Abstract
Intestinal-type adenocarcinoma (ITAC) is a rare form of sinonasal cancer characterized by an association with exposure to industrial dusts, aggressive clinical behavior, and histologic/immunophenotypic similarity to tumors of the gastrointestinal tract. ITAC is sometimes very poorly differentiated and difficult to distinguish from other sinonasal neoplasms, particularly in a limited biopsy. CDX-2 and cytokeratin 20 are consistently immunoreactive in ITAC and as a result, these immunostains are often used to support the diagnosis. However, CDX-2 and cytokeratin 20 have not been tested on a broad range of sinonasal tumors, so their specificities remain unknown. Immunohistochemistry for CDX-2 and cytokeratin 20 was performed on 6 sinonasal ITACs as well as 176 non-intestinal-type sinonasal neoplasms. CDX-2 and cytokeratin 20 were positive in all 6 cases of ITAC. CDX-2 immunoexpression was also observed in 17 of 176 (10 %) non-intestinal-type tumors including 6 of 16 (38 %) sinonasal undifferentiated carcinomas, 8 of 81 (10 %) squamous cell carcinomas (including 5 of 39 non-keratinizing variants), 2 of 20 (10 %) salivary-type adenocarcinomas, and 1 of 2 (50 %) small cell carcinomas. In contrast, among non-intestinal types of sinonasal tumors, cytokeratin 20 was only focally observed in 1 of 176 non-intestinal tumors (a non-keratinizing squamous cell carcinoma). All cases of non-intestinal surface-derived adenocarcinoma and esthesioneuroblastoma were negative for both markers. Both CDX-2 and cytokeratin 20 are highly sensitive for the diagnosis of sinonasal ITAC, but cytokeratin 20 is more specific. CDX-2 staining may be observed in other high grade tumor types, especially sinonasal undifferentiated carcinoma and non-keratinizing squamous cell carcinoma. As a result, in the setting of a poorly differentiated sinonasal carcinoma the diagnosis of ITAC should not be based on CDX-2 immunoexpression alone. Clear-cut glandular differentiation and cytokeratin 20 immunoexpression are more reliable features.
Keywords: CDX-2, Cytokeratin 20, Sinonasal adenocarcinoma, Sinonasal undifferentiated carcinoma
Introduction
Sinonasal intestinal-type adenocarcinoma (ITAC) is an uncommon form of head and neck cancer. Derived from the surface epithelium of the sinonasal passages, ITAC is characterized by its close histologic resemblance to carcinomas or adenomas of the gastrointestinal tract [1]. The diagnosis of ITAC is usually straightforward, with many ITACs forming glands and/or papillae with nuclear stratification and mucin production. However, approximately 20 % of ITACs are poorly differentiated, exhibiting a solid or trabecular architecture with only rare gland formation and scarce mucin [1–4]. By hematoxylin and eosin-stained sections alone, these high grade tumors may be difficult to distinguish from other poorly differentiated sinonasal neoplasms. Moreover, other sinonasal tumors may enter the differential diagnosis due to true (e.g., salivary-type adenocarcinoma) or false gland formation (e.g., Flexner–Wintersteiner rosettes in high grade esthesioneuroblastoma or acantholytic areas in squamous cell carcinoma). The difficulty is compounded by the fact that sinonasal biopsies are often very small with abundant crush artifact [5].
Despite the diagnostic challenges, it is important to diagnose ITAC correctly. From a prognostic standpoint, ITAC are usually locally aggressive tumors with a 5 year survival rate of only about 40 % [1–3, 6, 7]. In addition, ITACs and colorectal carcinomas share similar genetic profiles, so molecular testing (e.g., EGFR, KRAS, BRAF) could be similarly utilized to tailor a targeted therapeutic approach [8–10]. Finally, due to strong associations with occupational exposures, especially wood and leather dust, the diagnosis of ITAC may carry medicolegal ramifications [7, 11–14].
