Abstract
The human oviduct serves as a conduit for spermatozoa in the periovulatory phase and nurtures and facilitates transport of the developing embryo for nidation during the luteal phase. Interactions between the embryo and oviductal epithelial surface proteins and secreted products during embryo transit are largely undefined. This study investigated gene expression in the human oviduct in the early luteal versus follicular phases to identify candidate genes and biomolecular processes that may participate in maturation and transport of the embryo as it traverses this tissue. Oviductal RNA was hybridized to oligonucleotide arrays and resulting data were analysed by bioinformatic approaches. There were 650 genes significantly down-regulated and 683 genes significantly up-regulated (P < 0.05) in the luteal versus follicular phase. Quantitative real-time PCR, immunoblot analysis and immunohistochemistry confirmed selected gene expression and cellular protein localization. Down-regulated genes involved macrophage recruitment, immunomodulation and matrix-degeneration, and up-regulated genes involved anti-inflammatory, ion transport, anti-angiogenic and early pregnancy recognition. The oviduct displayed some similarities and differences in progesterone-regulated genes compared with the human endometrium. Together, these data suggest a unique hormonally regulated environment during embryo development, maturation and transport through human oviduct and some conservation of progesterone signalling in tissues of common embryological origin.
Keywords: angiogenesis, human Fallopian tube, immunomodulation, implantation, progesterone
Introduction
While the uterus plays an important role in implantation, the Fallopian tube plays an important role in gamete transport, fertilization and, with a growing body of evidence, early embryo development. The human oviduct and endometrium have common embryological origins that develop in parallel during fetal development (Valdés-Dapena, 1973; Witschi, 1970) from coelomic epithelium and surrounding mesenchyme (Sadler, 1979) and both are sensitive to changes in circulating ovarian-derived steroid hormones. However, adult cyclicity of the two tissues is distinct. In the oviduct, notable changes occur in epithelial ciliogenesis and secretory cell development, primarily in response to rising concentrations of oestradiol in the follicular phase of the cycle (Menezo and Guerin, 1997). In contrast, in the post-ovulatory, progesterone-dominant period, oviductal epithelial cell height and oviductal secretions decrease, accompanied by a reversal of the current of secretions, enabling directionality of the embryo towards the uterus, which is enhanced by tubal smooth muscle contractility (Menezo and Guerin, 1997).
Studies in humans as well as in animal models demonstrate that co-culture of oviductal epithelial monolayers with preimplantation embryos of the same species improves fertilization and quality of the inner cell mass, increases total embryo cell numbers and improves implantation rates (Bongso et al., 1992; Kervancioglu et al., 1997; Vlad, Walker, and Kennedy, 1996; Yeung et al., 1996; Yeung et al., 1992). Clinically, assisted reproduction technology such as IVF–embryo transfer and intracytoplasmic sperm injection have enabled bypassing the oviduct for conception with tubal factor infertility and severe male factor infertility, respectively (Palermo et al., 1992). However, pregnancy and take-home-baby rates with these technologies remain low (Stern et al., 2009), and frequently multiple embryos are transferred to overcome this shortcoming, resulting in complications of multiple gestation (Bergh et al., 1999). The development of sequential culture media (Gardner and Lane, 1997; Gardner et al., 1998) designed to imitate the changes in substrates (glucose-pyruvate-shift) during preimplantation embryo development and transport through the Fallopian tube has enhanced the blastocyst developmental rate in vitro comparable to that in vivo, although it still does not significantly ameliorate the pregnancy and take-home baby rates (Gardner and Lane, 2003). A better understanding of the physiological invivo conditions in the Fallopian tube is therefore important to gain insight into this complex biological interchange, as well as to improve culture media to increase pregnancy rates in IVF.
In contrast to the uterine endometrium that has enjoyed extensive investigation, especially in recent years by gene expression array techniques (Ace, Okulicz, William, 2004; Borthwick et al., 2003; Carson et al., 2002; Kao et al., 2003; Kao et al., 2002; Popovici, Kao, and Giudice, 2000; Talbi et al., 2006), most information about the oviduct in humans derives from limited studies on histology and biochemical evaluation of select oviductal proteins, receptors and growth factors (for review see Shaw et al. 2010), primarily because of the tissue's limited availability. Knowledge of global gene expression patterns could facilitate understanding the physiological processes and functions of the oviduct during the reproductive cycle. Thus, the aim of the current study was to investigate the transcriptome of full thickness human oviductal ampulla in the early luteal phase of the cycle, (i.e. at the equivalent time of embryo transport and at the onset of progesterone action on the tissue), compared with the oestrogen-dominant phase of the cycle. The data overall suggest that oviductal genes and biochemical processes during the time of embryo transport are hormonally regulated and involve a variety of immunomodulatory and anti-angiogenic factors, maintaining integrity of oviductal extracellular matrix components.
Materials and methods
Fallopian tubes were obtained in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from subjects, and the study was approved by the Stanford University Committee on the Use of Human Subjects in Medical Research (Protocol ID 77520, approved 3 August 2004 and 2 August 2005). Some tissues were also obtained through the Co-operative Human Tissue Network (Cleveland, OH, USA). Histologically normal Fallopian tube tissue samples were obtained from 10 cycling premenopausal subjects (28–43 years old) undergoing tubal ligation or hysterectomy for benign reasons, including fibroids, pelvic organ prolapse, pelvic pain or abnormal bleeding. All patients had regular menstrual cycles (27–31 days) and were documented as not being pregnant. Two subjects had a history of stage I endometriosis. None had been on hormonal treatment for at least 3 months before tissue acquisition.
The Fallopian tube samples were first assigned a cycle phase based on the last menstruation. This resulted in samples 621, 91, 94, 561 being classified as in the follicular phase and samples 95 and 617 in the early luteal phase. Subject 621 had a history of stage I endometriosis. Since endometrial histology in women with endometriosis is not always congruent to cycle phase, especially in the follicular–luteal transition (Burney et al. 2007), PCA analysis was performed, which showed that this sample should be classified as early luteal phase. Therefore, samples 91, 94, 561 were assigned to the follicular-phase group and samples 95, 617, 621 as the early luteal-phase group. As shown by the PCA analysis, two samples each of both groups clustered very close together (621 and 617 in the early luteal-phase group and 561 and 91 in the follicular-phase group) and one sample in each group clustered further away from the two other samples of the group but still in the same 3-dimensional space.
Thus, the samples were further investigated for differential gene expression through microarray analysis using these designations of phase. Samples were collected at room temperature in DMEM and directly processed as described below. Six samples (three in the follicular phase and three in the luteal phase) were used for the microarray analyses and for validation by quantitative real-time PCR (qRT-PCR). Four additional samples (two in the follicular phase and two in the luteal phase) were used for additional qRT-PCR validation. Western blots were carried out on the three follicular-phase and three luteal-phase samples investigated prior by microarray analysis. Immunohistochemistry validation was carried out on the three follicular-phase and three luteal-phase samples of the microarray analysis and two additional samples in each cycle phase.
Gene expression profiling
For microarray analysis, Fallopian tube samples from three subjects in the follicular phase and samples from three subjects in the early luteal phase of the cycle were processed. One subject, who had stage I endometriosis, had weakly follicular endometrial histology, although her oviduct sample clustered by principal component analysis with the luteal phase and was thus considered to be an early luteal-phase sample in the analysis. The ampullary portion of the oviduct was isolated and the specimens were comprised of serosa, smooth muscle and epithelium. Each Fallopian tube sample was processed individually for microarray hybridization following the Affymetrix protocol (Affymetrix, Santa Clara, CA, USA). Briefly, total RNA was isolated from the Fallopian tube tissue using Trizol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions, followed by DNase treatment. The purity and quality of the RNA were assessed spectrophotometrically by determining their absorbance ratio A260/A280 and electrophoresis of samples on 1.5% denaturing agarose gels. First and second-strand cDNA were prepared according to the Affymetrix microarray preparation protocol. Biotinylated-cRNA was then prepared using a BioArray High Yield T7 Transcript labelling kit (Enzo, Farmingdale, NY, USA) and chemically fragmented. Biotinylated cRNAs were hybridized overnight to a high-density Affimetrix Human Genome U133 Plus 2.0 oligonucleotide microarray genechip containing 54,600 probe sets (42,203 annotated genes and 12,397 expressed sequence tags (EST)) at the Stanford University School of Medicine Protein and Nucleic Acid Facility. Subsequently, fluorescent labelling and laser confocal scanning with an HR 3000 scanner was conducted. Generated data were extracted for analysis using the Affymetrix Gene-Chip Operating Software (GCOS) version 1.1.
