Cell identities can be stable over a long time due to a “cellular memory” of expression profiles achieved through epigenetic mechanisms. In this review, Stuwe et al. describe recent studies demonstrating that short noncoding RNAs can also provide molecular signals that define epigenetic states of cells, leading to transgenerational epigenetic inheritance.
Keywords: Argonautes, cellular memory, epigenetics, piRNA
Abstract
Cells in multicellular organisms have distinct identities characterized by their profiles of expressed genes. Cell identities can be stable over a long time and through multiple cellular divisions but are also responsive to extracellular signals. Since the DNA sequence is identical in all cells, a “cellular memory” of expression profiles is achieved by what are defined as epigenetic mechanisms. Two major molecular principles—networks of transcription factors and maintenance of cis-chromatin modifications—have been implicated in maintaining cellular memory. Here we describe recent studies demonstrating that short noncoding RNAs can also provide molecular signals that define epigenetic states of cells. Small RNAs can act independently or cooperate with chromatin modifications to achieve long-lasting effects necessary for cellular memory and transgenerational inheritance.
All cells in a multicellular organism contain the same DNA sequence and yet differ greatly in their morphologies and functions as a result of distinct gene expression profiles in each cell type. Cells can stably keep their identities over multiple divisions but are also responsive to extracellular factors, as is clearly seen during early development. It is believed that the DNA content of almost all cells within one organism is identical. Indeed, early experiments examining somatic cell nuclear transfer demonstrated that the nucleus of a differentiated cell has full developmental potential if it is transferred to oocyte cytoplasm (Briggs and King 1952). These observations led to cloning of a full organism from the nucleus of a differentiated cell—as in the case of the sheep Dolly (Campbell et al. 1996)—as well as the ability to reprogram multiple types of differentiated cells to induced pluripotent stem cells (iPSCs) (Takahashi and Yamanaka 2006; Okita et al. 2007; Takahashi et al. 2007; Wernig et al. 2007; Yu et al. 2007; Nakagawa et al. 2008).
If the DNA sequence of cells in an organism is the same, how can distinct cell types develop and maintain their identities? The mechanisms that provide long-term maintenance of distinct gene expression profiles despite the same genetic composition were named epi-(above)-genetic. Two major molecular modes of epigenetic regulation have been proposed: self-maintaining networks of transcription factors and chromatin modifications. Expression of particular sets of transcription factors that form gene regulatory networks can generate distinct patterns of gene expression that are stable over long time periods and impact the identity of the cell. This was demonstrated by the observation that inducing the expression of four transcription factors (the so-called Yamanaka factors) is sufficient to trigger a stable switch in cellular identity and generate iPSCs from differentiated cells (Takahashi and Yamanaka 2006; Okita et al. 2007; Takahashi et al. 2007; Wernig et al. 2007; Yu et al. 2007; Nakagawa et al. 2008).
A second mechanism of cell identity determination relies on the maintenance of distinct chromatin states. DNA methylation and certain histone modifications over the promoter and body of genes correlate with their transcriptional status (Jones and Takai 2001; Kouzarides 2007). The nonuniform distribution of histone marks along the genome (Barski et al. 2007; Mikkelsen et al. 2007; Kharchenko et al. 2011) led to the hypothesis that the stable maintenance of a particular pattern of chromatin mark distribution provides the physical basis for an epigenetic memory of gene expression. However, it is important to remember that chromatin profiling experiments can reveal only correlations between a specific chromatin mark and the transcriptional status of genes and cannot prove that a particular chromatin mark directly influences transcription or is stably maintained through cellular divisions, two properties necessary to provide a true epigenetic signal. In fact, it is likely that certain chromatin modifications are the consequence, rather than the cause, of the transcriptional state.
The maintenance of a distinct cell identity shows how epigenetic signals can be stable throughout mitotic cell divisions within one organism. There are, however, some examples for transmission of epigenetic signals not only through mitosis from one cell to its progeny but from one generation to the next. One such example of transgenerational epigenetic inheritance is fur coloration in the Agouti mouse strain (Argeson et al. 1996; Morgan et al. 1999). In this inbred strain, the fur color of animals can vary despite them being genetically identical. The differences in fur color depend on the DNA methylation status of a retrotransposon inserted close to the promoter of the Agouti gene, which is involved in determining fur color. The DNA methylation status of the Agouti locus is inherited from the mother and supports the role of DNA methylation as a transgenerational epigenetic mark. In other cases of transgenerational epigenetic inheritance, the underlying mechanism is not understood. Although transgenerational epigenetic inheritance has been described in just a handful of cases, like the Agouti mouse, these can provide invaluable insights into the mechanisms that likely also operate to maintain cellular identities. Here, we discuss how small RNAs can provide another molecular mechanism for cellular memory that can be transmitted through cellular divisions and even from one generation to the next.
The role of microRNAs (miRNAs) in specifying cell identity
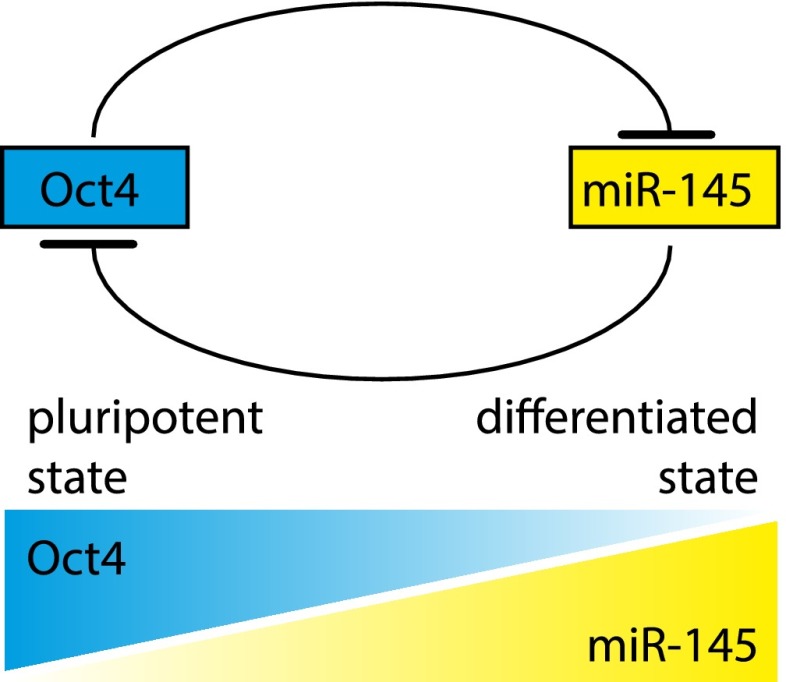
miRNAs regulate expression of protein-coding genes. This is achieved through recognition of binding sites usually located in the 3′ untranslated region (UTR) of target mRNAs followed by repression primarily through destabilization and degradation of the mRNA (Guo et al. 2010), although translational repression can also play a role in this process (Bazzini et al. 2012). The full cohort of miRNA targets is still unknown, but it is estimated that ∼60% of protein-coding genes in the human genome are regulated by miRNAs (Friedman et al. 2009). Importantly, the expression of many miRNAs is restricted to particular cell types, and miRNAs often target mRNAs of key transcription factors. On the other hand, expression of miRNAs is controlled by transcription factors, creating circuits that include both positive and negative feedback loops. Thus, miRNAs are essential components in the networks of transcription factors that are responsible for maintaining cellular identities. For example, miRNA-145 and OCT4, one of the key transcription factors required to maintain the pluripotent state of embryonic stem cells, suppress each other's expression (Fig. 1; Xu et al. 2009; Wu et al. 2011; Adammek et al. 2013). In embryonic stem cells, high levels of OCT4 lead to repression of miR-145 expression, while a reversed pattern is present in differentiated cells. Thus, a reciprocal negative regulatory loop that includes the transcription factor and the miRNA reinforces the switch between pluripotent and differentiated states. The importance of miRNAs in the specification of cellular identity was demonstrated in cell reprogramming experiments: Expression of the miR302/367 cluster reprograms mouse and human somatic cells to iPSCs without the requirement of exogenous transcription factors (Anokye-Danso et al. 2011).
