Abstract
Since the publication of the World Health Organization Classification of Head and Neck Tumors (Barnes et al., World Health Organization classification of tumours. Pathology and genetics head and neck tumours, IARC Press, Lyon, pp 10–80, 2001), a number of sinonasal lesions have been more completely described. This manuscript will focus on three such “new” lesions including sero mucinous hamartoma, HPV-related carcinoma with adenoid cystic-like features and low-grade sinonasal sarcoma with neural and myogenic features.
Keywords: Sinonasal hamartomas, Seromucinous hamartoma, Human papillomavirus-related carcinoma with adenoid cystic-like features, Low-grade sinonasal sarcoma with neural and myogenic differentiation
Sinonasal Hamartomas
Hamartomas of the sinonasal tract are uncommon. The majority of sinonasal hamartomas are of the pure epithelial type but mesenchymal hamartomas or mixed epithelial-mesenchymal hamartomas may occur. The classification of sinonasal hamartomas are listed in Table 1.
Table 1.
Classification of sinonasal hamartomas
| Epithelial |
| Respiratory epithelial adenomatoid hamartoma; |
| Seromucinous hamartoma |
| Mixed epithelial and mesenchymal |
| Chondroosseous and respiratory epithelial (CORE) hamartoma |
| Mesenchymal |
| Nasal chondromesenchymal hamartoma (NCH) |
Classification
The question arises whether the classification of these lesions as hamartomas is justified or whether they represent inflammatory-related lesions or even neoplastic proliferations (see below under Molecular Findings). While there are findings that weigh for as well as against classification as hamartomatous lesions, at present there are no definitive findings to negate classification as hamartomas and no findings that definitively allow classification as either an inflammatory lesion or neoplasm. As such, until more definitive evidence supports alternative considerations, these lesions should be classified as hamartomas.
Seromucinous Hamartoma (SH)
Seromucinous hamartoma of the sinonasal tract is a benign acquired nonneoplastic overgrowth of indigenous glands of the sinonasal tract, and rarely of the nasopharynx, arising from submucosally situated seromucinous glands. SH were initially described by Baille and Batsakis [2] in a case report of a patient with a nasopharyngeal lesion. Subsequent to that description, there have been a number of other case reports but only recently was SH the subject of larger studies, including 7 cases reported by Weinreb et al. [3] and 5 cases reported by Ambrosini-Spaltro et al. [4]. To date, there are less than 25 cases of SH identified in the world literature [2–10].
Table 2 details the comparison of various features among sinonasal hamartomas. Owing to cases showing histologic features of both respiratory epithelial adenomatoid hamartoma and seromucinous hamartoma, it is conceivable that these lesions represent a spectrum of the same lesion rather than different lesions.
Table 2.
Comparison of the Sinonasal Hamartomas
| READ Hamartoma | SH | CORE | NCH | |
|---|---|---|---|---|
| Age/Gender | M > F; 3rd to 9th decades, median 6th decade | M > F; 2nd decade to 9th decade | M = F; 2nd to 8th decades | M > F; most occur in newborns within the first 3 months of life but may occur in the second decade of life, and occasionally in adults |
| Site(s) of occurrence | Nasal cavity, in particular posterior nasal septum; involvement of other intranasal sites occurs less often and may be identified along the lateral nasal wall, middle meatus and inferior turbinate; other sites of involvement include the nasopharynx, ethmoid sinus, and frontal sinus | Posterior nasal septum although may occur in the lateral nasal wall, paranasal sinuses and nasopharynx | Nasal cavity most common; other sites include nasopharynx, ethmoid sinus and sphenoid sinus | Intranasal mass or facial swelling; may erode into the cranial cavity (through the cribriform plate area) |