Data analysis
Microarray gene expression data analysis
The intensity values of different probe sets (genes) generated by Affymetrix GCOS were imported into GeneSpring version 7.2 (Agilent Technologies, Santa Clara, CA, USA) for data analysis. The data were further processed applying the Robust Multiarray Analysis algorithm in GeneSpring for background adjustment, normalization and log2-transformation of perfect match values (Irizarry et al., 2003). By applying the GeneSpring normalization algorithms, the data were subjected to per chip and per gene normalization. By taking the median expression value of all probe sets on a chip and dividing each gene expression value by this median value, samples were subjected to a per chip normalization. As a second step, a transformation followed as a per gene normalization in which the median expression value of a given gene across all arrays was calculated and used to divide all gene expression values of that particular gene across all arrays. The pairwise comparison of the luteal compared with the follicular-phase Fallopian tube samples generated a gene list consisting of 1333 genes and expressed sequence tags (ESTs) including only genes that had a fold-change value of 1.5 or higher and by statistical one-way ANOVA P-value <0.05 and a parametric test using Benjamini–Hochberg multiple testing correction for false discovery rate. THe raw data files for these experiments are stored at the National Centre for Biotechnology Information Gene Expression Omnibus Database under the identifier GSE 33805.
Principal component analysis
Principal component analysis (PCA) is an unsupervised clustering and visualization approach for analysing data derived from array gene expression analyses (Joliffe and Morgen, 1992). It visualizes microarray data in three dimensions to display possible variation in the data, with each dimension representing a component to which a certain percentage of variance in the data is attributed. In this study, the unbiased PCA algorithm in GeneSpring was applied to all six samples of the luteal-phase and follicular-phase oviductal tissue, using all 54,600 probe sets on the Human Genome U133 Plus 2.0 chip to look for underlying cluster structures.
Gene Ontologies (GO) classification
Differentially expressed genes in the luteal versus follicular-phase oviducts were interrogated for their gene ontology classes using the Gene Ontology Tree Machine (GOTM) web-based software (Zhang et al., 2004). The GOTM was used to derive biological processes, molecular functions and cellular components involving differentially expressed genes between luteal-phase and follicular-phase Fallopian tube samples. Applying GO hierarchies leads to a possible division of a gene list into significant biological processes, molecular functions and cellular components. It further implements a statistical analysis of the GO categories for the gene list and suggests biological areas of interest for further investigation (Ashburner et al., 2000). When the observed number of genes in a GO category is greater than the expected number, this GO category is considered enriched. In addition to the tables and figures presented in the Results and as supplementary data, all gene expression arrays and raw.CEL files have been deposited with the National Center for Biotechnology Information in the gene expression and hybridization array data repository.
KEGG pathway analysis
Genes that were differently expressed in luteal-phase compared with follicular-phase oviductal samples were classified according to KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways. The KEGG pathway analysis organizes genes based on their biochemical pathways associated with the gene set (column 1), the number of genes in each pathway (column 2) and the Entrez Gene ID for the genes (column 3). A hypergeometric testing was applied to the down- and up-regulated gene sets to derive enriched genes in each pathway with a P-value < 0.05 (Zhang, Kirov, Snoddy, 2005).
Microarray validation
Samples were prepared for qRT-PCR as described previously (Hess et al., 2007). Briefly, total RNA was isolated from snap-frozen Fallopian tubes using Trizol (Invitrogen), following the manufacturer's protocol. RNA integrity was verified by denaturing agarose gel electrophoresis and spectrophotometry (OD260/280 absorption ratio greater than 1.95). Complementary DNA was generated from 2.5 g of total RNA isolated from each sample using the Omniscript Reverse Transcription Kit with a 1:1 ratio of oligo(dT)16–18 and random hexamers (Invitrogen). qRT-PCR was performed in triplicate using the QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. PCR reactions were run using the Mx4000 Q-PCR system (Stratagene, La Jolla, CA, USA). Intron–exon spanning primers for reference and target genes were designed using the PCR design software Primer3 (http://frodo.wi.mit.edu/primer3/). All oligosequences are listed in Table 1. Primers were used at the concentrations of 150 nmol/l. A standard curve for the normalizer (RPL19) and each gene of interest were run using serial dilutions of template to generate the curve. The efficiencies of amplification for both genes were calculated according to the formula 10(–1/slope] – 1.
Table 1.
Primer pairs used for real-time PCR validation
| Gene symbol | Forward primer (5 - 3 ) | Reverse primer (5 - 3 ) | Amplicon size (bp) |
|---|---|---|---|
| RPL19 | GTAAGCGGAAGGGTACAGCCA | TTGTCTGCCTTCAGCTTGTG | 211 |
| SCYA2 (CCL2) | CAAGGGCTCGCTCAGCCAGAT | GTCAGCACAGATCTCCTTG | 174 |
| SCYA18 (CCL18) | CTCCTTGTCCTCGTCTGCAC | TCTTATTGGGGTCAGCACAGAT | 205 |
| MMP7 | CAACTCATGAACTTGGCCATT | TGGATGTTCTGCCTGAAGTTT | 191 |
| CLDN1 | ATTTTAGTTGCCACAGCATGG | TTGGTGTTGGGTAAGAGGTTG | 193 |
| CXCL1 | ATAGCCACACTCAAGAATG | TCTGCAGCTGTGTCTCTCTT | 194 |
| CXCL2 | GGGCAGAAAGCTTGTCTCAAC | CCACCAATAAGCTTCCTCCTT | 113 |
| CXCL3 | ATAAAAAGGGTTCGCCGATCT | CCTGCAGGAAGTGTCAATGAT | 482 |
| TNFAIP2 | GCTGTTTCCGGGAGGATCCCATGGAGGCCTTGC | GGTGCTCACAGCTCGCCACA | 1008 |
| NRP1 | GGATTGCTGTGGATGACATTAG | TCACCTTCGTATCCTGGCGTGC | 132 |
| IL-S | GAAGAGAGCTCTGTCTGGAC | TCACTGGCATCTTCACTGAT | 131 |
| IL-1B | TGGGATAACGAGGCTTATAGTG | TGAAGACAAATCGCTTTTCCA | 331 |
| LIF | TGGTTCTGCACTGGAAACATG | TGTAATAGAGAATAAAGAGGGCATTGG | 164 |
| ISG15 | GCGAACTCATCTTTGCCAGTA | AGGGACACCTGGAATTCGTT | 118 |
| FOXO1 | GTCCTACGCCGACCTCATC | ATTCTGCACACGAATGAATTG | 196 |
| PAPPA | ACAACACAGAGGTCATTGCCAG | TGCACTCGGGGTCACAGTTCTCATC | 290 |
| ADAMTS6 | GCAGTGTGAAAGCAAATGTGA | GTGTCCTTGGCAGGTCTTACA | 154 |
| IL-6 | CCTTCTCCACAAGCGCCTTC | GGCAAGTCTCCTCATTGAATC | 327 |
Quantitative analysis was based on the relative quantification of each gene of interest in the luteal phase relative to the follicular phase. This ratio was corrected by the corresponding ratio of the RPL19 reference gene. Ct values were calculated by the Mx4000 software, based on fluorescence intensity values after normalization with an internal reference dye and baseline correction. All cDNA samples were diluted 1:4 immediately before each qRT-PCR experiment. The normalized repeated measures of expression data from all relevant experiments were tested for significance by applying the Relative Expression Software Tool version 2 as a pairwise fixed reallocation randomization test (Pfaffl, 2001; Pfaffl, Horgan, and Dempfle, 2002). A P-value < 0.05 was considered statistically significant.
Protein identification and cellular localization
Immunoblot
Frozen tissue was dissected and lysed with lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cell lysates were centrifuged at 14, 000 g for 10 min at 4 C (Centrifuge 5415Dl Eppendorf, Brinkmann, Westbury, NY, USA), and the supernatant was used for the immunoblots. Supernatant protein concentration was measured applying the Bradford protein assay (BioRad, Hercules, CA, USA) according to the manufacturer's instructions. Protein (30 g) was denatured using Laemmli buffer with DTT (Bio Rad) and subsequently separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (Gradipore, San Diego, CA, USA). Proteins were transferred to a nitrocellulose transfer and immobilization membrane (Schleicher and Schuell Bioscience, Keene, NH, USA) and incubated in Tris-buffered saline/Tween-20 (TBS-T; 20 mM Tris-HCl and 100 mM NaCl, pH 7.60, 0.1% Tween-20) containing 5% nonfat dry milk for 1 h to block non-specific binding sites. After three washes in TBS-T, the membranes were incubated overnight with the primary antibody (SCYA2, goat polyclonal IgG, final concentration 1 g/ml; Santa Cruz; ISG15, rabbit monoclonal Ig, clone no. RB02791, Abgent, San Diego, CA, USA, final concentration final concentration 2.5 g/ml) in TBS-T containing 5% nonfat dry milk. After overnight incubation, membranes were washed three times with TBS-T and incubated for 1h with a matching alkaline phosphatase-conjugated secondary antibody (all Amersham Bioscience, Little Chalfont Buckinghamshire, UK) diluted 1:1000 in TBS-T containing 5% nonfat dry milk. After several washings with TBS-T, bound antibodies were detected using the ECL+ chemiluminescent detection system (Enhanced chemiluminescence detergents; Amersham Bioscience) and exposed to X-ray films (Eastman Kodak, Rochester, NY, USA). The loading control -Actin served as a normalizer for the proteins of interest, SCYA2 and ISG 15.