Immunohistochemistry can be used to aid in the diagnosis of ITAC. While sinonasal ITAC shares the CDX-2 positive/cytokeratin 20 positive immunoprofile of its colorectal counterpart, low grade non-intestinal surface-type adenocarcinomas are consistently negative [15–20]. To our knowledge, however, these immunostains have not been tested across a broad range of sinonasal neoplasms, so their specificities for ITAC are not known. We sought to determine the immunoexpression of CDX-2 and cytokeratin 20 in non-intestinal sinonasal tumors, particularly high grade neoplasms that may enter the differential diagnosis of poorly differentiated sinonasal ITAC.
Materials and Methods
Paraffin-embedded formalin-fixed tissue from 182 sinonasal neoplasms was retrieved from the surgical pathology archives of The Johns Hopkins Hospital. The cases included 6 cases of sinonasal ITAC on whole slides and 176 non-intestinal sinonasal tumors present on previously constructed tissue microarrays [21]. Two to three cores 1 mm in diameter were taken from each donor block to address tumor heterogeneity. The non-intestinal sinonasal neoplasms included 81 squamous cell carcinomas and variants, 48 esthesioneuroblastomas, 20 salivary-type adenocarcinomas, 16 sinonasal undifferentiated carcinomas, 6 non-intestinal surface-type adenocarcinomas, 3 NUT midline carcinomas, and 2 small cell carcinomas. The human papillomavirus status for each sinonasal carcinoma was previously determined as previously described [21].
CDX-2 (clone EPR2764Y; Dako, Carpinteria, CA; prediluted by manufacturer) and cytokeratin 20 (clone Ks20.8; Dako; prediluted by manufacturer) immunohistochemistry was performed on each of the 4 micrometer thick sections on a Benchmark XT autostainer (Ventana Medical Systems, Inc. Tucson, AZ). The pattern of nuclear (for CDX-2) or cytoplasmic (for cytokeratin 20) staining for each tumor was recorded. Both intensity (weak, moderate, and strong) and extent (% of tumor nuclei positive) of staining was recorded. Positivity in ≥50 % of tumor cells was considered diffuse, while staining in <50 % of tumor cells was regarded as focal. Any tumor that was positive for either marker was closely re-reviewed to confirm the diagnosis. All neoplasms were classified by the histologic and immunophenotypic criteria set forth in the WHO Classification of Head and Neck Tumors [22].
Results
The results are summarized in Table 1. CDX-2 and cytokeratin 20 were both positive in all 6 cases of sinonasal ITAC (Fig. 1). CDX-2 immunoexpression was strong in 4 of 6 and diffuse in 5 of 6 ITACs. Cytokeratin 20 staining was strong in all ITACs, and diffuse in 4 of 6. Interestingly, in the tumors where immunostaining was focal for one antibody, diffuse positivity was seen for the other one (e.g., focal cytokeratin 20 but diffuse CDX-2 expression).