| Histology | Glandular proliferation composed of widely-spaced, small to medium-sized glands separated by stromal tissue; glands arise in direct continuity with the surface epithelium, which invaginate downward into the submucosa; glands are round to oval composed of multilayered ciliated respiratory epithelium often with admixed mucin-secreting (goblet) cells; characteristic finding is the presence of stromal hyalinization with envelopment of glands by a thick, eosinophilic basement membrane; atrophic glands may be lined by a single layer of flattened to cuboidal-appearing epithelium; reactive seromucinous gland proliferation present in between glandular proliferations can be seen | Dense serous gland proliferation with back-to-back appearance resemble a cribriform pattern of growth; glands are lined by low cuboidal to flat epithelium cells with round to oval nuclei and a variable amount of basophilic to eosinophilic to clear appearing cytoplasm; invagination of the surface lack a significant mucinous cell component although focal mucinous change may be found | Histologic features of READ hamartoma (although adenomatoid components tend to be of less prominent) and intimate association with cartilaginous and/or osseous trabeculae | Nodules of cartilage varying in size, shape and contour; loose spindle cell stroma or abrupt transition to hypocellular fibrous stroma present at the periphery of the cartilaginous nodules; mature adipose tissue can be present; proliferating epithelial elements are not a prominent feature |
| IHC | Cytokeratin positive (AE1/AE3, CAM 5.2, CK7); negative for CK20 and CDX2; p63 and CK903 (34βE12) staining of basal (myoepithelial) cells but may be absent; S100 may or may not be positive; low proliferation rate | Seromucinous glands reactive for cytokeratins (CK7, CK17, CK19), HMWK; negative for CK14, CK20; p63, calponin, MSA typically negative but in any given case may be positive; S100 protein staining is limited to the seromucinous glands; collagen type IV and laminin staining present around the glandular proliferation; low proliferation rate | None reported | Cartilaginous nodules and mesenchymal stromal component S100 protein staining (more intense staining in cartilaginous components); spindle cell stroma vimentin and smooth muscle actin reactivity; muscle specific actin (HHF35) may be present |
| Molecular findings | Increased fractional allelic loss (as compared to chronic sinusitis but less than that for adenocarcinoma) | Higher mutation rate in comparison to normal seromucinous glands | None reported | None reported |
| Associated Lesions | Sinonasal inflammatory polyps; hyperplasia and/or squamous metaplasia of the surface epithelium unrelated to the adenomatoid proliferation; osseous metaplasia; rare association with inverted type Schneiderian papilloma, and solitary fibrous tumor; reported instances of low-grade adenocarcinomas associated with READ hamartomas | Sinonasal inflammatory polyps; READ hamartoma | None reported | None reported |
READ respiratory epithelial adenomatoid (Hamartoma), SH seromucinous hamartoma, CORE chondro-osseous and respiratory epithelial (CORE) hamartoma, NCH nasal chondromesenchymal hamartoma, IHC immunohistochemistry
Clinical Findings
SH most commonly occurs as an incidental finding seen in surgical material from patients removed for clinical diagnoses such as chronic sinusitis and/or sinonasal inflammatory polyps. Symptomatic patients may present with nasal obstruction and epistaxis. There is a slight male predilection and SH occur over a wide age range including 2nd decade to 9th decade of life. The most common site of occurrence is the posterior nasal septum although SH may occur in the lateral nasal wall, paranasal sinuses and nasopharynx. Although generally limited in extent, SH may extend into adjacent sinuses (e.g., maxillary, ethmoid). There are no known etiologic factors, although some patients with SH have been reported in association with chronic sinusitis, inflammatory polyps, rheumatoid arthritis and Parkinson disease. Radiographically, SHs may appear as polypoid lesion without evidence of aggressive growth such as bone destruction or neuropathies.