Immunohistochemistry
Frozen sections were thawed quickly and fixed for 10 min on ice in fixation buffer containing 10% formaldehyde (Sigma-Aldrich, St Louis, MO, USA). Sections were washed with phosphate-buffered saline and incubated in 0.3% hydrogen peroxide (Sigma-Aldrich) for 20 min to quench endogenous peroxidase activity. According to the manufacturer's instructions for Vectastain Elite ABC Kits (Vector Laboratories, Burlingame, CA, USA), sections were incubated with diluted normal blocking serum from the species in which the secondary antibody was raised (anti-mouse, anti-goat or anti-rabbit) to minimize non-specific staining. Sections were washed and incubated with the primary antibody: monoclonal mouse anti-human CD45, clone no. 2B11 and PD7/26 (final concentration 2 g/ml; Dako Cytomation, Carpinteria, CA, USA), monoclonal mouse anti-human-cytokeratin 7, clone no. RCK105, monoclonal mouse anti-human interleukin (IL) 8, clone no. B-2, polyclonal goat anti-human MCP-1, or polyclonal goat anti-human MMP7 (final concentration 4 g/ml; Santa Cruz Biotechnologies) for 1 h at room temperature. To determine non-specific binding of the primary antibody, some slides were incubated with purified IgG fraction from the hosts in which the antibodies were raised. After washing with phosphate-buffered saline, the appropriate biotinylated secondary antibodies were applied, sections were rinsed and immunoperoxidase staining with diamonbenzidine tetrahydrochloride (Vector Laboratories) was performed. Sections were dehydrated with alcohol (70–98%) and xylene and mounted (Vector Laboratories). Leukocyte counts were performed by applying the ImageJ 1.46 programme for high-power field microscopes (WS Rasband, US National Institutes of Health, Bethesda, Maryland, USA; http://imagej.nih.gov/ij). Ten random fields per slide were counted incorporating all different anatomical parts of the Fallopian tube to gain a representative result. Each slide was treated in the same way to ensure comparability.
Results
Principal component analysis
PCA of the Fallopian tube expression profiles using all the genes and ESTs on the Affymetrix gene chip highlightsed a segregation of the samples (Supplementary Figure 1, available online). This unbiased tool visualized a distribution of the samples in a 3-dimensional space due to their similar gene expression profile. Interestingly, one out of three luteal-phase samples and one out of three follicular-phase samples clustered closer together than to their respective groups.
Regulation of Fallopian tube genes according to the cycle phase
Pair-wise comparison
Pair-wise analysis of genes regulated differently in luteal versus follicular-phase oviductal samples revealed 650 down-regulated and 683 up-regulated genes with P < 0.05 and a fold-change of at least 1.5. The full gene list is in Supplementary Table 1. Table 2 shows the most down-regulated genes included the chemokines CXCL1 (i.e. GRO1, CXCL2 (GRO2), CXCL3 (GRO3)), cytokines and angiogenic factors, including IL-8 and IL-1B, chemokine (C-C motif) ligand 18 (CCL18, also known as SCYA18) and CCL2 (also known as SCYA2 or monocyte chemotactic protein 1 (MCP1)). Out of the group of interferon-stimulated genes, tumour necrosis factor -induced protein 2 (TNFAIP2) was markedly down-regulated. Of note, IL-1B, CXCL1, CXCL2, CXCL3 and CCL2 are also down-regulated in human endometrium at the follicular–luteal transition (Supplementary Table 1; Talbi et al., 2006).
Table 2.
Most highly down- and up-regulated genes in the luteal versus follicular-phase oviduct.
| Gene | Gene ID | Fold-change |
|---|---|---|
| Down-regulated genes | ||
| CCL2 | 6347 | −11.1 |
| CXCL3 | 2921 | −11.0 |
| CXCL2 | 2920 | −10.0 |
| C4A | 720 | −9.1 |
| CXCL1 | 2919 | −8.3 |
| SOCS3 | 9021 | −8.3 |
| MMP7 | 4316 | −7.7 |
| NRP1 | 8829 | −4.2 |
| CCL18 | 6362 | −3.9 |
| IL-1 | 3553 | −3.6 |
| CLDN1 | 9076 | −2.9 |
| TNFAIP2 | 7127 | −2.8 |
| CLDN4 | 1364 | −2.2 |
| LIF | 3976 | −2.2 |
| SST | 6750 | −2.2 |
| OSMR | 9180 | −2.1 |
| IL-8 (CXCL8) | 3576 | −2.0 |
| Up-regulated genes | ||
| MGST1 | 4257 | 8.8 |
| PAPPA | 5069 | 8.1 |
| CADM2 | 253,559 | 8.0 |
| PDE4DIP | 83,679 | 7.0 |
| BTBD19 | 149,478 | 6.3 |
| NFATC2 | 4773 | 6.0 |
| SCGB1D2 | 10,647 | 5.5 |
| SAMD9L | 209,086 | 4.9 |
| ADAMTS6 | 11,174 | 4.5 |
| KCNF1 | 3754 | 4.1 |
| PTEN | 5728 | 4.1 |
| FOXO1 | 2308 | 3.0 |
| HAB1 | 55,547 | 2.8 |
| MUC5AC | 4586 | 2.3 |
| ISG15 | 9636 | 2.4 |
| ADAMTS14 | 140,766 | 2.2 |
Other significantly down-regulated genes included matrix metalloproteinase 7 (MMP7), endothelin and members of the claudin family, (CLDN) CLDN1 and CLDN4, which were also down-regulated in endometrium (Supplementary Table 1; Talbi et al., 2006). Also, LIF and somatostatin (SST) were down-regulated in oviduct in the luteal versus follicular phase, in contrast to endometrium where these genes are up-regulated in this phase of the cycle and are important in embryo implantation and in response to progesterone and activation of the PKA pathway, respectively (Supplementary Table 1; Talbi et al., 2006).
In addition, a number of transcription factors and signalling molecules and regulators of signalling were down-regulated, including GADD45B, FOSB2, ATF3 and DUSP5. These were similarly down-regulated in human endometrium at the follicular-to-luteal transition (Supplementary Table 1).
The most highly up-regulated genes (Table 2) included microsomal gluthathione S transferase 1 (MGST1), pregnancy-associated plasma protein A (PAPPA), secretoglobin family 1D member 2 (SCGB1D2), B1 for mucin (HAB1) and mucin 5AC (MUC5AC) and a disintegrin-like and metalloprotease domain with thrombospondin type I motif 6 (ADAMTS6). Also markedly up-regulated were members of the potassium voltage-gated subfamily (KCN), including KCNA6, KCNF1, KCNG1, KCNH6, KCNQ3, KCNS3 and KCNB1 as well as the interferon-induced protein 15 (ISG15) and the forkhead box O1 (FOXO1). While the latter is also an important target of progesterone action in endometrium, it is up-regulated in the window of implantation and not in the follicular-to-luteal shift in this tissue compared with what was observed in the oviduct) (Supplementary Table 1; Talbi et al., 2006; Aghajanova et al., 2009).
Compared with the gene families that consist of either down- or up-regulated genes, there were also families with genes represented in both groups (Table 3). The solute carrier (SLC) family displayed seven genes in the down-regulated group and 15 genes in the up-regulated group. The zinc finger (ZNF) family had five genes in the down-regulated group and 12 genes in the up-regulated gene group. These data show that, although for most gene families there is a clear segregation of either decreased or enhanced expression in the oviduct in the luteal phase compared with the follicular phase, there are also groups not following this pattern and are divided within their group – suggesting a very complex orchestration of the oviduct's composition throughout the cycle.
Table 3.
Genes in selected gene families represented in both down- and up-regulated groups.
| Family | Down-regulated genes | Up-regulated genes |
|---|---|---|
| Solute carrier (SLC) | SLC2A14 | SLC4A7 |
| SLC2A3 | SLC6A8 | |
| SLC5A1 | SLC7A1 | |
| SLC7A11 | SLC8A1 | |
| SLC13A5 | SLC9A3R1 | |
| SLC17A1 | SLC22A23 | |
| SLC25A27 | SLC27A3 | |
| SLC28A1 | ||
| SLC28A3 | ||
| SLC30A5 | ||
| SLC34A1 | ||
| SLC39A14 | ||
| SLC39A2 | ||
| SLC39A8 | ||
| SLC44A1 | ||
| Zinc finger protein (ZNF) | ZNF79 | ZNF7 |
| ZNF441 | ZNF148 | |
| ZNF442 | ZNF189 | |
| ZNF681 | ZNF41 | |
| ZNF765 | ZNF519 | |
| ZNF524 | ||
| ZNF542 | ||
| ZNF584 | ||
| ZNF652 | ||
| ZNF652 | ||
| ZNF687 | ||
| ZNF826P |
Gene ontology classifications
Gene ontology analysis of down-regulated genes (Supplementary Table 2) revealed biological processes in luteal-phase oviduct involved in cell communication, apoptosis, blood vessel development, angiogenesis, innate and humoral immune responses and complement activation. With regard to molecular functions, these involved regulation of transcription, chemokine/cytokine activity, growth factor activity as well as protease and caspase activator activity. Cellular components including down-regulated genes belonging to the cell and membrane fraction, T-cell receptor complex and the extracellular space and region (Supplementary Table 2).