Figure 1.
Model of a regulatory loop between Oct4 and miR-145. The transcription factor Oct4 is crucial for maintaining pluripotency of embryonic stem cells. Oct4 enforces its own expression by suppressing its negative regulator, miR-145. Pluripotent cells have high levels of Oct4 and thus low levels of miR-145. At the initiation of differentiation, declining levels of Oct4 allow miR-145 to be expressed, which further suppresses Oct4 and reinforces differentiation.
The functions of miRNAs extend beyond specifying and maintaining gene expression patterns in particular cell types of the adult organism. For example, miR-430 is expressed at the onset of zygotic transcription in zebrafish. It is responsible for repressing maternal mRNA during early zygotic development, thereby ensuring that the developing embryo will be cleared of maternal RNAs as it transitions to zygotic gene expression (Giraldez et al. 2006).
piRNAs provide long-term genetic memory of transposon invasions
Another class of small RNAs, piRNAs, associates with a distinct clade of the Argonaute protein family called the Piwi clade. Expression of both Piwi proteins and piRNAs seems to be restricted to germ cells of metazoans, with the exception of their expression in follicular cells of the Drosophila ovary that are of somatic origin (Khurana and Theurkauf 2010; Senti and Brennecke 2010; Siomi et al. 2011). In contrast to miRNAs that target a variety of host protein-coding genes, the main targets of piRNAs are diverse sets of transposable elements (TEs) present in metazoan genomes. Indeed, malfunction of the piRNA pathway leads to derepression of multiple TE families in germ cells of both Drosophila and mice (Aravin et al. 2007; Brennecke et al. 2007). In both species, TE activation is associated with sterility, likely caused by formation of dsDNA breaks generated by transposition events (Klattenhoff et al. 2007). The piRNA pathway can be compared with an immune system that recognizes and represses genomic parasites—TEs—instead of extracellular pathogens and retains a memory of previous host encounters with specific TEs.
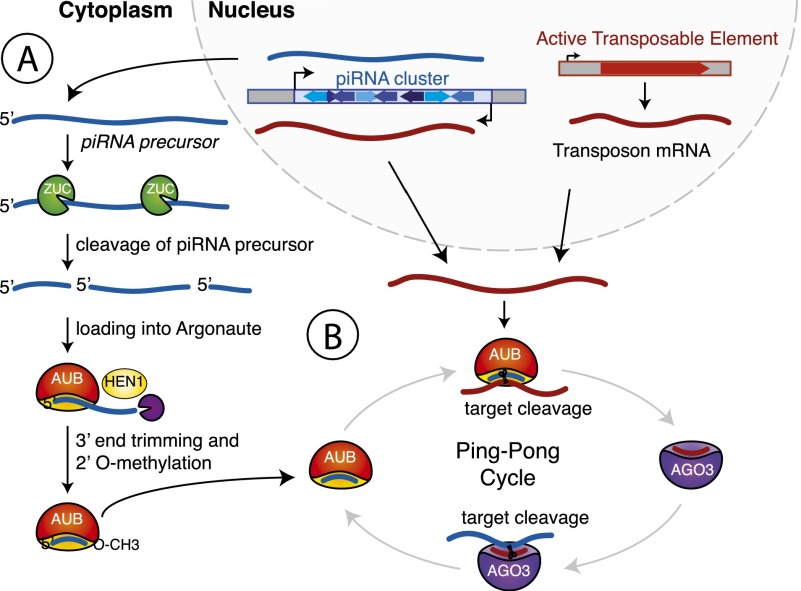
To perform its function, the piRNA pathway has to recognize a diverse set of TEs already present in the genome and also must be able to respond to new elements invading the host. Indeed, piRNA sequences are much more diverse than miRNAs, and the pool of piRNAs present in a given strain correlates well with the TE sequences present in the genome of this particular strain (Rozhkov et al. 2010; Kelleher et al. 2012). piRNAs are derived from distinct genomic regions called piRNA clusters, which are enriched in transposon sequences. piRNA precursors are transcribed from clusters as long transcripts, which are subsequently processed and loaded into Piwi proteins (Brennecke et al. 2007). The details of the processing mechanism are still largely unknown, although it seems to be a multistep process (Fig. 2A). In the first step, an endonuclease, possibly Zucchini, cleaves precursor transcripts to generate 5′ phosphorylated intermediate RNAs that are then loaded into Piwi proteins (Ipsaro et al. 2012; Nishimasu et al. 2012; Voigt et al. 2012). During the next step, another nuclease trims the intermediate from its 3′ end until it reaches the footprint protected by the Piwi protein (Kawaoka et al. 2011). Finally, the HEN1 methyltransferase modifies the 3′ end of mature piRNAs with a 2′ OMe group increasing their stability (Kirino and Mourelatos 2007; Kurth and Mochizuki 2009; Kamminga et al. 2010; Montgomery et al. 2012).