Pathologic Features
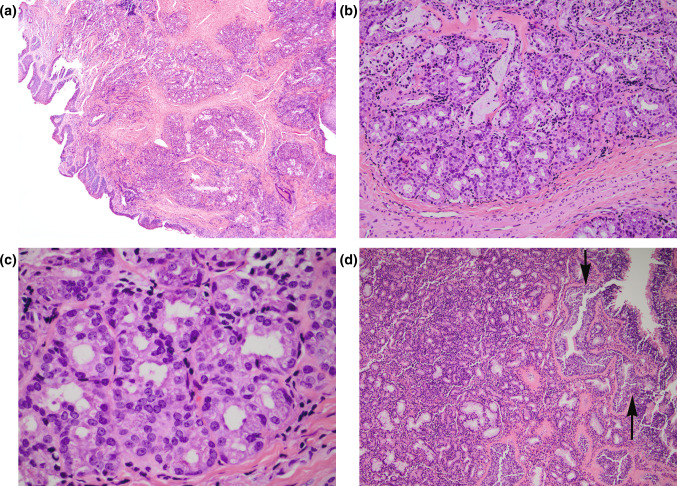
SH are polypoid to exophytic appearing lesions measuring from 6 mm to 4 cm in greatest dimension. Histologically, SH are covered by benign ciliated respiratory epithelium that may show squamous metaplasia and often have an associated hypocellular edematous, myxoid and/or fibrous stroma similar to that seen in sinonasal inflammatory polyps. The characteristic finding is the presence of a submucosal epithelial proliferation of small glands, serous acini and tubules growing in clusters and lobules (Fig. 1a, b). In some cases, haphazard arrangement of the glands characterized by absence of defined lobules comprised of irregularly shaped glands including larger glands and cysts may be present. In some cases, the serous glands may be densely packed with a back-to-back appearance that may resemble a cribriform pattern of growth (Fig. 1c). The glands are lined by low cuboidal to flat epithelium cells with round to oval nuclei and a variable amount of basophilic to eosinophilic to clear appearing cytoplasm. There is an absence of significant nuclear pleomorphism, increased mitotic activity and necrosis. Invagination of the surface respiratory epithelium may be seen with at least focal merging with the glandular proliferation and in conjunction with periglandular hyalinization shows features of respiratory epithelial adenomatoid hamartoma (Fig. 1d). The glands vary in appearance from round to oval to angulated to branching and stellate containing luminal mucin material. SHs lack a significant mucinous cell component although focal mucinous change may be found. A mixed chronic inflammatory cell infiltrate comprised of mature lymphocytes and plasma cells can be seen but a significant population of eosinophils is not typically present.
Fig. 1.
a, b. Seromucinous hamartoma characterized by the presence of a submucosal epithelial proliferation of small glands, serous acini and tubules growing in clusters and lobules; c in areas the glands may be densely packed with a back-to-back (cribriform) appearance that may suggest a possible diagnosis of an adenocarcinoma. d Invagination of the surface respiratory epithelium may be seen (arrows) that in conjunction with periglandular hyalinization are features seen in the READ hamartoma. These histologic features in conjunction with the submucosal seromucinous glandular proliferation that typify features of SH raise the notion that SH and READ hamartoma represent a spectrum of the same lesion rather than different lesions
Immunohistochemistry
Surface epithelium and the seromucinous glands are reactive for cytokeratins, including CK7, CK17, CK19 and high molecular weight cytokeratin (HMWK) but negative for CK14 and CK20. The surface respiratory epithelium shows p63 positive basal cells but the seromucinous glands are typically negative for p63 [2, 4, 5, 10]. In addition, calponin and muscle specific actin are negative in both the surface respiratory epithelium and the seromucinous glands. Fleming et al. [8] reported a case of SH showing p63, calponin and actin staining indicating that not all SHs lack p63 reactivity. S100 protein staining is limited to the seromucinous glands. Collagen type IV and laminin staining is present around the glandular proliferation. Ki67 (MIB1) staining is either absent or shows a very low proliferation rate (1–2 %).
Molecular Findings
Most authorities view SH as a nonneoplastic proliferation. However, Ozolek and Hunt [9] evaluated the molecular profile of sinonasal hamartoma, sinonasal adenocarcinomas and chronic sinusitis. These authors found a fractional allelic loss of 31 % in hamartomas, 64 % in sinonasal adenocarcinoma and 2 % in chronic sinusitis. The authors indicated that based on the molecular profile of hamartomas which would be considered unusually high for a non-neoplastic entity, the appreciable allelic loss within hamartomas suggests the possibility that they may be a benign neoplasm rather than a hamartoma. Ambrosini-Spaltro et al. [4] performed DNA mutation analysis on SH that showed a higher mutation rate in comparison to normal seromucinous glands which exhibited a lower mutation frequency (0.83 %) supporting SH as representing a benign process although no comments relative to their neoplastic nature was offered. These authors did state the similarities of SH to micro glandular adenosis of the breast.
Treatment and Prognosis
The treatment for SH include simple but complete surgical resection which is curative with no reported recurrences over extended periods of time.