Table 1.
CDX-2 and cytokeratin 20 immunoexpression in sinonasal neoplasms
| CDX-2 | Cytokeratin 20 | |
|---|---|---|
| Intestinal-type adenocarcinoma | 6/6 (100) | 6/6 (100) |
| Squamous cell carcinoma | 8/81 (10) | 1/81 (1) |
| Non- or partially-keratinizing | 5/39 (13) | 1/39 (3) |
| Keratinizing | 2/21 (10) | 0/21 (0) |
| Papillary | 1/5 (20) | 0/5 (0) |
| Other variants | 0/16 (0) | 0/16 (0) |
| Sinonasal undifferentiated carcinoma | 6/16 (38) | 0/16 (0) |
| Salivary-type adenocarcinoma | 2/20 (10) | 0/20 (0) |
| Adenoid cystic carcinoma | 1/12 (8) | 0/12 (0) |
| Myoepithelial carcinoma | 1/3 (33) | 0/3 (0) |
| Other types | 0/5 (0) | 0/5 (0) |
| Small cell carcinoma | 1/2 (50) | 0/2 (0) |
| Esthesioneuroblastoma | 0/48 (0) | 0/48 (0) |
| Non-intestinal surface-type adenocarcinoma | 0/6 (0) | 0/6 (0) |
| NUT midline carcinoma | 0/3 (0) | 0/3 (0) |
| Total | 23/182 (13) | 7/182 (4) |
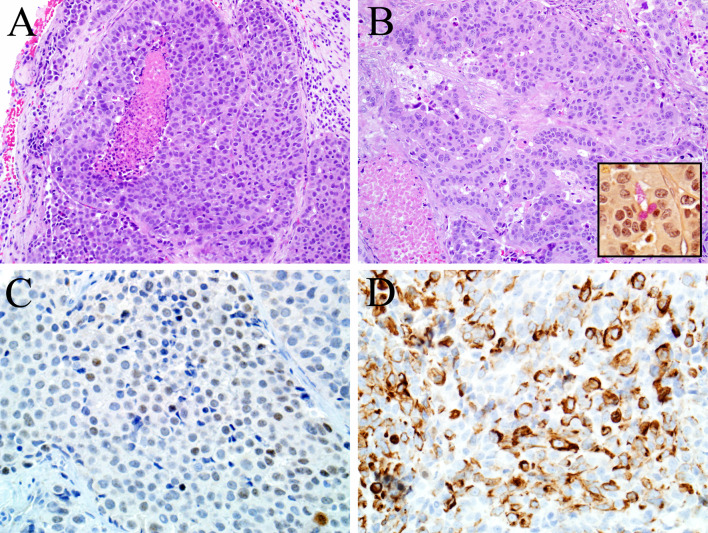
Fig. 1.
This example of sinonasal intestinal-type adenocarcinoma is very poorly differentiated, growing as nests and sheets of pleomorphic cells with necrosis (a) and exhibiting only focal gland formation (b) and mucin production demonstrated by a mucicarmine stain (inset of b). CDX-2 (c) and cytokeratin 20 (d) immunostains were consistently positive in the sinonasal intestinal-type adenocarcinomas
CDX-2 immunoexpression was also observed in 17 of 176 (10 %) non-intestinal type tumors, where CDX-2 staining was usually diffuse (10 of 17 cases) and weak (14 of 17 cases) (Fig. 2). The CDX-2 positive tumors included 6 of 16 (38 %) sinonasal undifferentiated carcinomas, 8 of 81 (10 %) squamous cell carcinomas, 2 of 20 (10 %) salivary-type adenocarcinomas, and 1 of 2 (50 %) small cell carcinomas. The CDX-2 positive squamous cell carcinomas included 5 non-keratinizing, 2 keratinizing, and 1 papillary variants; 4 of them were human papillomavirus-related carcinomas. The two CDX-2 positive salivary-type carcinomas included one adenoid cystic carcinoma (where the positivity was peripheral suggestive of a myoepithelial distribution) and one myoepithelial carcinoma. In contrast, cytokeratin 20 was only observed in 1 of 176 non-intestinal-type tumors: a non-keratinizing squamous cell carcinoma that was also diffusely and weakly CDX-2 positive (Fig. 3). The cytokeratin 20 staining was focal but strong. All cases of non-intestinal surface-type adenocarcinoma and esthesioneuroblastoma were negative for both CDX-2 and cytokeratin 20. Overall, CDX-2 was 100 % sensitive and 90 % specific for the diagnosis of sinonasal ITAC. In comparison, cytokeratin 20 was also 100 % sensitive but 99 % specific for ITAC.