Figure 2.
Biogenesis of piRNAs. (A) Long RNAs that constitute substrates for piRNA processing are transcribed from distinct genomic regions called piRNA clusters. These transcripts are cleaved, presumably by the endonuclease Zucchini (ZUC), to produce 5′ phosphorylated piRNA precursors. The precursors are anchored into Piwi proteins with their 5′ end, and their 3′ end is trimmed by an unknown nuclease. Methylation of the 3′ end 2′OH group by the methyltransferase HEN1 produces mature piRNAs. (B) The Ping-Pong cycle amplifies piRNA sequences. piRNA-loaded Aubergine (AUB) initiates the Ping-Pong cycle by cleaving a complementary target transcript derived from either an active TE or the opposite strand of the initial cluster. This produces the 5′ end of a new piRNA precursor. This precursor is loaded into Argonaute 3 (AGO3) and guides cleavage of a piRNA cluster transcript complementary to its sequence.
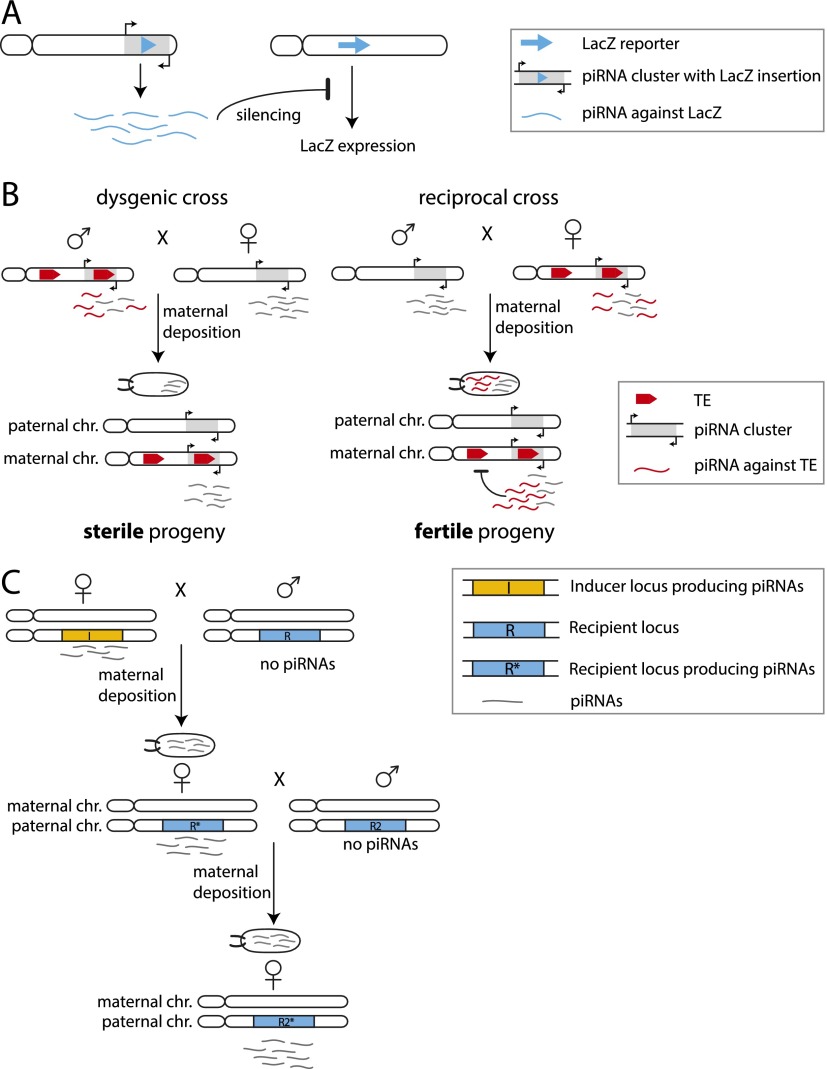
Although the specificity of piRNA processing is not fully understood, it seems like any sequence can be processed into piRNAs as long as it is located inside a piRNA cluster region. Indeed, an exogenous sequence (LacZ) inserted into a piRNA cluster is processed into piRNAs that match and repress a LacZ reporter (Fig. 3A; Roche and Rio 1998; Ronsseray et al. 2003; Muerdter et al. 2012). Similarly, upon exposure of the organism to a new TE, transposition of the TE will eventually lead to its insertion into a piRNA cluster and generate immunity against the new transposon (Khurana et al. 2011). Thus, piRNA clusters contain genetically encoded information about sequences that have to be recognized as foreign and need to be silenced.
Figure 3.
Transgenerational effects of piRNAs in Drosophila. (A) Insertion of a LacZ sequence into a subtelomeric piRNA cluster leads to production of piRNAs corresponding to the LacZ sequence. These piRNAs can silence the expression of a LacZ reporter gene in trans. Maternal inheritance of the lacZ-containing piRNA cluster leads to silencing of the reporter, while paternal inheritance does not. (B) Scheme of hybrid dysgenesis. The presence of a potentially active TE in the genome correlates with expression of piRNAs targeting this element derived from piRNA clusters that contain the TE sequence. piRNAs targeting the element are transmitted from the maternal germline into the embryo and silence the TE in the progeny, keeping it fertile. If the TE is present in the paternal but not the maternal genome, no piRNAs targeting the TE are deposited into the embryo. The presence of the TE sequences in the paternally inherited piRNA cluster is not sufficient to protect the progeny against sterility. (C) The inducer locus I gives rise to piRNAs, which are transmitted into the next generation through the female germline. The maternally transmitted piRNAs from the I locus suffice to convert the recipient locus R, which did not produce piRNAs, into a piRNA-producing locus R*. If a female fly containing R* is crossed to a male containing the unproductive R locus (R2), the maternally deposited piRNAs can again switch R2 into R2*. Conversion to a piRNA-producing locus is stable over many generations.
Transgenerational effects of piRNAs in Drosophila
While the sequence content of piRNA clusters provides the genetic component of the piRNA pathway, the pathway also has an epigenetic component that was revealed during studies of transgenically encoded piRNAs and a phenomenon called hybrid dysgenesis. piRNAs corresponding to lacZ, which are generated as a result of a lacZ transgene insertion into a telomeric piRNA cluster, can silence a lacZ reporter in trans (Fig. 3A). Unexpectedly, silencing is observed only if the lacZ-generating cluster is maternally inherited, while no repression is seen if the transgene is inherited from the father despite the fact that the progeny are genetically identical (Josse et al. 2007). This result indicates that the presence of the piRNA cluster in the genome is not sufficient to induce silencing and that an additional, epigenetic signal transmitted through the maternal germline is required for repression.