Differential Diagnosis
The differential diagnosis for SH includes other benign lesions such as respiratory epithelial adenomatoid hamartoma (READ Hamartoma) [11] and Schneiderian papillomas as well as sinonasal adenocarcinomas. As previously indicated, owing to cases showing histologic features of both READ hamartoma and SH, it is conceivable that these lesions represent a spectrum of the same lesion rather than different lesions. Nevertheless, there are contrasting histologic features between SH and READ hamartoma including the presence of characteristic submucosal epithelial proliferation of small glands, serous acini and tubules growing in clusters and lobules in SH typically devoid of a mucinous cell component as well as absence of surface invagination and periglandular hyalinization. Further, the serous glands in SH may show a cribriform pattern of growth, a feature not typically seen in READ hamartoma. In contrast, READ hamartomas include surface respiratory epithelial invagination with at least focal merging with a submucosal mucinous glandular proliferation with periglandular hyalinization. READ hamartomas may also show atrophic appearing glands lined by a single layer of flattened to cuboidal-appearing epithelium, albeit with retention of the periglandular hyalinization, a feature not usually identified in SH. Relative to immunohistochemical staining, there are no specific markers differentiating SH from READ hamartoma.
The presence of residual seromucinous glands with retention of their lobular architecture represents important findings in differentiation of SH from sinonasal low-grade adenocarcinoma. There are no specific immunohistochemical markers differentiating SH from low-grade sinonasal adenocarcinoma.
Human Papillomavirus (HPV)-Related Carcinoma with Adenoid Cystic-Like Features
HPV-related carcinoma with adenoid cystic-like features is a recently described and defined type of sinonasal carcinoma with morphologic features suggestive of adenoid cystic carcinoma, immunohistochemical evidence of myoepithelial differentiation but with features distinctly unusual for adenoid cystic carcinoma, including the presence of surface intraepithelial dysplasia, absence of MYB gene rearrangement and association with HPV [12]. Given features considered distinctly unusual for adenoid cystic carcinoma, classification within the spectrum of adenoid cystic carcinoma is not justified and until and when additional evidence is reported, this entity merits distinction as a unique and recently described sinonasal neoplasm.
Clinical Findings
HPV-related carcinoma with adenoid cystic-like features is a rare tumor with less than 10 cases reported in the literature to date [12]. It is more common in women than in men and occurs over a wide age range including 40–73 years (mean 55 years). The presenting symptoms include nasal obstruction and epistaxis; less commonly, epiphora may occur. The sites of occurrence include nasal cavity and/or paranasal sinuses, including ethmoid > maxillary and/or sphenoid; orbital involvement may occur.
Pathologic Features
The histologic features include the presence of an invasive hypercellular lesion with solid, lobular and nested growth separated by fibrous stroma (Fig. 2a). Cribriform (Fig. 2b) and microcystic (Fig. 2c) growth, as well as true ductal structures are present (Fig. 2d); ductal structures represent a minor component. The microcystic spaces are filled with basophilic appearing material. The neoplastic infiltrate is comprised of two cells types, including basaloid cells and true duct cells. The basaloid cells predominate characterized by hyperchromatic and angulated nuclei, scant cytoplasm and increased nuclear-to-cytoplasmic ratio with marked increase in mitotic activity; tumor necrosis and perineural invasion may be present but lymph-vascular invasion not reported. The true duct cells appear cuboidal with eosinophilic cytoplasm and are often located at center of lobules surrounded by zones of basaloid cells. Intraepithelial dysplasia of the surface epithelium is present in a majority of cases but may be absent (Fig. 2e). The invasive component lacks squamous differentiation.
Fig. 2.
Human papillomavirus-related carcinoma with adenoid cystic-like features. a The histologic features include the presence of an invasive hypercellular lesion with solid, lobular and nested growth separated by fibrous stroma. Mixed growth patterns seen include: b cribriform, c microcystic, and d true ductal structures, the latter representing a minor component. The microcystic spaces are filled with basophilic appearing material. The neoplastic infiltrate is predominantly comprised of basaloid cells with hyperchromatic and angulated nuclei while the true duct cells appear cuboidal with eosinophilic cytoplasm often located at center of lobules surrounded by zones of basaloid cells. e Intraepithelial dysplasia of the surface epithelium is present in a majority of cases. f. Strong and diffuse p16 reactivity (nuclear and cytoplasmic) is present
Immunohistochemistry
The lesional cells are cytokeratin (AE1/AE3) positive in all cases with strong staining in duct cells and relatively weaker staining in the basaloid cells. The basaloid cells express one or more myoepithelial related markers including p63, calponin, S100 protein and/or actin. CD117 (C-kit) is consistently positive and is usually limited to ductal cells but occasionally may be diffuse. Strong and diffuse p16 reactivity is present (Fig. 2f).
