E, Saavedra JM. Telmisartan ameliorates lipopolysaccharide-induced innate immune response through PPARγ activation in human monocytes. J. Hypertens. 2012a;30:87–96. doi: 10.1097/HJH.0b013e32834dde5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang T, Wang J, Benicky J, Sánchez-Lemus E, Saavedra JM. Telmisartan directly ameliorates the neuronal inflammatory response to IL-1β partly through the JNK/c-Jun and NADPH oxidase pathways. J. Neuroinflammation. 2012b;9:102. doi: 10.1186/1742-2094-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J. Mol. Med. 2008;86:715–722. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Jacob A, Wang P, Wu R. Peroxisome proliferator activated receptor-γ and traumatic brain injury. Int. J. Clin. Exp. Med. 2010;3:283–292. [PMC free article] [PubMed] [Google Scholar]
- Rotman N, Wahli W. PPAR modulation of kinase-linked receptor signaling in physiology and disease. Physiology (Bethesda) 2010;25:176–185. doi: 10.1152/physiol.00018.2010. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin. Sci. (Lond) 2012;123:567–590. doi: 10.1042/CS20120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology. 2011;36:1–18. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamura H, Hein L, Krieger JE, Pratt RE, Kobilka BK, Dzau VJ. Cloning, characterization, and expression of two angiotensin receptor (AT-1) isoforms from the mouse genome. Biochem. Biophys. Res. Commun. 1992;185:253–259. doi: 10.1016/s0006-291x(05)80983-0. [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp. Neurol. 2011;227:128–135. doi: 10.1016/j.expneurol.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin. Sci. 2007;112:375–384. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- Stangier J, Su CA, Roth W. Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J. Int. Med. Res. 2000;28:149–167. doi: 10.1177/147323000002800401. [DOI] [PubMed] [Google Scholar]
- Strauss KI, Marini AM. Cyclooxygenase-2 inhibition from glutamate-mediated cell death. J. Neurotrauma. 2002;19:627–638. doi: 10.1089/089771502753754091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suksomboon N, Poolsup N, Prasit T. Systematic review of the effect of telmisartan on insulin sensitivity in hypertensive patients with insulin resistance or diabetes. J. Clin. Pharm. Ther. 2012;37:319–327. doi: 10.1111/j.1365-2710.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- Takaki J, Fujimori K, Miura M, Suzuki T, Sekino Y, Sato K. L-glutamate released from activatedmicroglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: the 'collusion' hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J. Neuroinflammation. 2012;23:275–291. doi: 10.1186/1742-2094-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham DM, Martin-McNulty B, Wang YX, Wilson DW, Vergona R, Sullivan ME, Dole W, Rutledge JC. Angiotensin II is associated with activation of NF-kappaB mediated genes and downregulation of PPARs. Physiol. Genomics. 2002;11:21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- Tian C, Erdmann N, Zhao J, Cao Z, Peng H, Zheng J. HIV-infected macrophages mediate neuronal apoptosis through mitochondrial glutaminase. J. Neurochem. 2008;105:994–1005. doi: 10.1111/j.1471-4159.2007.05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timaru-Kast R, Wyschkon S, Luh C, Schaible EV, Lehmann F, Merk P, Werner C, Engelhard K, Thal SC. Delayed inhibition of angiotensin II receptor type 1 reduces secondary brain damage and improves functional recovery after experimental brain trauma. Crit. Care Med. 2012;40:935–944. doi: 10.1097/CCM.0b013e31822f08b9. [DOI] [PubMed] [Google Scholar]
- Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol. Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- Tsukuda K, Mogi M, Iwanami J, Min LJ, Sakata A, Jing F, Iwai M, Horiuchi M. Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension. 2009;54:782–787. doi: 10.1161/HYPERTENSIONAHA.109.136879. [DOI] [PubMed] [Google Scholar]
- Villapol S, Yaszemski AK, Logan TT, Sanchez-Lemus E, Saavedra JM, Symes AJ. Candesartan, an Angiotensin II AT1-Receptor Blocker and PPAR-[gamma] Agonist, Reduces Lesion Volume and Improves Motor and Memory Function After Traumatic Brain Injury in Mice. Neuropsychopharmacology. 2012;37:2817–2829. doi: 10.1038/npp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1 beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, Humala N, Seror I, Bartholomew S, Rosendorff C, Pasinetti GM. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J. Clin. Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa H, Jezova M, Saavedra JM. Normalization of endothelial and inducible nitric oxide synthase expression in brain microvessels of spontaneously hypertensive rats by Angiotensin II AT1 receptor inhibition. J. Cereb. Blood Flow Metab. 2003;23:371–380. doi: 10.1097/01.WCB.0000047369.05600.03. [DOI] [PubMed] [Google Scholar]
- Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008;1244:164–172. doi: 10.1016/j.brainres.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao SM, Shen LH, Li HW, Wang L, Chen H, Wang YL, Guo CY. Downregulation of the expression of angiotensin II type 1 receptor in neonatal rat cardiac fibroblast by activation of PPARgamma signal pathway. Chin. J. Physiol. 2008;51:357–362. [PubMed] [Google Scholar]
- Zhao Y, Foryst-Ludwig A, Bruemmer D, Culman J, Bader M, Unger T, Kintscher U. Angiotensin II induces peroxisome proliferator-activated receptor gamma in PC12W cells via angiotensin type 2 receptor activation. J. Neurochem. 2005;94:1395–14011. doi: 10.1111/j.1471-4159.2005.03275.x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ando H, Macova M, Dou J, Saavedra JM. Angiotensin II AT(1) receptor blockade abolishes brain microvascular inflammation and heat shock protein responses in hypertensive rats. J. Cereb. Blood Flow Metab. 2005;25:878–886. doi: 10.1038/sj.jcbfm.9600082. [DOI] [PubMed] [Google Scholar]
- Zhu D, Shi J, Zhang Y, Wang B, Liu W, Chen Z, Tong Q. Central angiotensin II stimulation promotes β amyloid production in Sprague Dawley rats. PLoS One. 2011;6:e16037. doi: 10.1371/journal.pone.0016037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorad S, Dou J, Benicky J, Hutanu D, Tybitanclova K, Zhou J, Saavedra JM. Long-term Angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARγ. Eur. J. Pharm. 2006;552:112–122. doi: 10.1016/j.ejphar.2006.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]