Silencing of native transposons in the progeny also depends on the direction in which two fly strains are crossed, as was observed a long time ago in a phenomenon called hybrid dysgenesis. Hybrid dysgenesis is observed in crosses between two fly strains where a particular TE is present in the genome of one but not the other strain. In the progeny of crosses between two such strains, the TE is activated if it was inherited from the father, leading to sterile, dysgenic progeny. However, if the TE is inherited from the mother, then the TE remains repressed, and the progeny is fertile (Fig. 3B; Bucheton 1979; Bingham et al. 1982; Rubin et al. 1982). Recent studies revealed that derepression of the TE in a dysgenic cross correlates with the absence of cognate piRNAs targeting this element, thereby implicating a failure of piRNA-mediated silencing as a primary reason for element activation (Brennecke et al. 2008; Khurana et al. 2011; Grentzinger et al. 2012). Similar to the crosses with the lacZ transgene described above, the genotypes of the progeny in the two crosses are identical, pointing to the involvement of a maternally transmitted epigenetic signal that is required to produce piRNAs targeting the element.
It seems that the piRNAs themselves are the epigenetic signal that is transmitted from one generation to the next and is required to mount the silencing response. Indeed, Piwi proteins loaded with piRNAs are deposited from the maternal germline into the oocyte and are present in the early embryo before the start of zygotic transcription (Brennecke et al. 2008). The maternal deposition of piRNAs correlates with the ability of the adult progeny to repress cognate sequences in its ovaries (Brennecke et al. 2008). In the dysgenic cross, no piRNAs against the particular TE are transmitted to the embryos, as the mother lacks both the element and the cognate piRNAs, and the father does not transmit piRNAs.
How can piRNAs inherited from the previous generation ensure repression of targets in the germ cells of the progeny? It is unlikely that the piRNA molecules deposited into the oocyte are able to endure until the adult stage and can be sufficient to mediate repression. Therefore, piRNAs inherited from the previous generation must somehow trigger production of new cognate piRNAs in the next generation. The proof that maternally provided piRNAs are indeed able to activate production of piRNAs from cognate regions in the next generation was found in experiments with transgenic piRNA-generating loci (de Vanssay et al. 2012). Ronsseray and colleagues (de Vanssay et al. 2012) found that the exposure of a naïve transgenic locus that does not produce piRNAs to a cognate piRNA species leads to the generation of piRNAs from that locus (Fig. 3C). After initial activation, the ability to generate piRNAs is inherited if the activated locus is transmitted through the maternal germline, further supporting the idea that maternally deposited piRNAs are required to maintain piRNA production. Thus, at least in Drosophila, piRNAs can provide an epigenetic signal that is transmitted from one generation to the next.
Mechanisms of transgenerational effects of piRNAs
How piRNAs inherited from the previous generation can activate piRNA generation in the progeny is not yet clear. However, two possible mechanisms can be proposed. First, the inherited piRNAs might induce a change in the chromatin state over piRNA clusters in the progeny. This change might be necessary to ensure transcription of these regions in order to generate piRNA precursor transcripts or might facilitate their channeling into the piRNA processing machinery after transcription. Alternatively, the inherited piRNAs might be necessary to initiate efficient processing of the precursor molecules into piRNAs in the cytoplasm.
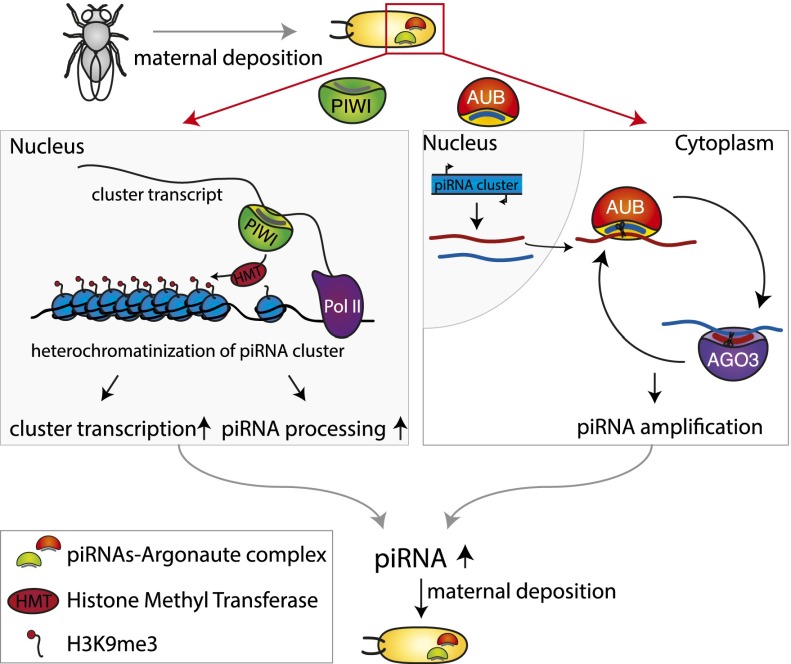
Two requirements need to be met in order for inherited piRNAs to regulate new piRNA production in the progeny by a chromatin-mediated mechanism. piRNA clusters must possess a unique chromatin signature that leads to the production of piRNAs, and piRNAs need to be able to induce establishment of such a chromatin state. piRNA clusters are located at the borders of euchromatic and heterochromatic regions or within heterochromatic domains and are enriched in the heterochromatic H3K9me3 mark (Brennecke et al. 2007; Rangan et al. 2011). The heterochromatic nature of piRNA clusters seems to be not only a signature of piRNA clusters but actually a requirement for their function. The histone methyltransferase dSETDB1 (also called “EGG”), which deposits the methylation mark onto Lys9 of the histone 3 tails, was identified as an essential factor for the transcription of piRNA clusters (Rangan et al. 2011). Additionally, Rhino, a germline-specific paralog of the heterochromatin protein HP1 in Drosophila, is required for production of piRNAs from dual-strand clusters (Klattenhoff et al. 2009). These data support the rather counterintuitive idea that piRNA clusters need to have what is thought of as repressive chromatin in order to generate piRNAs. Besides the possibility that the H3K9me3 mark might be required for piRNA cluster transcription (Rangan et al. 2011), it was also proposed that the heterochromatic nature of clusters ensures their physical location in specific regions of the nucleus, which in turn warrants effective channeling of piRNA precursors to the perinuclear compartment of piRNA processing (Fig. 4; Zhang et al. 2012).