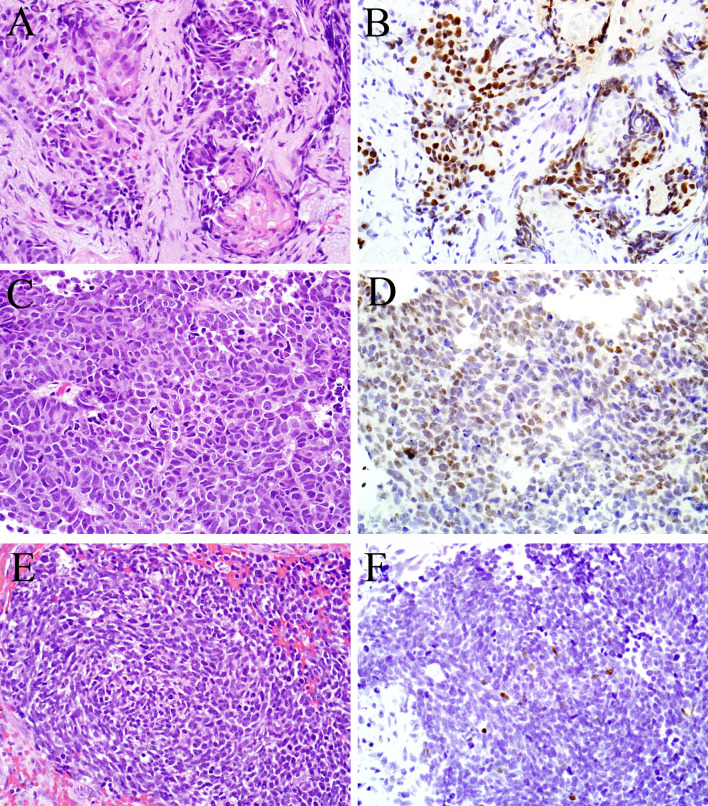
Fig. 2.
CDX-2 immunohistochemistry was not limited to sinonasal intestinal-type adenocarcinoma. Varying degrees of staining were also observed in some cases of squamous cell carcinoma (a, b) and sinonasal undifferentiated carcinoma (c, d) as well as one small cell carcinoma (e, f)
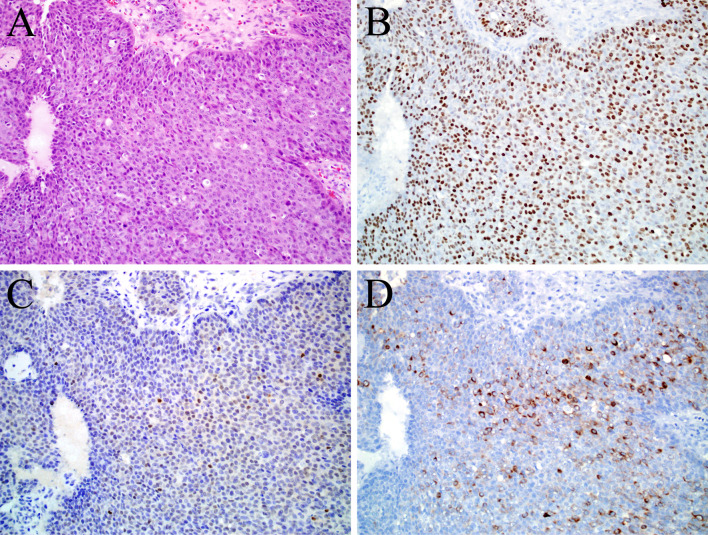
Fig. 3.
This is a case of non-keratinizing squamous cell carcinoma (a) as supported by diffuse and strong staining for the squamous marker p40 (b), but it also expressed CDX-2 (c) and cytokeratin 20 (d). This was the only sinonasal tumor aside from the intestinal-type adenocarcinomas that was cytokeratin 20 positive
Discussion
Sinonasal ITAC is an uncommon form of head and neck cancer that is characterized by an enteric phenotype, aggressive behavior, and association with exposures to industrial agents. ITAC is important to recognize for prognostic and potentially therapeutic and medicolegal reasons. ITAC has a variable histologic appearance. At one end of the spectrum, it has well-formed glands and/or papillae with abundant mucin. At the other extreme, however, ITAC may be very poorly differentiated and may therefore be in the differential diagnosis with other high grade sinonasal malignancies like poorly differentiated squamous cell carcinoma or sinonasal undifferentiated carcinoma. Immunohistochemistry for CDX-2 and cytokeratin 20 is often used to confirm the diagnosis of sinonasal ITAC, but the usefulness of these immunostains is not entirely clear because they have not been previously performed on a wide range of sinonasal malignancies.