In contrast, GO categories involving up-regulated genes comprised focal adhesion formation, regulation of cell growth, fatty acid metabolism, negative regulation of cellular metabolism and physiological processes. Molecular functions involved ion binding, ion channel activity and ion-transporter activity, and cellular components included membrane fractions and voltage-gated potassium channel complexes (Supplementary Table 3).
KEGG pathway analysis
KEGG pathway analysis enables analysis and organization of genes according to their biochemical signalling pathways. The greatest number of down-regulated genes in the luteal-phase oviduct belonged to signalling pathways involved in cytokine–cytokine receptor interaction, MAPK and- Jak-STAT signalling, natural killer cell-mediated cytotoxiticy, T-cell receptor signalling, apoptosis, insulin signalling, TGF signalling, antigen processing and presentation, and cysteine metabolism. In addition, down-regulated genes were observed to be involved in the B-cell receptor and Hedgehog and the toll-like receptor signalling pathways (Supplementary Table 4).
Supplementary Table 5 lists several pathways containing up-regulated genes in luteal-phase oviduct. The largest number of genes was involved in the Wnt signalling pathway and in focal adhesion, insulin signalling, calcium signalling and fatty acid metabolism. Additionally, genes involved in tight junction signalling and ubiquitin-mediated proteolysis cascades were up-regulated in luteal-phase versus follicular-phase oviduct. Note that some pathways (e.g. the Jak/STAT pathway) are listed in both the up- and down-regulated pathway table, because different genes of the same pathway may be regulated in different directions.
Validation of gene expression
Different approaches were used to validate the changes in gene expression according to the phase of the menstrual cycle. These included qRT-PCR at the mRNA level (Figure 1) and immunoblotting (Figure 2) and immunohistochemistry (Figure 3) at the protein level.
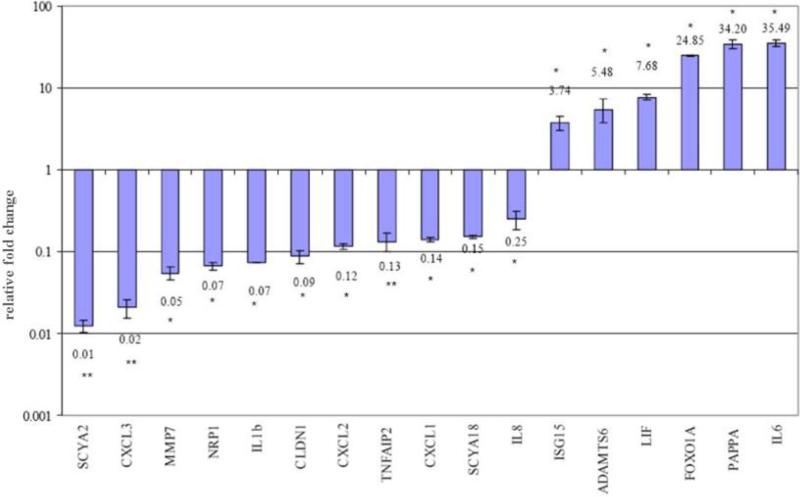
Figure 1.
Validation of microarray data by quantitative real-time PCR of 11 representative genes down-regulated and six genes up-regulated in human Fallopian tubes in the luteal compared with the follicular phase. Each fold-change value is represented as the mean SEM. Asterisks next to each fold-change value illustrate the results obtained by Relative Expression Software Tool statistical analysis. *P < 0.05; **P < 0.01.
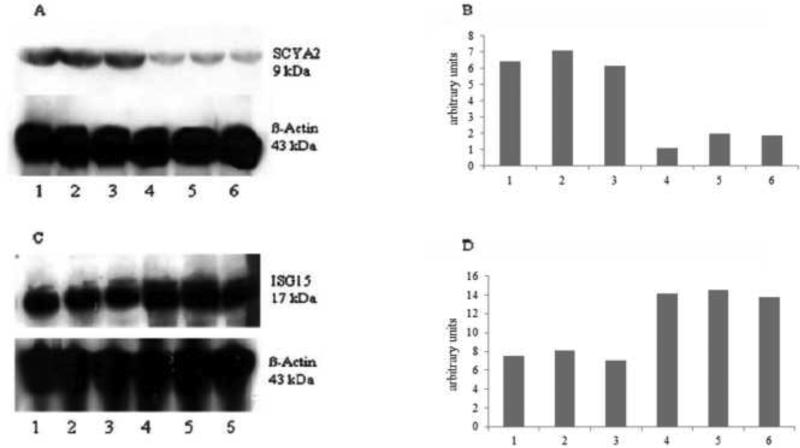
Figure 2.
Immunoblot protein analysis (A, C) and corresponding densitometric analysis (B, D) of the down-regulated protein SCYA2 (A, B) and the up-regulated protein ISG15 (C, D) in luteal versus follicular-phase human Fallopian tubes. Lanes 1–3 represent Fallopian tubes samples of the follicular phase whereas lanes 4–6 represent Fallopian tube samples of the luteal phase.
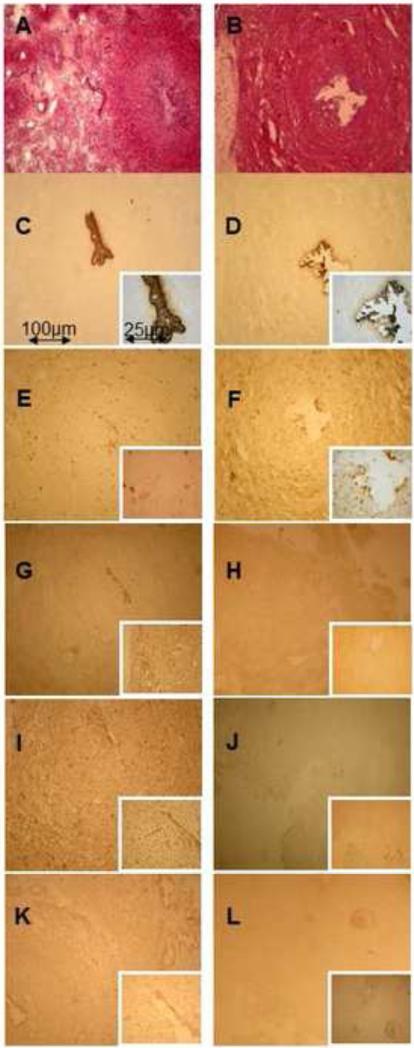
Figure 3.
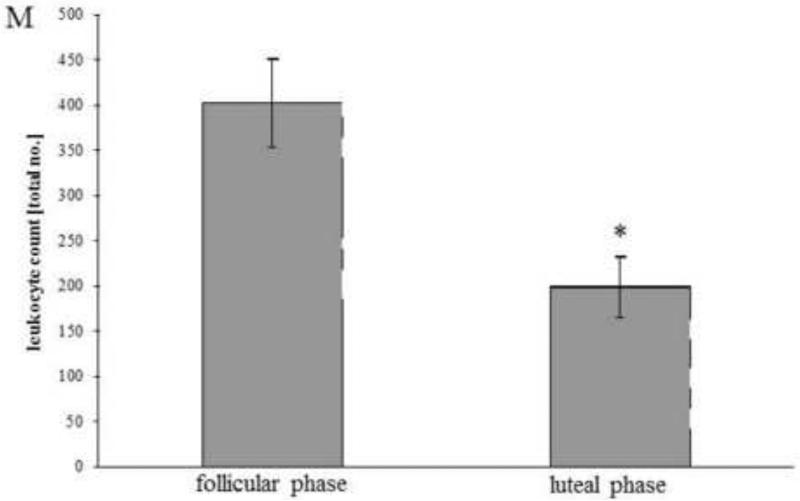
Immunohistochemistry analysis of human Fallopian tube samples of the follicular phase (A, C, E, G, I, K) versus the luteal phase (B, D, F, H, J, L). Images were taken at 100 magnification ( 400 for the inserts). (A, B) Haematoxylin eosin. (C, D) Cytokeratin antibody as an epithelial cell marker. (E, F) Leukocyte marker antibody CD45, (G, H) IL-8 antibody. (I, J) SCYA2 antibody. (K, L) Antibody against MMP7. Lu = lumen. (M) cell count of CD45+ cells/high-power field for all samples investigated in the follicular (n = 5)and luteal phases (n = 5); values are mean SEM; *P < 0.001 (Student's t-test).