Molecular Testing
HPV DNA in situ hybridization evaluation was identified in all cases with hybridization signals distributed throughout basaloid cells and ductal cells, in surface dysplasia and invasive component [12]. Quantitative PCR confirmatory of high-risk HPV verified HPV33 in 6 of 8 cases and HPV35 in 1 of 8 cases [12]. The presence of high risk HPV by PCR essentially excludes the possibility of incidental (non-specific) colonization with HPV.
Treatment and Prognosis
Treatment options include surgical resection alone, surgery plus postoperative radiotherapy, or combined chemoradiation therapy. Given the absence of long term follow-up the true biologic nature of this neoplasm is yet to be determined but in cases identified in the literature, albeit with limited follow-up periods (median 15 months). Most patients were reported to be alive without evidence of disease or with local recurrence [12].
Low-Grade Sinonasal Sarcoma with Neural and Myogenic Differentiation (LGSSNMF)
LGSSNMF is also a recently described distinct spindle cell sarcoma of the sinonasal region characterized by concomitant neural and myogenic differentiation [13]. Whether in fact LGSSNMF truly represents a bona fide independent neoplasm rather than a neoplasm that should be subsumed in another defined tumor category (e.g., fibrosarcoma or malignant Schwannoma) [14] remains to be determined requiring the identification and description of additional cases in the literature. However, on the basis of relatively unique findings, LGSSNMF merits classification as an independent recently described entity with the proviso that classification may or may not change in the future dependent on the reporting of additional cases that may support its unique classification or affirm subclassification within a larger category of sinonasal sarcomas.
Clinical Findings
To date, there are only 28 cases of LGSSNMF reported in the literature [13]. LGSSNMF is more common in women than men and occurs over a wide age range from 24 to 85 years (mean, 52 years). In fact, there is a striking predilection for women in the fifth decade of life (30 % of cases). The presenting symptoms include difficulty breathing, facial pressure, and congestion: facial pain and mild epiphora occasionally present. Some patients were reported to have had a history of sinonasal surgery for apparently benign processes. Most of the tumors involve multiple sites in the sinonasal region; the nasal cavity (54 %) and ethmoid sinus (57 %) were the most commonly involved areas, either singly or in combination. The tumors may extend to orbit, cribriform plate or into cranial vault. There are no known etiologic factors and no evidence of association with neurofibromatosis.
Pathologic Features
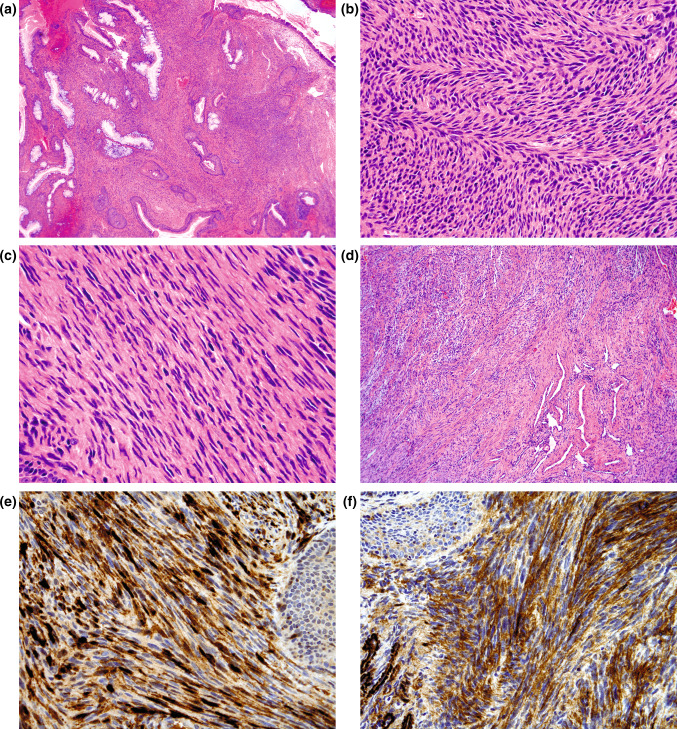
These tumors have been described as polypoid measuring up to approximately 4 cm in greatest dimension. Histologically, these tumors are poorly circumscribed and unencapsulated comprised of a cellular spindle cell proliferation organized into medium-to-long fascicles (Fig. 3a). Areas of classic “herringbone” pattern are identified in most of the cases (Fig. 3b). Intercellular collagen is typically scanty to moderate and arrayed in delicate strands. There is an absence of dense or ropey collagenous matrix. The overall cellularity of the tumor is quite high with only a mild degree of variability within a given tumor. The tumor cells are highly uniform with elongated nuclei that are focally wavy to buckled (Fig. 3c). Despite the cellularity, only rare mitotic figures are identified. A striking and characteristic finding in the majority of cases includes a concomitant benign epithelial proliferation composed of surface-type respiratory epithelium, forming invaginations of small glands or cystic spaces beneath the mucosal surface (Fig. 3a). The glandular structures are intimately admixed with neoplastic spindle cells (imparting a morphologic picture reminiscent of adenosarcoma of the female genital tract). A prominent hemangiopericytomatous vascular pattern may also be present (Fig. 3d). Focal rhabdomyoblastic differentiation identified in a minority of cases (11 %) is characterized by the presence of large cells with bright, eosinophilic cytoplasm, prominent nuclei, and focal cross-striations. There is no evidence to suggest origin from a nerve. An infiltrative growth can be appreciated at low magnification, including infiltration of sinonasal bones.