Figure 4.
Possible mechanisms of transgenerational effects of piRNAs. Maternally transmitted piRNAs can lead to stable piRNA production in the next generation by two mechanisms. In the nucleus, piRNAs in complex with PIWI could ensure piRNA precursor production by inducing establishment of a chromatin state over cognate piRNA clusters that permits piRNA production. Such a chromatin environment can either enhance transcription of the locus or result in channeling of the cluster transcript into the piRNA processing machinery or both. In the cytoplasm, the maternally deposited piRNAs associated with AUB could initiate cleavage of piRNA cluster transcripts in the Ping-Pong cycle.
Although it is not yet clear whether piRNAs are indeed required for determining the chromatin state on piRNA clusters, piRNAs associated with PIWI do guide deposition of the H3K9me3 mark on their genomic targets (Klenov et al. 2007; Wang and Elgin 2011; Sienski et al. 2012; Gu and Elgin 2013; Huang et al. 2013; Le Thomas et al. 2013; Rozhkov et al. 2013; Yakushev et al. 2013). According to the model that emerged from studies of nuclear PIWI function, piRNAs guide the nuclear PIWI protein to complementary nascent transcripts followed by recruitment of a histone methyltransferase (e.g., dSETDB1) and heterochromatinization of the target locus (Fig. 4). Accordingly, it is expected that PIWI will be able to induce recruitment of the chromatin-modifying machinery to piRNA clusters that produce transcripts complementary to PIWI-associated piRNAs. Thus, heterochromatin seems to be the prerequisite for piRNA production but also the consequence of piRNA-mediated regulation on the transcriptional level. This positive feedback loop could explain why maternally deposited piRNAs are needed for proper expression of clusters in the progeny.
The alternative hypothesis of how maternally deposited piRNAs can trigger expression of cognate piRNAs in the progeny and thus ensure epigenetic transmission of the defense against TEs is based on the ability of piRNAs to be amplified in the so-called Ping-Pong cycle (Fig. 2B; Brennecke et al. 2007; Gunawardane et al. 2007). Two Piwi proteins, Argonaute 3 (AGO3) and Aubergine (AUB), have endonuclease activities and team up to produce piRNAs if target substrates are available. AUB loaded with a piRNA recognizes a complementary transcript and cleaves it to generate the 5′ end of a new piRNA, which is then incorporated into AGO3. AGO3 associated with this piRNA can in turn recognize complementary transcripts and cleave them to generate new piRNAs that are identical in sequence to the initial piRNA that started the cycle. Major piRNA clusters in germ cells are bidirectionally transcribed, generating RNAs from both strands that can be substrates for the Ping-Pong cycle. In this scenario, maternally deposited piRNAs bound to AUB together with zygotically transcribed piRNA clusters could lead to the amplification of piRNAs without changes to the piRNA cluster chromatin or its level of transcription (Fig. 4). It should be noted, though, that the Ping-Pong amplification cycle can only work in germ cells that express the AUB and AGO3 proteins. However, the role of maternally deposited piRNAs in TE suppression was also described in the somatic follicular cells surrounding the germ cells in Drosophila ovarioles, which express only PIWI and not AUB and AGO3 (Akkouche et al. 2013). This suggests that—at least in follicular cells—the maternally transmitted piRNAs can induce production of new cognate piRNAs without signal amplification through the Ping-Pong cycle.
The two proposed mechanisms for the transmission of epigenetic information via maternally deposited piRNAs are not mutually exclusive and might even be working cooperatively: piRNA-induced heterochromatin formation is required for adequate transcription of piRNA clusters, while piRNAs incorporated into AUB are required for efficient processing of the cluster transcripts.
Small RNA-based memory of gene expression in Caenorhabditis elegans
Recent studies in C. elegans uncovered a remarkable system that uses transgenerational memory of gene expression to find and repress foreign, nonself sequences in germ cells (Ashe et al. 2012; Lee et al. 2012; Luteijn et al. 2012; Shirayama et al. 2012). This system leads to the repression of sequences in germ cells unless they were expressed in the germ cells of the previous generation. Indeed, experimentally introduced transgenes with new sequences become repressed in germ cells (Ashe et al. 2012; Lee et al. 2012; Luteijn et al. 2012; Shirayama et al. 2012). Importantly, expression of such transgenes can be rescued in the next generation by injecting a fragment of the transgene's RNA into the parental gonad, indicating that the presence of RNA per se (and not the act of transcription) is sufficient to generate a transmittable epigenetic signal (Johnson and Spence 2011). This result indicates that C. elegans uses the history of gene expression of the previous generation to identify and repress nonself sequences.
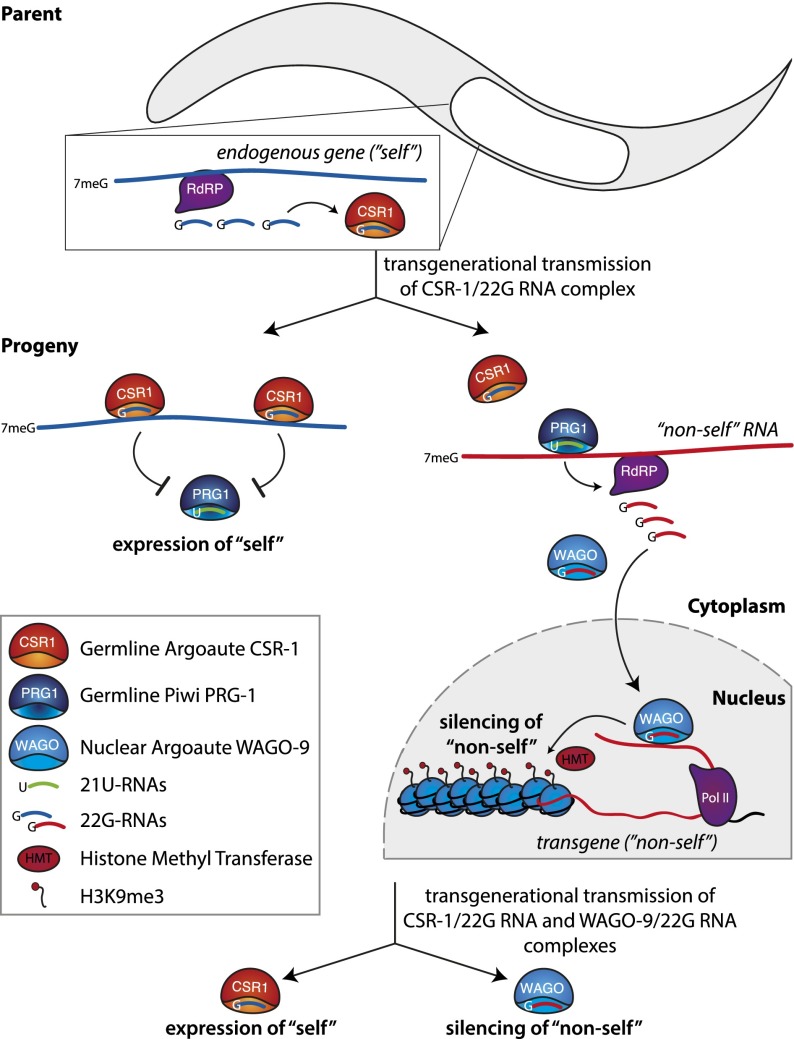
It seems that the correct identification and silencing of nonself sequences in C. elegans requires the cooperation of three different small RNA pathways (Fig. 5). First, a diverse population of 22G-RNAs associated with the CSR-1 protein marks all transcripts expressed in germ cells (Claycomb et al. 2009). This is achieved by the generation of complementary RNA to transcripts by an RNA-dependent RNA polymerase and subsequent processing into 22G-RNAs. 22G-RNAs that are loaded into CSR-1 are transmitted into the progeny and are able to recognize complementary transcripts. In the progeny, genes that have CSR-1-bound complementary 22G-RNAs are expressed, while all others are silenced, indicating that the population of CSR-1-bound 22G-RNAs provides an epigenetic signal of gene expression (Claycomb et al. 2009).