Quantitative real-time PCR
This study validated expression of selected down- and up-regulated genes with qRT-PCR. The qRT-PCR results for 11 down-regulated (SCYA2, SCYA18, CXCL1, CXCL2, CXCL3, IL-8, IL-1B, CLDN1, MMP7, NRP1, TNFAIP2) and six up-regulated genes (PAPPA, ADAMTS6, FOXO1, ISG15, IL-6, LIF) are shown in Figure 1. These genes were selected for validation because of their involvement in immune and inflammatory processes, known or presumed oviductal function, embryo implantation and signalling in response to progesterone. For all genes tested except two, the fold-change values derived from the microarray data and the qRT-PCR occurred in the correct direction (down or up) (Figure 1). There were differences in absolute magnitude, most likely due to the high sensitivity of the qRT-PCR approach and the tendency for Robust Multiarray Analysis normalization to understimate fold-change (Irizarry et al., 2003). LIF and IL-6, both down-regulated on array, were found to be up-regulated using qRT-PCR. For LIF, two alternatively spliced transcripts exist (isoforms 1 and 2) and careful assessment of primer- and probe-binding sites showed that the qRT-PCR fold-change was specific for LIF isoform 1, while the array data represented the total LIF, providing a likely explanation for the discrepancy between qRT-PCR and array results. As no known splice variants are currently known for IL-6, the inconsistency might have been due to unrecognized splice variants or single nucleotide polymorphisms within the region, leading to altered annealing for either PCR primers or array probes. All genes showed statistically significant fold-changes by Relative Expression Software Tool analysis (P < 0.05 or < 0.01; Pfaffl, 2001; Pfaffl, Horgan and Dempfle, 2002).
Immunoblot
To confirm translation of selected mRNAs, immunoblot analysis was conducted (Figure 2). Use of an antibody against SCYA2 – down-regulated on the mRNA level – showed a specific protein band at 9 kDa (Figure 2A). The oviduct samples from the luteal phase of the cycle showed lower SCYA2 protein expression compared with samples from the follicular phase by densitometric analysis (Figure 2B), which was consistent with the gene array and qRT-PCR analyses. Oviduct samples from the luteal phase showed a higher intensity of the ISG protein at 17 kDa compared with follicular-phase samples (Figure 2C, D), confirming the microarray and qRT-PCR analyses.
Immunohistochemistry
While the qRT-PCR and the immunoblot results confirmed the robustness of the microarray data, cellular localization of representative proteins within the Fallopian tube warranted investigation. Therefore, selected proteins were studied by immunohistochemistry in sections of the same Fallopian tube samples used for the gene chip analysis and two additional samples per group to give a final n = 5 for each group. IL-8, SCYA2, MMP7 and CD45 were investigated. CD45 is a common leukocyte marker that recognizes the human CD45 antigen and therefore reflects macrophages and lymphocytes such as B and T cells. CD45 appeared more prominent in the follicular-phase samples, showing staining in the labyrinth-like mucous membrane folds, especially close to the epithelium and in small vessels of the lamina propria (Figure 3E). The absolute count of CD45+ cells/high power field (Figure 3M) confirmed that the number of oviductal leukocytes was decreased in the luteal compared with follicular phase of the cycle. IL-8 staining was primarily observed in follicular samples with greatest intensity in mucosal epithelium and more moderately in the remaining mucosal folds (Figure 3G). SCYA2 was abundantly expressed in follicular samples, whereas only sparse staining in the luteal-phase mucosa was observed (Figure 3I). MMP7 showed a similar pattern, with high expression in the epithelium and the mucosal folds of follicular-phase samples (Figure 3K), consistent with the microarray data.
Discussion
The Fallopian tube is divided anatomically into four parts: the infundibulum for oocyte pick up, the ampulla (investigated herein) where fertilization occurs and embryo transport begins, the isthmus which is key in regulating sperma passage and embryo entry into the uterus, and the intramural portion that resides within the uterine wall (Dodd, 1979). Histologically, the oviduct is composed of three layers: the inner mucosa, the muscularis and the outer serosa. The mucosa is comprised of a single layer of cuboidal cells with two major cell types – ciliated and nonciliated – with branching and folding that is most pronounced in the infundibulum and ampulla (Crow et al., 1994). It is a dynamic tissue whose cellular components respond throughout the menstrual cycle to changes in circulating oestrogen and progesterone (Critoph, Dennis, 1977; Novak and Everett, 1928). Both ciliated and non-ciliated cells gain height as the follicular phase proceeds, and shortly after ovulation, the secretory cells begin secreting their contents into the tubal lumen, leaving prominent-appearing ciliated cells. The latter are believed to facilitate movement of the secretions along the tubal lumen, presumably to nourish the developing embryo and facilitate its transit towards the uterus. The current study investigated the transcriptome of whole oviductal tissue (epithelium, mucosa, serosa) and immunohistochemical analysis confirmed the microarray and validation data at the cellular level. While >1200 genes were up- and down-regulated in this tissue in the early luteal versus follicular phase, striking were the gene families and biological processes associated with immune cells, angiogenesis and the extracellular matrix.
Leukocyte recruitment and extracellular matrix integrity
This study demonstrated that the oviduct has a distinct pattern of leukocyte regulation including down-regulation of leukocyte recruitment during the progesterone-dominant luteal phase of the cycle. The complement of immune cells in this tissue is a function of the steroid hormone environment, and although it is well accepted that T lymphocytes comprise a major constituent in the Fallopian tube, there are conflicting reports about granulocytes and macrophages and the steroid dependence of leukocytes within this tissue. The data herein support down-regulation of leukocytes which teleologically may be important to ensure appropriate embryo transport and development and avoid abnormal implantation within the oviduct. At the same time, at a more mechanical level, the down-regulation of matrix-degrading and cell adhesion-promoting genes support mechanisms of molecular guidance towards the uterus and thereby potentially preventing ectopic tubal implantation.
Down-regulated genes
Immune modulators
Within the group of immune modulatory genes, chemokines, interferon-regulated genes and tumour necrosis -induced proteins such as CXCL1, CXCL2, CXCL3, IL-1B, TNFAIP2 and monocyte chemotactic protein-1 MCP1 (SCYA2 or CCL2) were significantly down-regulated in the luteal-phase oviduct.
CXCL1, -2 and -3 are members of a gene superfamily encoding a set of related cytokines with inflammatory and growth regulatory properties (Haskill et al., 1990; Nathan, 1987) and are believed to play an important role in maternal acceptance of the semi-allograft embryo and induction of angiogenesis (Baston-Buest et al., 2010). They belong to the CXC family of chemokines produced by activated monocytes and neutrophils that have a pivotal role in leukocyte recruitment, control of inflammation and control of angiogenesis (Romagnani et al., 2004). In this study CXCL1, -2 and -3 were down-regulated 8-, 10- and 11-fold, respectively, by microarray and 7-, 8-, and 50-fold, respectively, using qRT-PCR, suggesting a down-regulation of the inflammatory milieu and thus down-regulation of neutrophil traffic into the Fallopian tube. While these chemokines are also down-regulated in human endometrium during the follicular–luteal transition (Supplementary Table 1; Talbi et al., 2006), they are up-regulated in the endometrium during the window of implantation (Talbi et al., 2006), a proreceptive environment to the blastocyst. These data overall support lack of maternal acceptance to embryo implantation in the oviduct during its transit in the early luteal phase. Additionally, down-regulation of leukocytes per se in the luteal- phase oviduct, as observed herein (Figure 3M), may prevent recognition of the blastocyst as an allograft and normally prohibit ectopic implantation within this tissue. This hypothesis is supported by a recent study showing an increase of CD45+ leukocytes in Fallopian tubes in the mid-luteal phase of women with tubal ectopic pregnancy compared with Fallopian tubes from healthy women (Shaw et al., 2011). While there were no differences in CD45+ leukocytes (or CD68+ macrophages) in Fallopian tubes in the mid-luteal compared with the follicular phase, these findings are not necessarily in conflict with the current data where samples were from the early luteal phase. Subtle hormonally driven regulation of individual cell types in the Fallopian tube was described by Ulziibat et al. (2006), who found an increase of intraepithelial lymphocytes in the late follicular phase followed by a decrease in the early luteal phase and again an increase in the late luteal phase. These observations underscore the importance of knowing in which cycle-phase samples are obtained, since leukocyte trafficking within the Fallopian tube is highly diverse and appears to change according to the steroid hormone milieu.
SCYA2, another immunomodulatory chemokine that was highly down-regulated (10 fold) in the luteal-phase oviduct belongs to the CC-chemokine subgroup involved in immunoregulatory and inflammatory processes (Mackay, 1996; Mehrabian et al., 1991). It is a potent chemoattractant and activator of monocytes, macrophages, T cells and natural killer cells (Caballero-Campo et al., 2002) and is secreted by endothelial (Sica et al., 1990), epithelial and stromal cells, monocytes and lymphocytes (Yoshimura and Leonard, 1990). It was shown previously to be expressed in cultured human oviductal epithelial cells (Fahey et al., 2005). SCYA2 influences innate immunity through its effects on monocytes, as well as adaptive immunity through control of T helper cell polarization (Gu et al., 2000). Therefore, a marked down-regulation of SCYA2 may contribute to allogenic tolerance to the preimplantation embryo as it traverses the Fallopian tube. Additionally, down-regulation of SCYA2, as with CXCL1,-2, and -3, may prevent leukocyte influx into the oviduct. It is noteworthy that media conditioned by blastocysts that subsequently implanted versus non-implanted blastocysts also show a lower abundance of SCYA2, suggesting a comparable mechanism on the embryo's side to promote allogenic tolerance (Dominguez et al., 2008).