We confirmed that CDX-2 and cytokeratin 20 are highly sensitive for sinonasal ITAC, as has been previously shown [15–20]. It is worth noting that the immunostaining was not always diffuse in ITACs: focal positivity for CDX-2 and cytokeratin 20 was seen in 1 of 6 and 2 of 6 cases, respectively. In addition, the two immunostains were complementary; in the 3 cases where one was only focally positive, the other was diffuse. Both markers were consistently negative in non-intestinal surface-type adenocarcinomas, as others have previously demonstrated [15–20]. On the other hand, we found that neither marker is entirely specific. Cytokeratin 20 staining in non-intestinal tumors was very rare (i.e., a single squamous cell carcinoma), but CDX-2 immunoexpression was observed in examples of keratinizing and non-keratinizing squamous cell carcinoma, sinonasal undifferentiated carcinoma, small cell carcinoma, adenoid cystic carcinoma, and myoepithelial carcinoma. Practically speaking, most cases of adenoid cystic carcinoma, myoepithelial carcinoma, and keratinizing or papillary squamous cell carcinoma would not be mistaken for ITAC, but poorly differentiated ITAC could be confused with sinonasal undifferentiated carcinoma, small cell carcinoma, or non-keratinizing squamous cell carcinoma. CDX-2 immunoexpression in these high grade tumors is a potential diagnostic pitfall, particularly when histologic examination is limited by sample size or artifact, and therefore immunohistochemistry is relied upon heavily. Accordingly, in a poorly differentiated carcinoma of the sinonasal tract, the diagnosis of ITAC should not be made on the basis of CDX-2 staining alone. True glandular differentiation at the histologic level is a reliable feature which virtually excludes those other high grade tumors; a positive mucicarmine stain may also be helpful in highlighting mucin production. In addition, an immunohistochemical panel that includes the more specific intestinal marker cytokeratin 20, the squamous markers p40 and cytokeratin 5/6, and the neuroendocrine markers synaptophysin and chromogranin should resolve the differential diagnosis in most cases.
The CDX-2 immunoexpression in non-intestinal sinonasal tumors is curious. One potential possibility for this unexpected finding is that these tumors actually represent ITACs that were misclassified. However, all of the CDX-2 positive tumors were closely re-reviewed, and none of them exhibited gland formation or mucin production. Moreover, all but one of these carcinomas were negative for cytokeratin 20. It is possible that some or all of the CDX-2 positive sinonasal undifferentiated carcinomas actually represented de-differentiated ITACs, but without any concurrent or prior evidence of adenocarcinoma it is difficult to confirm that hypothesis. CDX-2 immunoexpression in non-intestinal types of tumors could represent primitive intestinal differentiation that is the result of pluripotency of the sinonasal mucosa stem cells. The fact that one case of squamous cell carcinoma co-expressed CDX-2 and cytokeratin 20 lends some support to this possibility. Finally, perhaps the most likely explanation is that CDX-2 is simply not as specific for intestinal differentiation as it was once thought to be. Indeed, CDX-2 immunoexpression has recently been increasingly reported in human tissues that lack other evidence of intestinal differentiation, including leukemias [23, 24], endometrioid carcinomas [25], thyroid carcinomas [26–29], and even reactive urothelium [30].
To summarize, both CDX-2 and cytokeratin 20 are highly sensitive for sinonasal ITAC, but cytokeratin 20 is more specific. Despite its lack of absolute specificity for the intestinal phenotype, CDX-2 immunohistochemistry is still useful for the diagnosis of sinonasal ITAC; after all, we did find the marker to be 100 % sensitive and 90 % specific. In the setting of a high grade sinonasal carcinoma, though, CDX-2 should not be used indiscriminately as evidence of a poorly differentiated ITAC. Instead, in the absence of overt intestinal differentiation at the histologic level, the diagnosis of ITAC should be reserved for tumors that do exhibit at least focally clear-cut gland formation along with CDX-2/cytokeratin 20 co-expression.
References
- 1.Franchi A, Santucci M, Wenig BM. Intestinal-type adenocarcinomas. In: Barnes L, Eveson JW, Reichart P, Sidranksy D, editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. p. 20−2.