Figure 5.
Three small RNA pathways cooperate to repress nonself sequences in C. elegans. The expression of a gene (blue) in the germline leads to generation of complementary 22G-RNAs by an RNA-dependent RNA polymerase (RdRP). 22G-RNAs associated with the CSR-1 protein are transmitted to the next generation through the female germline. PRG-1 guided by 21U-RNAs has a potential to recognize and repress any sequence; however, transcripts targeted by the transgenerationally inherited CSR-1/22G-RNA complex are excluded from this repression. Accordingly, only newly expressed nonself sequences (red) are recognized for repression by 21U-RNAs and PRG-1. Recognition of a sequence by PRG-1 causes generation of 22G-RNAs by an RdRP, and these 22G-RNAs associate with the nuclear WAGO-9 protein. The recognition of nascent transcripts by WAGO-9/22G-RNAs leads to deposition of the H3K9me3 mark on the target and its transcriptional silencing. WAGO-9 and the associated 22G-RNAs are transmitted to the next generation, ensuring a memory of nonself sequences that have to be repressed.
Repression of nonself sequences is initiated by the C. elegans Piwi-clade protein PRG-1 and the associated 21U-RNAs (the C. elegans piRNAs). Recognition of nonself sequences seems to be achieved by targeting of all sequences that are not protected by the CSR-1 system (Ashe et al. 2012; Lee et al. 2012; Luteijn et al. 2012; Shirayama et al. 2012). To be able to identify any nonself sequence, 21U-RNAs need to be able to recognize and silence any possible sequence. Indeed, 21U-RNAs are highly diverse and, unlike Drosophila piRNAs, are not particularly enriched in sequences that correspond to TEs. Therefore, it was proposed that 21U-RNAs are able to bind many, if not all, transcripts through incomplete sequence complementarity (Ruby et al. 2006; Bagijn et al. 2012). Binding of CSR-1-associated 22G-RNAs to “self” transcripts is thought to protect them from binding of 21U-RNA/PRG-1, leading to specific recognition of new, nonself sequences by PRG-1 and 21U RNAs (Ashe et al. 2012; Luteijn et al. 2012; Shirayama et al. 2012).
Binding of the PRG-1/21U-RNA complex to a target transcript leads to recruitment of an RNA-dependent RNA polymerase and production of antisense 22G-RNAs (similar to the 22G-RNAs in CSR-1 but mapping to the foreign sequence), which get loaded into WAGO-9 (Ashe et al. 2012; Lee et al. 2012; Luteijn et al. 2012; Shirayama et al. 2012). WAGO-9 enters the nucleus and—together with other factors, including chromatin proteins—induces transcriptional silencing of the sequence along with deposition of the H3K9me3 mark. Once established, silencing of the target is independent of the 21U-RNAs and PRG-1. In fact, silencing is maintained throughout mitotic divisions even in the absence of WAGO-9, possibly through propagation of repressive chromatin marks. However, absence of the WAGO pathway during meiosis leads to derepression of the foreign sequences in the progeny (Ashe et al. 2012; Lee et al. 2012; Luteijn et al. 2012; Shirayama et al. 2012). These results indicate that the WAGO pathway is also transmitting epigenetic information to the next generation, but unlike CSR-1, which identifies “self” sequences for expression, WAGO-associated sequences mark their targets for silencing in the progeny.
Together, these three systems, in concert with chromatin factors, allow for stable transgenerational transmission of information regarding self and nonself and ensure an immediate silencing of new foreign sequences. The promiscuity of targeting guided by 21U-RNAs associated with PRG1 allows the piRNA pathway to recognize and induce silencing of new foreign elements of any sequence. To assure that the worm's own genes are not silenced by PRG-1, sequences that were expressed in the germline of the previous generation are protected by CSR-1-associated 22G-RNAs, which are inherited from the previous generation and interfere with binding of PRG-1 to these transcripts. Finally, small RNAs associated with WAGO-9 mark previously identified foreign sequences (such as TEs) and provide a transgenerationally inherited signal for transcriptional repression.
Conclusion
Gregor Mendel described the fundamental principles of inheritance in the 1860s. He tracked visible traits of plants and formulated rules that later were explained by the distribution of the genetic material, DNA, during meiosis. However, subsequent studies in various systems occasionally described patterns of inheritance that did not follow those rules; they were named “non-Mendelian” or “epigenetic.” Recent studies showed that small RNAs are molecular signals that can be transmitted from one generation to the next and underlie many cases of epigenetic inheritance in flies and worms. It seems to be a common theme that small RNAs and chromatin modifications act together to form a stable mechanism of epigenetic inheritance. The rather rare examples of transgenerational effects of small RNAs likely represent the tip of the iceberg in comparison with their role in specifying and maintaining cellular identities within an organism. Therefore, small RNA pathways might play crucial roles in providing cellular memory of gene expression even in organisms such as mammals, where involvement of small RNAs in transgenerational inheritance was not described.