Fig. 3.
Low-grade sinonasal sarcoma with neural and myogenic differentiation. a Polypoid appearing sinonasal mucosa showing a poorly circumscribed and unencapsulated submucosal cellular spindle cell proliferation intimately associated with variably sized benign glands invaginating from the surface respiratory epithelium; b most examples show foci of classic “herringbone” pattern; c wavy to buckled appearing nuclei may be focally identified. Despite the hypercellularity, the tumor cells are highly uniform lacking significant nuclear pleomorphism and increased mitotic activity; d a hemangiopericytomatous vascular pattern may also be present; immunohistochemical staining shows the lesional cells to be reactive for e S100 protein (diffuse and strong) and f muscle specific actin
Immunohistochemistry
The lesional cells show S100 protein positive in all tumors (Fig. 3e) and may be diffuse (57 %), patchy (36 %) or restricted to isolated tumor cells (7 %). In any event, in all cases there is at least focal S100 protein staining. There is also concomitant expression of actins (muscle specific actin [Fig. 3f] and smooth muscle actin) seen in 96 % of the cases including diffuse (52 %), patchy (39 %) or restricted to isolated tumor cells (9 %). Additional reactivity may include: CD34 (reported in 5 cases: 4 focal; 1 diffuse); focal desmin (4 cases); weak EMA (3 cases); keratin (AE1/AE3) very focal (2 cases). There is no reactivity for myogenin, estrogen receptor (ER) and progesterone receptor (PR).
Cytogenetics and Molecular Genetics
RT-PCR for SYT-SSX1 and SYT-SSX2 negative in 18 cases evaluated [13]. t(2;4)(q37.1;q31.3) chromosomal translocation were identified in 2 cases [13]. Distinctive cytogenetic signature has not been reported in any of the neoplasms supporting the notion that LGSSNMF is a separate biological entity likely characterized by a specific fusion gene.
Treatment and Prognosis
Surgery is the treatment of choice with or without radiotherapy and may include craniofacial resection with orbital exenterations depending on the extent of the disease. Local recurrences have been reported in 44 % of cases with follow-up ranging from less than 1–28 years (mean, 8.3 year) [13]. Disease recurred locally from less than 1 year to a maximum of 9 years after treatment. 14 patients were reported to be alive and 2 had died from other causes [13]. There are no reported instances of known regional or distant metastases, and no patients died of disease.
Conclusion
Given the 10 year span since the last edition of the World Health Organization (WHO) Classification of Head and Neck Tumors [1], the time has arrived for a new edition of this book to be published. The identification of recently described head and neck lesions, in general, and sinonasal tract lesions, in specific, based on combinations of light microscopic, immunohistochemical and molecular findings raises the specter of adding new entities in the proposed new edition of the World Health Organization Classification of Head and Neck Tumors. I have attempted to delineate and justify the inclusion of three sinonasal tract lesions not included in the prior iteration of the WHO Classification of Head and Neck Tumors. Admittedly, an argument could be put forth that each of these 3 “new” lesion types, including seromucinous hamartoma, HPV-related carcinoma with adenoid cystic-like features and low-grade sinonasal sarcoma with neural and myogenic features do not demonstrate sufficient features as to allow independent classification rather than being subsumed within previously described entities. However, in my view based on personal experience as well as information culled from the literature, at the present time there are unique pathologic findings as described in this manuscript to merit their description as unique entities with independent classification in the next edition of the WHO Classification of Head and Neck Tumors.