- 2.Franchi A, Gallo O, Santucci M. Clinical relevance of the histological classification of sinonasal intestinal-type adenocarcinomas. Hum Pathol. 1999;30(10):1140–1145. doi: 10.1016/S0046-8177(99)90029-1. [DOI] [PubMed] [Google Scholar]
- 3.Barnes L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am J Surg Pathol. 1986;10(3):192–202. doi: 10.1097/00000478-198603000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Urso C, Ninu MB, Franchi A, Paglierani M, Bondi R. Intestinal-type adenocarcinoma of the sinonasal tract: a clinicopathologic study of 18 cases. Tumori. 1993;79(3):205–210. doi: 10.1177/030089169307900310. [DOI] [PubMed] [Google Scholar]
- 5.Stelow EB, Mills SE. Neural, neuroectodermal, and neuroendocrine neoplasms. Biopsy interpretation of the upper aerodigestive tract and ear. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 149. [Google Scholar]
- 6.Franquemont DW, Fechner RE, Mills SE. Histologic classification of sinonasal intestinal-type adenocarcinoma. Am J Surg Pathol. 1991;15(4):368–375. doi: 10.1097/00000478-199104000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kleinsasser O, Schroeder HG. Adenocarcinomas of the inner nose after exposure to wood dust. Morphological findings and relationships between histopathology and clinical behavior in 79 cases. Arch Otorhinolaryngol. 1988;245(1):1–15. doi: 10.1007/BF00463541. [DOI] [PubMed] [Google Scholar]
- 8.Franchi A, Fondi C, Paglierani M, Pepi M, Gallo O, Santucci M. Epidermal growth factor receptor expression and gene copy number in sinonasal intestinal type adenocarcinoma. Oral Oncol. 2009;45(9):835–838. doi: 10.1016/j.oraloncology.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Szablewski V, Solassol J, Poizat F, Larrieux M, Crampette L, Mange A, et al. EGFR Expression and KRAS and BRAF Mutational Status in Intestinal-Type Sinonasal Adenocarcinoma. Int J Mol Sci. 2013;14(3):5170–5181. doi: 10.3390/ijms14035170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Inclan C, Lopez F, Perez-Escuredo J, Cuesta-Albalad MP, Vivanco B, Centeno I, et al. EGFR status and KRAS/BRAF mutations in intestinal-type sinonasal adenocarcinomas. Cell Oncol (Dordr). 2012;35(6):443–450. doi: 10.1007/s13402-012-0103-7. [DOI] [PubMed] [Google Scholar]
- 11.Health. NIfOSa. Health effects of exposure to wood dust: a summary of the literature. Washington, D.C.: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, Division of Standards Development and Technology Transfer; 1987.
- 12.Kleinsasser O, Schroeder HG, Mayer-Brix J. Preinvasive stages of adenocarcinoma of the nose after exposure to wood dust. Eur Arch Otorhinolaryngol. 1991;248(4):222–229. doi: 10.1007/BF00173661. [DOI] [PubMed] [Google Scholar]
- 13.d’Errico A, Pasian S, Baratti A, Zanelli R, Alfonzo S, Gilardi L, et al. A case-control study on occupational risk factors for sino-nasal cancer. Occup Environ Med. 2009;66(7):448–455. doi: 10.1136/oem.2008.041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonneterre V, Deschamps E, Persoons R, Bernardet C, Liaudy S, Maitre A, et al. Sino-nasal cancer and exposure to leather dust. Occup Med (Lond). 2007;57(6):438–443. doi: 10.1093/occmed/kqm050. [DOI] [PubMed] [Google Scholar]
- 15.Cathro HP, Mills SE. Immunophenotypic differences between intestinal-type and low-grade papillary sinonasal adenocarcinomas: an immunohistochemical study of 22 cases utilizing CDX2 and MUC2. Am J Surg Pathol. 2004;28(8):1026–1032. doi: 10.1097/01.pas.0000126856.09058.71. [DOI] [PubMed] [Google Scholar]