Acknowledgments
We thank members of the Aravin laboratory for discussion. E.S. is supported by a Ph.D. fellowship of the Boehringer Ingelheim Fonds. Work on this topic in the Aravin laboratory is supported by grants from the National Institutes of Health (R00 HD057233, R01 GM097363, and DP2 OD007371A) and the Searle Scholar and the Packard Fellowship Awards.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.236414.113.
References
- Adammek M, Greve B, Kassens N, Schneider C, Bruggemann K, Schuring AN, Starzinski-Powitz A, Kiesel L, Gotte M 2013. MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertil Steril 99: 1346–1355.e5 [DOI] [PubMed] [Google Scholar]
- Akkouche A, Grentzinger T, Fablet M, Armenise C, Burlet N, Braman V, Chambeyron S, Vieira C 2013. Maternally deposited germline piRNAs silence the tirant retrotransposon in somatic cells. EMBO Rep 14: 458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, et al. 2011. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 8: 376–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ 2007. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316: 744–747 [DOI] [PubMed] [Google Scholar]
- Argeson AC, Nelson KK, Siracusa LD 1996. Molecular basis of the pleiotropic phenotype of mice carrying the hypervariable yellow (Ahvy) mutation at the agouti locus. Genetics 142: 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Sapetschnig A, Weick EM, Mitchell J, Bagijn MP, Cording AC, Doebley AL, Goldstein LD, Lehrbach NJ, Le Pen J, et al. 2012. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell 150: 88–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagijn MP, Goldstein LD, Sapetschnig A, Weick EM, Bouasker S, Lehrbach NJ, Simard MJ, Miska EA 2012. Function, targets, and evolution of Caenorhabditis elegans piRNAs. Science 337: 574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K 2007. High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837 [DOI] [PubMed] [Google Scholar]
- Bazzini AA, Lee MT, Giraldez AJ 2012. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 336: 233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham PM, Kidwell MG, Rubin GM 1982. The molecular basis of P-M hybrid dysgenesis: The role of the P element, a P-strain-specific transposon family. Cell 29: 995–1004 [DOI] [PubMed] [Google Scholar]
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ 2007. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128: 1089–1103 [DOI] [PubMed] [Google Scholar]
- Brennecke J, Malone CD, Aravin AA, Sachidanandam R, Stark A, Hannon GJ 2008. An epigenetic role for maternally inherited piRNAs in transposon silencing. Science 322: 1387–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R, King TJ 1952. Transplantation of living nuclei from blastula cells into enucleated frogs' eggs. Proc Natl Acad Sci 38: 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheton A 1979. Non-Mendelian female sterility in Drosophila melanogaster: Influence of aging and thermic treatments. III. Cumulative effects induced by these factors. Genetics 93: 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KH, McWhir J, Ritchie WA, Wilmut I 1996. Sheep cloned by nuclear transfer from a cultured cell line. Nature 380: 64–66 [DOI] [PubMed] [Google Scholar]
- Claycomb JM, Batista PJ, Pang KM, Gu W, Vasale JJ, van Wolfswinkel JC, Chaves DA, Shirayama M, Mitani S, Ketting RF, et al. 2009. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell 139: 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vanssay A, Bouge AL, Boivin A, Hermant C, Teysset L, Delmarre V, Antoniewski C, Ronsseray S 2012. Paramutation in Drosophila linked to emergence of a piRNA-producing locus. Nature 490: 112–115 [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP 2009. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, Schier AF 2006. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312: 75–79 [DOI] [PubMed] [Google Scholar]
- Grentzinger T, Armenise C, Brun C, Mugat B, Serrano V, Pelisson A, Chambeyron S 2012. piRNA-mediated transgenerational inheritance of an acquired trait. Genome Res 22: 1877–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T, Elgin SC 2013. Maternal depletion of Piwi, a component of the RNAi system, impacts heterochromatin formation in Drosophila. PLoS Genet 9: e1003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC 2007. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 315: 1587–1590 [DOI] [PubMed] [Google Scholar]
- Guo H, Ingolia NT, Weissman JS, Bartel DP 2010. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466: 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XA, Yin H, Sweeney S, Raha D, Snyder M, Lin H 2013. A major epigenetic programming mechanism guided by piRNAs. Dev Cell 24: 502–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsaro JJ, Haase AD, Knott SR, Joshua-Tor L, Hannon GJ 2012. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature 491: 279–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Spence AM 2011. Epigenetic licensing of germline gene expression by maternal RNA in C. elegans. Science 333: 1311–1314 [DOI] [PubMed] [Google Scholar]
- Jones PA, Takai D 2001. The role of DNA methylation in mammalian epigenetics. Science 293: 1068–1070 [DOI] [PubMed] [Google Scholar]
- Josse T, Teysset L, Todeschini AL, Sidor CM, Anxolabehere D, Ronsseray S 2007. Telomeric trans-silencing: An epigenetic repression combining RNA silencing and heterochromatin formation. PLoS Genet 3: 1633–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamminga LM, Luteijn MJ, den Broeder MJ, Redl S, Kaaij LJ, Roovers EF, Ladurner P, Berezikov E, Ketting RF 2010. Hen1 is required for oocyte development and piRNA stability in zebrafish. EMBO J 29: 3688–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka S, Izumi N, Katsuma S, Tomari Y 2011. 3′ end formation of PIWI-interacting RNAs in vitro. Mol Cell 43: 1015–1022 [DOI] [PubMed] [Google Scholar]
- Kelleher ES, Edelman NB, Barbash DA 2012. Drosophila interspecific hybrids phenocopy piRNA-pathway mutants. PLoS Biol 10: e1001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharchenko PV, Alekseyenko AA, Schwartz YB, Minoda A, Riddle NC, Ernst J, Sabo PJ, Larschan E, Gorchakov AA, Gu T, et al. 2011. Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature 471: 480–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana JS, Theurkauf W 2010. piRNAs, transposon silencing, and Drosophila germline development. J Cell Biol 191: 905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana JS, Wang J, Xu J, Koppetsch BS, Thomson TC, Nowosielska A, Li C, Zamore PD, Weng Z, Theurkauf WE 2011. Adaptation to P element transposon invasion in Drosophila melanogaster. Cell 147: 1551–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino Y, Mourelatos Z 2007. 2′-O-methyl modification in mouse piRNAs and its methylase. Nucleic Acids Symp Ser 51: 417–418 [DOI] [PubMed] [Google Scholar]