References
- 1.Barnes L, Eveson JW, Reichart P, Sidransky D. Tumours of the nasal cavity and paranasal sinuses. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours. Pathology and genetics head and neck tumours. IARC Press; Lyon: 2005:10–80.
- 2.Baillie EE, Batsakis JG. Glandular (seromucinous) hamartoma of the nasopharynx. Oral Surg Oral Med Oral Pathol. 1974;38:760–762. doi: 10.1016/0030-4220(74)90397-1. [DOI] [PubMed] [Google Scholar]
- 3.Weinreb I, Gnepp DR, Laver NM, et al. Seromucinous hamartomas: a clinicopathological study of a sinonasal glandular lesion lacking myoepithelial cells. Histopathology. 2009;54:205–213. doi: 10.1111/j.1365-2559.2008.03198.x. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosini-Spaltro A, Morandi L, Spagnolo DV, et al. Nasal seromucinous hamartoma (microglandular adenosis of the nose): a morphological and molecular study of five cases. Virchows Arch. 2010;457:727–734. doi: 10.1007/s00428-010-0984-7. [DOI] [PubMed] [Google Scholar]
- 5.Khan RA, Chernock RD, Lewis JS., Jr Seromucinous hamartoma of the nasal cavity: a report of two cases and review of the literature. Head Neck Pathol. 2011;5(3):241–247. doi: 10.1007/s12105-011-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinreb I. Low grade glandular lesions of the sinonasal tract: a focused review. Head Neck Pathol. 2010;4:77–83. doi: 10.1007/s12105-009-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Ordoñez B. Hamartomas, papillomas and adenocarcinomas of the sinonasal tract and nasopharynx. J Clin Pathol. 2009;62:1085–1095. doi: 10.1136/jcp.2007.053702. [DOI] [PubMed] [Google Scholar]
- 8.Fleming KE, Perez-Ordoñez B, Nasser JG, Paooy B, Bullock MJ. Sinonasal seromucinous hamartoma: a review of the literature and a case report with focal myoepithelial cells. Head Neck. 2102;6:395–399. doi: 10.1007/s12105-012-0339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozolek JA, Hunt JL. Tumor suppressor gene alterations in respiratory epithelial adenomatoid hamartoma (REAH): comparison to sinonasal adenocarcinoma and inflamed sinonasal mucosa. Am J Surg Pathol. 2006;30:1576–1580. doi: 10.1097/01.pas.0000213344.55605.77. [DOI] [PubMed] [Google Scholar]
- 10.Ozolek JA, Barnes EL, Hunt JL. Basal/myoepithelial cells in chronic sinusitis, respiratory epithelial adenomatoid hamartoma, inverted papilloma, and intestinal-type and nonintestinal-type sinonasal adenocarcinoma: an immunohistochemical study. Arch Pathol Lab Med. 2007;131:530–537. doi: 10.5858/2007-131-530-MCICSR. [DOI] [PubMed] [Google Scholar]
- 11.Wenig BM, Heffner DK. Respiratory epithelial adenomatoid hamartomas of the sinonasal tract and nasopharynx: a clinicopathologic study of 31 cases. Ann Otol Rhinol Laryngol. 1995;104:639–645. doi: 10.1177/000348949510400809. [DOI] [PubMed] [Google Scholar]
- 12.Bishop JA, Ogawa T, Stelow EB, Moskaluk CA, Koch WM, Westra WH. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal trac. Am J Surg Pathol. 2013;37:836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis JT, Oliveira AM, Nascimento AG, et al. Low-grade sinonasal sarcoma with neural and myogenic features: a clinicopathologic analysis of 28 cases. Am J Surg Pathol. 2012;36:517–525. doi: 10.1097/PAS.0b013e3182426886. [DOI] [PubMed] [Google Scholar]
- 14.Heffner DK, Gnepp DR. Sinonasal fibrosarcomas, malignant schwannomas, and “Triton” tumors. A clinicopathologic study of 67 cases. Cancer. 1992;70:1089–1101. doi: 10.1002/1097-0142(19920901)70:5<1089::AID-CNCR2820700513>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]