- 16.Franchi A, Massi D, Baroni G, Santucci M. CDX-2 homeobox gene expression. Am J Surg Pathol. 2003;27(10):1390–1391. doi: 10.1097/00000478-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Franchi A, Massi D, Palomba A, Biancalani M, Santucci M. CDX-2, cytokeratin 7 and cytokeratin 20 immunohistochemical expression in the differential diagnosis of primary adenocarcinomas of the sinonasal tract. Virchows Archiv Int J pathol. 2004;445(1):63–67. doi: 10.1007/s00428-004-1030-4. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy MT, Jordan RC, Berean KW, Perez-Ordonez B. Expression pattern of CK7, CK20, CDX-2, and villin in intestinal-type sinonasal adenocarcinoma. J Clin Pathol. 2004;57(9):932–937. doi: 10.1136/jcp.2004.016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortiz-Rey JA, Alvarez C, San Miguel P, Iglesias B, Anton I. Expression of CDX2, cytokeratins 7 and 20 in sinonasal intestinal-type adenocarcinoma. Appl Immunohistochem Mol Morphol. 2005;13(2):142–146. doi: 10.1097/01.pai.0000133058.00588.15. [DOI] [PubMed] [Google Scholar]
- 20.Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27(3):303–310. doi: 10.1097/00000478-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37(2):185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes L, Tse LLY, Hunt JL, Brandwein-Gensler M, Curtin HD, Boffetta P. Tumours of the nasal cavity and paranasal sinuses: introduction. In: Barnes L, Eveson JW, Reichart P, Sidranksy D, editors. World Health Organization classification of tumours: Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 12–15. [Google Scholar]
- 23.Scholl C, Bansal D, Dohner K, Eiwen K, Huntly BJ, Lee BH, et al. The homeobox gene CDX2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis. J Clin Investig. 2007;117(4):1037–1048. doi: 10.1172/JCI30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riedt T, Ebinger M, Salih HR, Tomiuk J, Handgretinger R, Kanz L, et al. Aberrant expression of the homeobox gene CDX2 in pediatric acute lymphoblastic leukemia. Blood. 2009;113(17):4049–4051. doi: 10.1182/blood-2008-12-196634. [DOI] [PubMed] [Google Scholar]
- 25.Wani Y, Notohara K, Saegusa M, Tsukayama C. Aberrant Cdx2 expression in endometrial lesions with squamous differentiation: important role of Cdx2 in squamous morula formation. Hum Pathol. 2008;39(7):1072–1079. doi: 10.1016/j.humpath.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Bongiovanni M, Piana S, Frattini M, Giovanella L, Spitale A, Ragazzi M, et al. CDX2 expression in columnar variant of papillary thyroid carcinoma. Thyroid. 2013 doi: 10.1089/thy.2013.0146. [DOI] [PubMed] [Google Scholar]
- 27.Sujoy V, Pinto A, Nose V. Columnar cell variant of papillary thyroid carcinoma: a study of 10 cases with emphasis on CDX2 expression. Thyroid. 2013;23(6):714–719. doi: 10.1089/thy.2012.0455. [DOI] [PubMed] [Google Scholar]
- 28.Cameselle-Teijeiro J, Alberte-Lista L, Peteiro-Gonzalez D, Abdulkader-Nallib I, Reyes-Santias R, Soares P, et al. CDX2 expression in some variants of papillary thyroid carcinoma. Am J Clin Pathol. 2012;138(6):907. doi: 10.1309/AJCP1BGCA6MFCNKH. [DOI] [PubMed] [Google Scholar]
- 29.Enriquez ML, Baloch ZW, Montone KT, Zhang PJ, LiVolsi VA. CDX2 expression in columnar cell variant of papillary thyroid carcinoma. Am J Clin Pathol. 2012;137(5):722–726. doi: 10.1309/AJCPXE3PUBWVZCGZ. [DOI] [PubMed] [Google Scholar]
- 30.Steininger H, Mueller H, Marquardt L. Aberrant expression of CDX2 in metaplastic and inflammatory epithelium of the urinary bladder. Am J Surg Pathol. 2005;29(9):1252. doi: 10.1097/01.pas.0000162242.31810.d4. [DOI] [PubMed] [Google Scholar]