- Klattenhoff C, Bratu DP, McGinnis-Schultz N, Koppetsch BS, Cook HA, Theurkauf WE 2007. Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response. Dev Cell 12: 45–55 [DOI] [PubMed] [Google Scholar]
- Klattenhoff C, Xi H, Li C, Lee S, Xu J, Khurana JS, Zhang F, Schultz N, Koppetsch BS, Nowosielska A, et al. 2009. The Drosophila HP1 homolog Rhino is required for transposon silencing and piRNA production by dual-strand clusters. Cell 138: 1137–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenov MS, Lavrov SA, Stolyarenko AD, Ryazansky SS, Aravin AA, Tuschl T, Gvozdev VA 2007. Repeat-associated siRNAs cause chromatin silencing of retrotransposons in the Drosophila melanogaster germline. Nucleic Acids Res 35: 5430–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T 2007. Chromatin modifications and their function. Cell 128: 693–705 [DOI] [PubMed] [Google Scholar]
- Kurth HM, Mochizuki K 2009. 2′-O-methylation stabilizes Piwi-associated small RNAs and ensures DNA elimination in Tetrahymena. RNA 15: 675–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Gu W, Shirayama M, Youngman E, Conte D Jr, Mello CC 2012. C. elegans piRNAs mediate the genome-wide surveillance of germline transcripts. Cell 150: 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Thomas A, Rogers AK, Webster A, Marinov GK, Liao SE, Perkins EM, Hur JK, Aravin AA, Toth KF 2013. Piwi induces piRNA-guided transcriptional silencing and establishment of a repressive chromatin state. Genes Dev 27: 390–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luteijn MJ, van Bergeijk P, Kaaij LJ, Almeida MV, Roovers EF, Berezikov E, Ketting RF 2012. Extremely stable Piwi-induced gene silencing in Caenorhabditis elegans. EMBO J 31: 3422–3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, et al. 2007. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448: 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery TA, Rim YS, Zhang C, Dowen RH, Phillips CM, Fischer SE, Ruvkun G 2012. PIWI associated siRNAs and piRNAs specifically require the Caenorhabditis elegans HEN1 ortholog henn-1. PLoS Genet 8: e1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan HD, Sutherland HG, Martin DI, Whitelaw E 1999. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet 23: 314–318 [DOI] [PubMed] [Google Scholar]
- Muerdter F, Olovnikov I, Molaro A, Rozhkov NV, Czech B, Gordon A, Hannon GJ, Aravin AA 2012. Production of artificial piRNAs in flies and mice. RNA 18: 42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S 2008. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26: 101–106 [DOI] [PubMed] [Google Scholar]
- Nishimasu H, Ishizu H, Saito K, Fukuhara S, Kamatani MK, Bonnefond L, Matsumoto N, Nishizawa T, Nakanaga K, Aoki J, et al. 2012. Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature 491: 284–287 [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S 2007. Generation of germline-competent induced pluripotent stem cells. Nature 448: 313–317 [DOI] [PubMed] [Google Scholar]
- Rangan P, Malone CD, Navarro C, Newbold SP, Hayes PS, Sachidanandam R, Hannon GJ, Lehmann R 2011. piRNA production requires heterochromatin formation in Drosophila. Curr Biol 21: 1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche SE, Rio DC 1998. Trans-silencing by P elements inserted in subtelomeric heterochromatin involves the Drosophila Polycomb group gene, Enhancer of zeste. Genetics 149: 1839–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsseray S, Josse T, Boivin A, Anxolabehere D 2003. Telomeric transgenes and trans-silencing in Drosophila. Genetica 117: 327–335 [DOI] [PubMed] [Google Scholar]
- Rozhkov NV, Aravin AA, Zelentsova ES, Schostak NG, Sachidanandam R, McCombie WR, Hannon GJ, Evgen'ev MB 2010. Small RNA-based silencing strategies for transposons in the process of invading Drosophila species. RNA 16: 1634–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhkov NV, Hammell M, Hannon GJ 2013. Multiple roles for Piwi in silencing Drosophila transposons. Genes Dev 27: 400–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Kidwell MG, Bingham PM 1982. The molecular basis of P-M hybrid dysgenesis: The nature of induced mutations. Cell 29: 987–994 [DOI] [PubMed] [Google Scholar]
- Ruby JG, Jan C, Player C, Axtell MJ, Lee W, Nusbaum C, Ge H, Bartel DP 2006. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell 127: 1193–1207 [DOI] [PubMed] [Google Scholar]
- Senti KA, Brennecke J 2010. The piRNA pathway: A fly's perspective on the guardian of the genome. Trends Genet 26: 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Seth M, Lee HC, Gu W, Ishidate T, Conte D Jr, Mello CC 2012. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell 150: 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienski G, Donertas D, Brennecke J 2012. Transcriptional silencing of transposons by piwi and maelstrom and its impact on chromatin state and gene expression. Cell 151: 964–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi MC, Sato K, Pezic D, Aravin AA 2011. PIWI-interacting small RNAs: The vanguard of genome defence. Nat Rev Mol Cell Biol 12: 246–258 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872 [DOI] [PubMed] [Google Scholar]
- Voigt F, Reuter M, Kasaruho A, Schulz EC, Pillai RS, Barabas O 2012. Crystal structure of the primary piRNA biogenesis factor Zucchini reveals similarity to the bacterial PLD endonuclease Nuc. RNA 18: 2128–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Elgin SC 2011. Drosophila Piwi functions downstream of piRNA production mediating a chromatin-based transposon silencing mechanism in female germ line. Proc Natl Acad Sci 108: 21164–21169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R 2007. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448: 318–324 [DOI] [PubMed] [Google Scholar]
- Wu Y, Liu S, Xin H, Jiang J, Younglai E, Sun S, Wang H 2011. Up-regulation of microRNA-145 promotes differentiation by repressing OCT4 in human endometrial adenocarcinoma cells. Cancer 117: 3989–3998 [DOI] [PubMed] [Google Scholar]
- Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS 2009. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 137: 647–658 [DOI] [PubMed] [Google Scholar]
- Yakushev EY, Sokolova OA, Gvozdev VA, Klenov MS 2013. Multifunctionality of PIWI proteins in control of germline stem cell fate. Biochemistry Biokhimiia 78: 585–591 [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920 [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang J, Xu J, Zhang Z, Koppetsch BS, Schultz N, Vreven T, Meignin C, Davis I, Zamore PD, et al. 2012. UAP56 couples piRNA clusters to the perinuclear transposon silencing machinery. Cell 151: 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]