Extracellular matrix integrity
MMP7 is a member of the family of matrix-degrading enzymes involved in the breakdown of extracellular matrix in embryonic development, reproduction and tissue remodelling. The observation of down-regulation of MMP7 in the luteal-phase oviduct is consistent with maintaining oviductal matrix integrity, particularly important as an embryo traverses the ampulla en route to the uterus. Of note, two members of the tissue inhibitor of metalloproteinase family (TIMP1 and TIMP2) are more highly secreted in implanted versus non-implanted blastocysts, suggesting inhibition of matrix degradation is regulated by both the oviduct and embryo to prevent implantation within the Fallopian tube (Dominguez et al., 2008).
Progesterone signalling and growth response genes
Herein, we found that some transcription factors and pathways involved in progesterone signalling in the follicular–luteal transition are similarly down-regulated in oviduct and endometrium, including GADD45B, DUSP5 and the cyclic AMP-dependent transcription factor ATF-3 (Supplementary Table 1). Of interest and new to oviduct biology is the down-regulation of DUSP5, a member of a family of terminal kinase-specific phosphatases in the ERK1/2, p38 and JNK1/2 mediating signalling pathways in cell cycle and growth responses and other functions in response to progesterone (Patterson et al., 2009). In addition, GADD45B, a transcription factor important in the p38/JNK pathway, is similarly down-regulated. However, there are also progesterone-regulated genes (EGFR, FOSL2) that are down-regulated in oviduct in the follicular–luteal transition that are up-regulated in endometrium in the same phase of the cycle (Supplementary Table 1). These suggest signalling pathways in response to progesterone during the follicular–luteal transition that are tissue specific. It is noteworthy that progesterone receptors A and B are differentially expressed in different regions of the Fallopian tube (e.g. increased progesterone receptor B in the ampulla) and are cycle-dependent (e.g. decreased mRNA of both receptors in the mid-luteal phase compared with the follicular phase) (Briton-Jones et al., 2005). Progesterone receptors A and B have been found in nuclei of all oviductal epithelial cells and in about half of stromal and smooth muscle cells (Horne et al., 2009), supporting the importance of progesterone signalling in this tissue and cell type-specific responses.
Up-regulated genes
A total of 683 genes were up-regulated in the oviduct in the early luteal phase – the beginning of progesterone action on the tissue. Among this group there are genes for protection from oxidative stress (MGST1), genes and gene families known in endometrium to be mediators of progesterone action (Talbi et al. 2006), including the transcription factors NFATC2, FOXO-1A, the secreted proteins (secretoglobin) and proliferative processes (PAPPA), as well as ion transport and modifications of carbohydrate moieties on cell surface glycoproteins (N-acetylglucosamine-6-O-sulphotransferase4 (CHST4)).
MGST1 is a member of the MAPEG (membrane-associated proteins in eicosanoid and glutathione metabolism) family and exhibits glutathione S-transferase and peroxidase activities involved in cellular defence against toxic, carcinogenic and other electrophilic compounds (Johannson et al., 2010). The protein product is localized to the endoplasmic reticulum and outer mitochondrial membrane where it is thought to protect these membranes from oxidative stress. The fact that this is the most highly up-regulated gene in the human oviduct during transit of an embryo supports, teleologically, the importance of protecting the oviduct from oxidative stress and maintaining its structural and functional integrity during this period.
SCGB1D2 (secretoglobin family 1D, member 2 (ScgbB1D2)) is a member of the lipophilin subfamily, part of the uteroglobin superfamily (Mukherjee et al., 2007). Uteroglobin, the only SCGB extensively studied displays tissue-specific expression in most mucosa and in the uterus and oviduct is regulated by steroid hormones. It is a multifunctional protein with anti-inflammatory/immunomodulatory properties and it inhibits phospholipase A2 activity and binds progesterone, retinols, polychlorinated biphenyls, phospholipids and prostaglandins (Mukherjee et al., 2007). In addition to its anti-inflammatory activities, uteroglobin displays anti-chemotactic and embryonic growth-stimulatory activities (Riffo et al., 2007).
FOXO1-A is a member of the FOXO subfamily of Forkhead/winged-helix family of transcription factors which belongs to one of the earliest induced genes in endometrium in response to progesterone and is a key participant in endometrial stromal decidualization (Brar et al., 2001). Furthermore, it associates with other nuclear transcription factors resulting in repression or transactivation of genes (Kim and Fazleabas, 2004).
CHST4 is a sulphotransferase that catalyses the transfer of sulphate to position 6 of non-reducing N-acetylglucosamine (GlcNAc) residues within mucin-associated glycans that ultimately serve as l-selectin ligands. l-Selectin, expressed on lymphocytes and other leukocytes, is essential for lymphocyte homing from the blood stream to lymph nodes. The initial process of this interaction, ‘rolling’, is mediated by l-selectin on lymphocytes and sulphated glycans on venules (Kawashima, 2006). Similarities between transendothelial lymphocyte migration and embryonic implantation have been invoked (Campbell et al., 1995; Dominguez et al., 2005), as human trophectoderm expresses l-selectin (Genbacev et al., 2006; Prakobphol et al., 2006). It is speculated that, similar to the lymphocytes/leukocytes rolling along vessels until the right area for attachment and subsequent transmigration is found, it is possible that the human embryo interacts via l-selectin and the l-selectin ligand in the oviduct, to continue transit as a ‘rolling’ phenomenon in the oviduct until it reaches the endometrial cavity for subsequent nidation. This hypothesis is further supported by the fact that chemokines, chemokine receptors and adhesion molecules like MCP1, RANTES, CCR2, CCR5, ICAM1, VCAM1, MUC1 and integrins, which are known to facilitate steps in blastocyst attachment and migration into the uterus, are not up-regulated in the Fallopian tube although embryo expression of MCP-1, RANTES and VCAM1 has been demonstrated (Dominguez et al., 2008).
Conclusion
Successful embryonic implantation requires precisely timed processes of ovulation, fertilization and early embryo development and transport within the oviduct before implanting into the uterine endometrium. The current study has demonstrated that a variety of genes belonging to the families of immunomodulatory and inflammatory chemokines and anti-angiogenic factors are suppressed in the ampulla of the oviduct during the time corresponding to embryo transit. Furthermore, genes associated with secretory proteins, K+ transport, the anti-angiogenic ADAMTS6 and CHST4 known to be involved in lymphocyte rolling are up-regulated in this tissue. Interestingly, although oviductal cellular responses to oestradiol and progesterone throughout the menstrual cycle differ from endometrial cellular responses, some signalling pathways may be conserved.
These observations suggest that these steroid-hormone dependent tissues respond to fulfil unique roles in facilitating successful implantation and pregnancy with spatiotemporal fidelity. These data give insight into an anti-implantation, anti-inflammatory, anti-angiogenic, matrix-stable environment of the human oviduct as it facilitates transit of a developing embryo en route to the uterine endometrium. This study provides a basis for further validation and understanding of genes and proteins expressed in the human oviduct that facilitate embryo development and transport as well as conditions in which these are compromised leading to infertility and ectopic pregnancy and abnormalities leading to Fallopian tube cancers.
Supplementary Material
Summary.
The oviduct serves as a conduit for spermatozoa in the peri-ovulatory phase and it nurtures and facilitates transport of the developing embryo during the luteal phase of the menstrual cycle, although precise interactions between the embryo and oviductal epithelium and secreted products are largely undefined. Herein, we investigated gene expression in human oviduct to identify candidate genes and processes that may participate in maturation and transport of the embryo as it develops implantation competence. Total RNA from human ampullary oviducts in the early luteal versus follicular phases was isolated and hybridized to oligonucleotide arrays. The data, analysed by bioinformatic approaches, revealed that 650 genes were significantly down- and 683 genes were significantly up-regulated in the luteal phase. Quantitative real-time PCR, immunoblot analysis and immunohistochemistry confirmed selected gene expression and cellular protein localization. The data demonstrated down-regulation of genes involved in macrophage recruitment, immunomodulation and matrix degeneration and up-regulation of ion transport and secretions, as well as anti-angiogenic and early pregnancy recognition. Together, these data suggest a unique hormonally regulated environment during embryo development, maturation and transport through the human oviduct and provide insight into mechanisms influencing acquisition of implantation competence of the human embryo during its passage through the oviduct en route to the uterine endometrium.
Acknowledgements
The authors thank all the women who participated in this study, as well as the staff at Stanford Hospital and Clinics and especially Kim Chi Vo for their help in tissue acquisition. They gratefully acknowledge the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; He3544/2-1 and He3544/2-2) to APH and the NIH Co-operative Program on Trophoblast: Maternal Tissue Interactions (NICHD UO1 HD42298) to LCG for financial support for this study.
Biography
 Alexandra Hess received her MD from the Heinrich-Heine University in Düsseldorf, Germany in 2000. After completing her residency, she pursued a post-doctoral fellowship at Stanford University. In 2005, she completed her training and became a consultant in obstetrics and gynaecology at the University's Medical Hospital where she specialized in reproductive endocrinology and infertility. In 2011 she received her PhD in the field of embryo–maternal cross-talk. She is currently an associate professor and vice-coordinator of the University's fertility clinic UniKiD.
Alexandra Hess received her MD from the Heinrich-Heine University in Düsseldorf, Germany in 2000. After completing her residency, she pursued a post-doctoral fellowship at Stanford University. In 2005, she completed her training and became a consultant in obstetrics and gynaecology at the University's Medical Hospital where she specialized in reproductive endocrinology and infertility. In 2011 she received her PhD in the field of embryo–maternal cross-talk. She is currently an associate professor and vice-coordinator of the University's fertility clinic UniKiD.
REPRODUCTIVE BIOMEDICINE ONLINE
AUTHOR DECLARATION
Submission of an article implies that the work described has not been published previously (except in the form of an abstract or as part of a published lecture or academic thesis), that it is not under co nsideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in E nglish or in any other language, without the written consent of the copyright-holder.
By attaching this Declaration to the submission , the corresponding author certifies that:
The manuscript represents original and valid work and that neither this manuscri pt nor one with substantially similar content under the same authorship has been published or is being considered for publication elsewhere.
Every author has agreed to allow the corresponding author to serve as the primary correspondent with the editorial office, and to review the edited typescript and proof.
Each author has given final approval of the submitted manuscript and order of authors. Any subsequent change to authorship will be approved by all authors.
Each author has participated sufficiently in the work to take public responsibility for all the content.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration: The authors report no financial or commercial conflicts of interest.
References
- Ace CI, Okulicz WC. Microarray profiling of progesterone-regulated endometrial genes during the rhesus monkey secretory phase. Reprod. Biol. Endocrinol. 2004;2:54. doi: 10.1186/1477-7827-2-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol. Reprod. 2009;80(1):105–14. doi: 10.1095/biolreprod.108.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene Ontology: tool for the unification of biology. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baston-Buest DM, Goette M, Janni W, Kruesssel JS, Hess AP. Syndecan-1 knock-down in decidualized endometrial stromal cells leads to significant changes in cytokine and angiogenic factor expression patterns. Reprd. Biol. Endocrinol. 2010;8:133. doi: 10.1186/1477-7827-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh T, Ericson A, Hillensjö T, Nygren KG, Wennerholm UB. Deliveries and children born after in-vitro fertilisation in Sweden 1982–95: a retrospective cohort study. Lancet. 1999;354(9190):1579–1585. doi: 10.1016/S0140-6736(99)04345-7. [DOI] [PubMed] [Google Scholar]
- Bongso A, Ng SC, Fong CY, Anadakumar C, Marshall B, Edirisinghe R, Ratnam S. Improved pregnancy rate after transfer of embryos grown in human fallopian tubal cell coculture. Fertil. Steril. 1992;58(3):569–574. doi: 10.1016/s0015-0282(16)55265-0. [DOI] [PubMed] [Google Scholar]
- Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, Smith SK. Determination of the transcript profile of human endometrium. Mol. Hum. Reprod. 2003;9(1):19–33. doi: 10.1093/molehr/gag004. 10.1093/molehr/gag004. [DOI] [PubMed] [Google Scholar]
- Brar AK, Handwerger S, Kessler CA, Aronow BJ. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol. Genomics. 2001;7(2):135–148. doi: 10.1152/physiolgenomics.00061.2001. [DOI] [PubMed] [Google Scholar]
- Briton-Jones C, Kok IH, Cheung CK, Po AL, Chiu TT, Haines C. Ratio of mRNA expression of progesterone receptor isoforms AB is to B in human oviduct mucosal cells during the ovulatory cycle. J. Assist. Reprod. Genet. 2005;22(11–12):429–435. doi: 10.1007/s10815-005-7203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- Caballero-Campo P, Dominguez F, Coloma J, Meseguer M, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation. Mol. Hum. Reprod. 2002;8(4):375–384. doi: 10.1093/molehr/8.4.375. 10.1093/molehr/8.4.375. [DOI] [PubMed] [Google Scholar]
- Campbell S, Swann HR, Seif MW, Kimber SJ, Aplin JD. Integrins and adhesion mlecules: Cell adhesion molecules on the oocyte and preimplantation human embryo. Hum. Reprod. 1995;10(6):1571–1578. doi: 10.1093/humrep/10.6.1571. 10.1093/HUMREP/10.6.1571. [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol. Hum. Reprod. 2002;8(9):871–879. doi: 10.1093/molehr/8.9.871. 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- Critoph FN, Dennis KJ. The cellular composition of the human oviduct epithelium. Br. J. Obstet. Gynaecol. 1977;84(3):219–221. doi: 10.1111/j.1471-0528.1977.tb12559.x. [DOI] [PubMed] [Google Scholar]
- Crow J, Amso NN, Lewin J, Shaw RW. Physiology: Morphology and ultrastructure of Fallopian tube epithelium at different stages of the menstrual cycle and menopause. Hum. Reprod. 1994;9(12):2224–2233. doi: 10.1093/oxfordjournals.humrep.a138428. [DOI] [PubMed] [Google Scholar]
- de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, Silverman RH, Williams BRG. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 2001;69(6):912–920. [PubMed] [Google Scholar]
- Dodd EE. ‘Atlas of histology.’. McGraw-Hill; New York: 1979. [Google Scholar]
- Dominguez F, Yanez-Mo M, Sanchez-Madrid F, Simon C. Embryonic implantation and leukocyte transendothelial migration: different processes with similar players? FASEB J. 2005;19(9):1056–1060. doi: 10.1096/fj.05-3781hyp. 10.1096/fj.05–3781hyp. [DOI] [PubMed] [Google Scholar]
- Dominguez F, Gadea B, Esteban FJ, Horcajades JA, Pellicer A, Simon C. Comparative protein- profile analysis of implanted versus non-implanted human blastocysts. Hum. Reprod. 2008;23(9):1993–2000. doi: 10.1093/humrep/den205. 10.1093/humrep/den205. [DOI] [PubMed] [Google Scholar]
- Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum. Reprod. 2005;20(6):1439–1446. doi: 10.1093/humrep/deh806. 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- Farrell PJ, Broeze RJ, Lengyel P. Accumulation of mRNA and protein in interferon-treated Ehrlich ascites tumor cells. Nature. 1979;279:523–525. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- Fowler AK, Reed CD, Giron DJ. Identification of an interferon in murine placentas. Nature. 1980;286:266–267. doi: 10.1038/286266a0. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum. Reprod. Update. 1997;3(4):367–382. doi: 10.1093/humupd/3.4.367. 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M. Towards a single embryo transfer. Reprod. Biomed. Online. 2003;6(4):470–81. doi: 10.1016/s1472-6483(10)62170-0. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil. Steril. 1998;69(1):84–88. doi: 10.1016/s0015-0282(97)00438-x. [DOI] [PubMed] [Google Scholar]
- Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2006;299(5605):405–8. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404(6776):407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- Haskill S, Piece A, Morris J, Sporn SA, Anisowicz A, Lee SW, Smith T, Martin G, Ralph P, Sager R. Identification of three related GRO genes encoding cytokine functions. Proc. Natl. Acad. Sci. USA. 1990;87(19):7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, Giudice LC. Decidual Stromal Cell Response to Paracrine Signals from the Trophoblast: Amplification of Immune and Angiogenic Modulators. Biol. Reprod. 2007;76(1):102–117. doi: 10.1095/biolreprod.106.054791. 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- Horne AW, King AE, Shaw E, Mc Donald SE, Williams ARW, Saunders PT, Critchley HOD. Attenuated sex steroid receptor expression in fallopian tube of women with ectopic pregnancy. J. Clin. Endocrinol. Metab. 2009;94:5146–5154. doi: 10.1210/jc.2009-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Carpizo D, Luque A. ADAMTS1: A Matrix Metalloprotease with Angioinhibitory Properties. Ann. NY Acad. Sci. 2003;995:183–190. doi: 10.1111/j.1749-6632.2003.tb03221.x. (TISSUE REMODELING) [DOI] [PubMed] [Google Scholar]
- Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. USA. 1999;96(13):7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson PJ, Morgenstern R, Mancini J, Ford-Hutchinson A, Persson B. Membrane-associated proteins in eicosanoid and glutathione metabolism (MAPEG). A widespread protein superfamily. Am. J. Respir. Crit. Care Med. 2000;161(2PT2):S20–4. doi: 10.1164/ajrccm.161.supplement_1.ltta-5. [DOI] [PubMed] [Google Scholar]
- Jentsch S, Pyrowolakis G. Ubiquitin and its kin: how close are the family ties? Trends in Cell Biology. 2000;10(8):335–342. doi: 10.1016/s0962-8924(00)01785-2. [DOI] [PubMed] [Google Scholar]
- Johansson K, Järvliden J, Gogvadze V, Morgenstern R. Multiple roles of microsomal glutathione transferase 1 in cellular protection: a mechanistic study. Free Radic.Biol. Med. 2010;49(11):1638–1645. doi: 10.1016/j.freeradbiomed.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Joliffe IT, Morgen BJ. Principal component analysis and exploratory factor analysis. Stat. Methods Med. Res. 1992;1(1):69–95. doi: 10.1177/096228029200100105. [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression Profiling of Endometrium from Women with Endometriosis Reveals Candidate Genes for Disease-Based Implantation Failure and Infertility. Endocrinology. 2003;144(7):2870–2881. doi: 10.1210/en.2003-0043. 10.1210/en.2003–0043. [DOI] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global Gene Profiling in Human Endometrium during the Window of Implantation. Endocrinology. 2002;143(6):2119–2138. doi: 10.1210/endo.143.6.8885. 10.1210/en.143.6.2119. [DOI] [PubMed] [Google Scholar]
- Kawashima H. Roles of Sulfated Glycans in Lymphocyte Homing. Biological and Pharmaceutical Bulletin. 2006;29(12):2343–2349. doi: 10.1248/bpb.29.2343. [DOI] [PubMed] [Google Scholar]
- Kervancioglu ME, Saridogan E, Atasu T, Camlibel T, Demircan A, Sarikamis B, Djahanbakhch O. Human Fallopian tube epithelial cell co-culture increases fertilization rates in male factor infertility but not in tubal or unexplained infertility. Hum. Reprod. 1997;12(6):1253–1258. doi: 10.1093/humrep/12.6.1253. 10.1093/humrep/12.6.1253. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fazleabas AT. Uterine receptivity and implantation: the regulation and action of insulin-like growth factor binding protein-1 (IGFBP-1), HOXA10 and forkhead transcription factor-1 (FOXO-1) in the baboon endometrium. Reprod. Biol. Endocrinol. 2004;16(2):34. doi: 10.1186/1477-7827-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb KR, Haas AL. Conjugates of ubiquitin cross-reactive protein distribute in a cytoskeletal pattern. Mol. Cell. Biol. 1994;14(12):8408–8419. doi: 10.1128/mcb.14.12.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay CR. Chemokine receptors and T cell chemotaxis. J. Exp. Med. 1996;184(3):799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian M, Sparkes RS, Mohandas T, Fogelman AM, Lusis AJ. Localization of monocyte chemotactic protein-1 gene (SCYA2) to human chromosome 17q11.2-q21.1. Genomics. 1991;9(1):200–203. doi: 10.1016/0888-7543(91)90239-b. [DOI] [PubMed] [Google Scholar]
- Menezo Y, Guerin P. The mammalian oviduct: biochemistry and physiology. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997;73(1):99–104. doi: 10.1016/s0301-2115(97)02729-2. [DOI] [PubMed] [Google Scholar]
- Morey JS, Ryan JC, Van Dolah FM. Microarray validation: factors influencing correlation between oligonucleotide microarrays and real-time PCR. Biol. Proced. Online. 2006;8(1):175–193. doi: 10.1251/bpo126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee AB, Zhang Z, Chilton BS. Uteroglbin: a steroid-inducible immunomodulatory protein that founded the Secertoglobin superfamily. Endocr. Rev. 2007;28(7):707–725. doi: 10.1210/er.2007-0018. [DOI] [PubMed] [Google Scholar]
- Nasu K, Itoh H, Yuge A, Nishida M, Narahara H. Human oviductal epithelial cells express Toll-like receptor 3 and respond to double-stranded RNA: Fallopian tube-specific mucosal immunity against viral infection. Hum. Reprod. 2007;22:356–361. doi: 10.1093/humrep/del385. [DOI] [PubMed] [Google Scholar]
- Nathan CF. Secretory products of macrophages. J. Clin. Invest. 1987;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak E, Everett HS. Cyclical and other variations in the tubal epithelium. Amer. J. Obstet. Gynecol. 1928;16:499–530. [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–28. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem. J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RTPCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl. Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovici RM, Kao L-C, Giudice LC. Discovery of New Inducible Genes in in vitroDecidualized Human Endometrial Stromal Cells Using Microarray Technology. Endocrinology. 2000;141(9):3510–3515. doi: 10.1210/endo.141.9.7789. 10.1210/en.141.9.3510. [DOI] [PubMed] [Google Scholar]
- Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem. J. 2005;386(Pt 1):15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakobphol A, Genbacev O, Gormley M, Kapidzic M, Fisher SJ. A role for the L-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev. Biol. 2006;298(1):107–117. doi: 10.1016/j.ydbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Ritchie KJ, Zhang DE. ISG15: the immunological kin of ubiquitin. Semin. Cell. Dev. Biol. 2004;15(2):237–246. doi: 10.1016/j.semcdb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Riffo M, González KD, Nieto A. Uteroglobin induces the development and celleluar proliferation of the mouse early embryo. J. Exp. Zool. A. Ecol. Genet. Physiol. 2007;307(1):28–34. doi: 10.1002/jez.a.342. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends in Immunology. 2004;25(4):201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Sadler T. ‘Langman's Medical Embryology.’. Lippincott Williams and Wilkins; 1979. [Google Scholar]
- Shaw JL, Dey SK, Critchley HO, Home AW. Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum. Reprod. Update. 2010;16(4):432–444. doi: 10.1093/humupd/dmp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JL, Fitch P, Cartwright J, Entrican G, Schwarze J, Critchley HO, Horne AW. Lymphoid and myeloid cell populations in the non-pregnant human Fallopian tube and in ectopic pregnancy. J. Reprod. Immunol. 2011;89(1):84–91. doi: 10.1016/j.jri.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Wang JM, Colotta F, Dejana E, Mantovani A, Oppenheim JJ, Larsen CG, Zachariae CO, Matsushima K. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J. Immunol. 1990;144(8):3034–3038. [PubMed] [Google Scholar]
- Stern JE, Goldman M, Hatasaka H, MacKenzie TA, Surrey ES, Racowsky C, Society for Reproductive Technology Writing Group Optimizing the number of cleavage stage embryos to transfer on day 3 in women 38 years of age and older: a Society for Assisted Reproductive Technology database study. Fertil. Steril. 2009;91(3):767–76. doi: 10.1016/j.fertnstert.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Guidice LC. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Ulziibat S, Ejima K, Shibata Y, Hishikawa Y, Kitajima M, Fujishita A, Ishimaru T, Koji T. Identification of estrogen receptor beta-positive intraepithelial lymphocates and their possible roles in normal and tubal pregnancy oviducts. Hum. Reprod. 2006;21(9):2281–2289. doi: 10.1093/humrep/del176. [DOI] [PubMed] [Google Scholar]
- Valdés-Dapena M. Histologic and pathologic features of the fetal and neonatal lung. Ann. Clin. Lab. Sci. 1973;3(2):108–117. [PubMed] [Google Scholar]
- Vlad M, Walker D, Kennedy RC. Fertilization and early embryology: Nuclei number in human embryos co-cultured with human ampullary cells. Hum. Reprod. 1996;11(8):1678–1686. doi: 10.1093/oxfordjournals.humrep.a019469. [DOI] [PubMed] [Google Scholar]
- Witschi E. Development and differentiation of the uterus. In: M HC, editor. Proceedings of the Third Annual Symposium on Physiology and Pathology of Human Reproduction:1967 Prenatal Life. Wayne State University Press; 1970. pp. 11–35. [Google Scholar]
- Yeung WS, Lau EY, Chan AY, Ho PC. The production of interleukin-1 alpha immunoreactivity by human oviductal cells in an coculture system. J. Assist. Reprod. Genet. 1996;13(10):772–775. doi: 10.1007/BF02066496. [DOI] [PubMed] [Google Scholar]
- Yeung WS, Ho PC, Lau EYL, Chan STH. Improved development of human embryos in vitro by a human oviductal cell co-culture system*. Hum. Reprod. 1992;7(8):1144–1149. doi: 10.1093/oxfordjournals.humrep.a137810. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Leonard EJ. Secretion by human fibroblasts of monocyte chemoattractant protein (MCP-1), the product of the gene JE. J. Immunol. 1990;144:2377–2383. [PubMed] [Google Scholar]
- Zhang B, Schmoyer D, Kirov S, Snoddy J. GOTree Machine (GOTM): a web-based platform for interpreting sets of interesting genes using Gene Ontology hierarchies. BMC Bioinformatics. 2004;5(1):16. doi: 10.1186/1471-2105-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.